Interpreting the evolution of SARS-CoV-2
Jesse Bloom
Fred Hutch Cancer Center / HHMI
These slides at https://slides.com/jbloom/unc2023
Disclosures
I am on the scientific advisory boards of Apriori Bio, Aerium Therapeutics, Invivyd, and the Vaccine Company.
I am an inventor on Fred Hutch licensed patents related to deep mutational scanning of viral proteins

The Faroe Islands


"Measles had not prevailed on the Faroes since 1781, then it broke out early in April 1846."
"Of the 7782 inhabitants, about 6000 were taken with measles."
"Of the many aged people still living in the Faroes who had measles in 1781, not one was attacked the second time."
Panum is describing immune memory, which provides lifelong protection from measles.
But we are repeatedly infected by some other viruses. Typical person infected by H3N2 influenza ~5 years. Why?
History offers natural experiment with influenza like Panum's study of measles
In 1977, old H1N1 strain from ~1954 was inadvertently re-released and caused pandemic. So re-introduction of identical virus after a few decades.
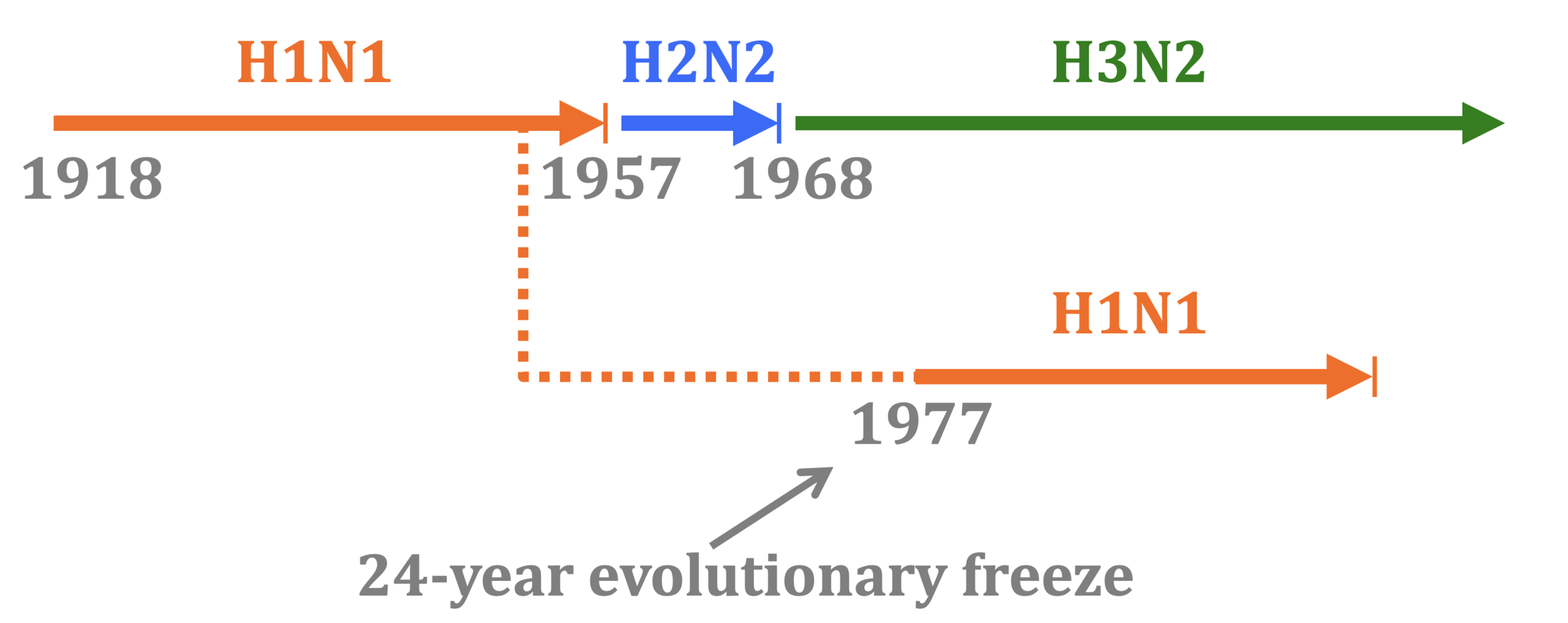


"One boy from Hong Kong had a transient febrile illness from 15 to 18 January. On Sunday 22 January, three boys were in the college infirmary… 512 boys (67%) spent between three and seven days away from class."

"Of about 130 adults who had some contact with the boys, only one, a house matron, developed similar symptoms."
Influenza infection also elicits multi-decade immunity, but only if virus is evolutionarily frozen
Some human RNA respiratory viruses evolve to escape immunity
Why some viruses evolve to escape immunity while others don't is a deep question outside scope of this talk. See here for some possible explanations.
Rate of viral antigenic evolution
Measles
Influenza

CoV-229E causes common colds and has been circulating in humans for a long time.
The typical person is infected every ~3 to 5 years.
How do other human coronaviruses evolve?
Evolution of CoV-229E spike

We experimentally generated CoV-229E spikes at ~8 year intervals so we could study them in the lab:
- 1984
- 1992
- 2001
- 2008
- 2016
Evolution of CoV-229E spike erodes neutralization by human serum antibodies
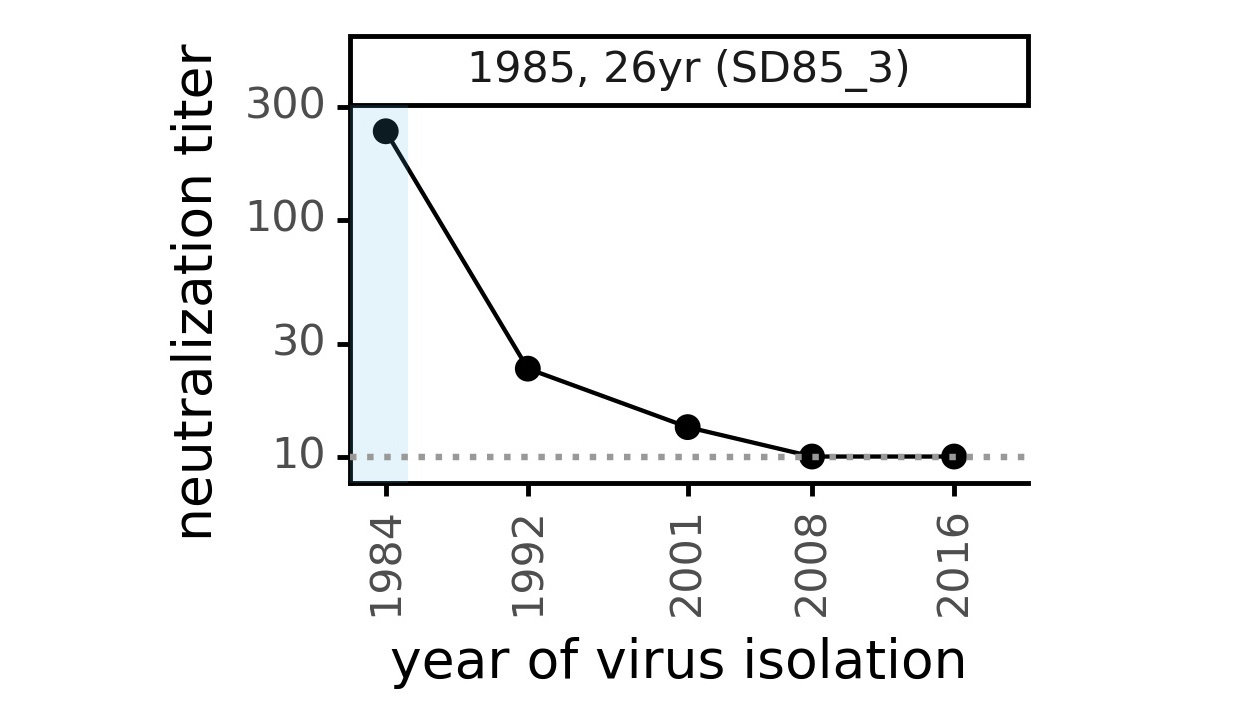
Evolution erodes CoV-229E neutralization by different sera at different rates

Ideally vaccines would elicit evolution-resistant neutralizing antibodies (like those naturally made by person at right) rather than evolution-sensitive antibodies (like those naturally made by person at left)
Strongest evolutionary selection is in RBD
Sites of evolutionary change in the spike of CoV-229E over the last four decades
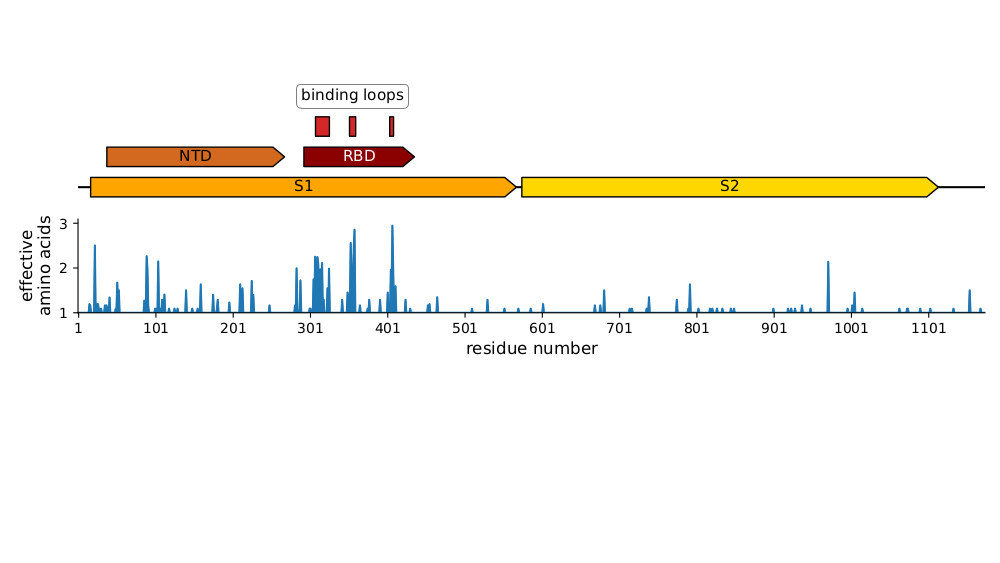
Strongest evolutionary selection is in RBD
Sites of evolutionary change in the spike of CoV-229E over the last four decades

Sites of mutations in SARS-CoV-2 Omicron BQ.1.1 spike relative to Wuhan-Hu-1
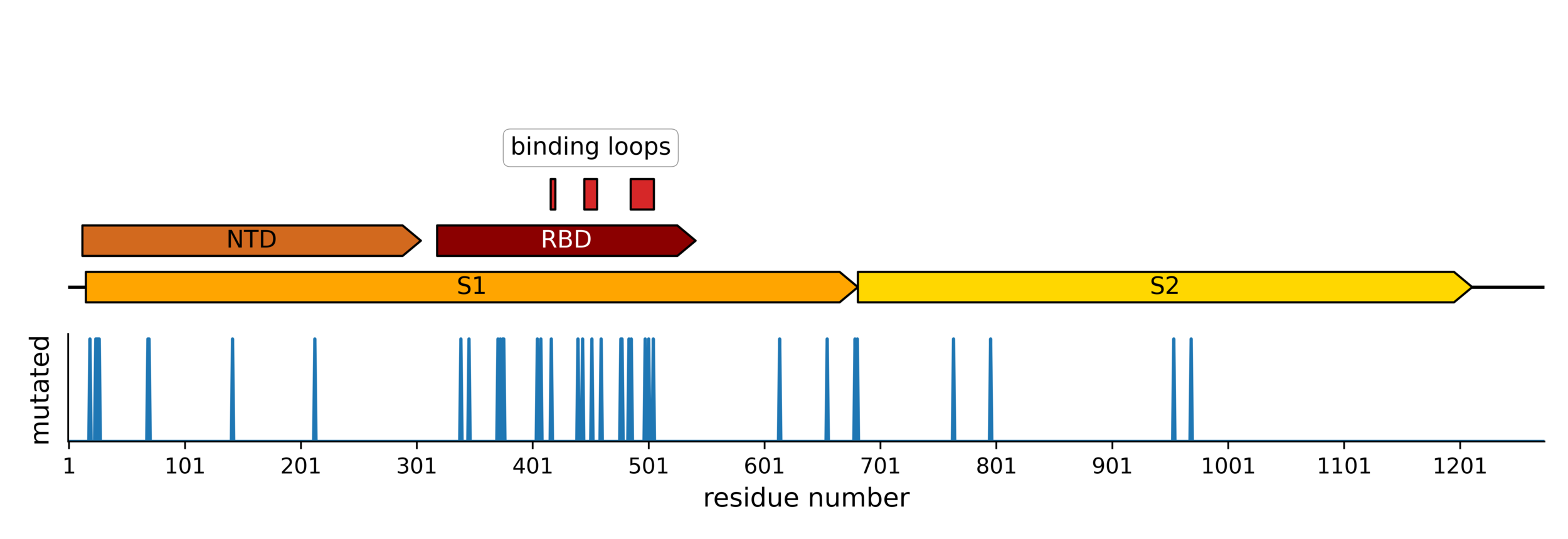
RBD does not run out of evolutionary space
25 of 31 residues in CoV-229E RBD that contact receptor varied during virus's evolution in humans over last ~50 years (Li et al, 2019)
Phylogenetic tree shape & vaccine strategy
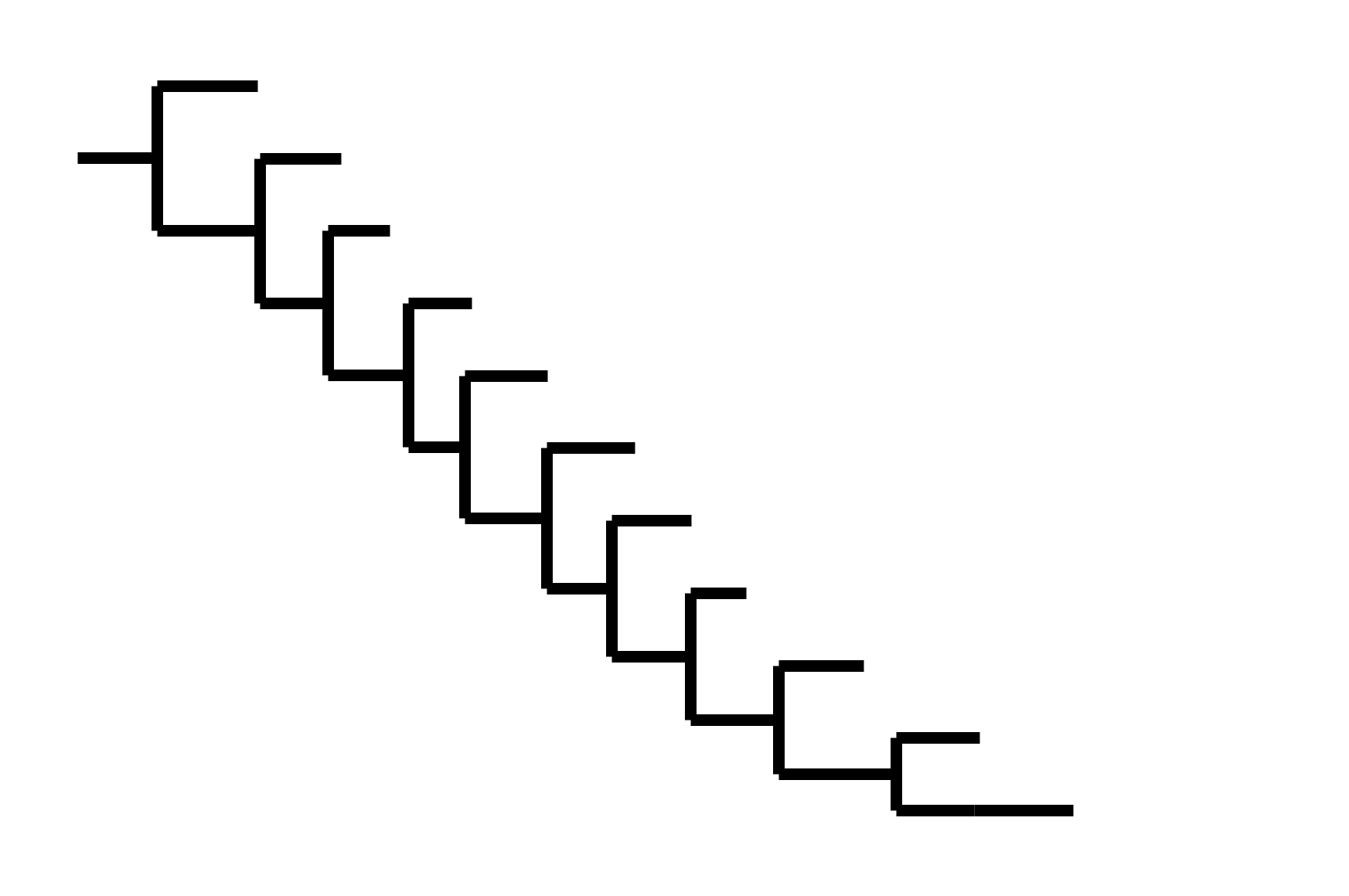
CoV-229E has ladder-like tree:
- new variants displace old ones
- new variants descend from recent successful ones
Human influenza A evolves this way too. It's theoretically possible to pick single well-matched vaccine strain.
Phylogenetic tree shape & vaccine strategy
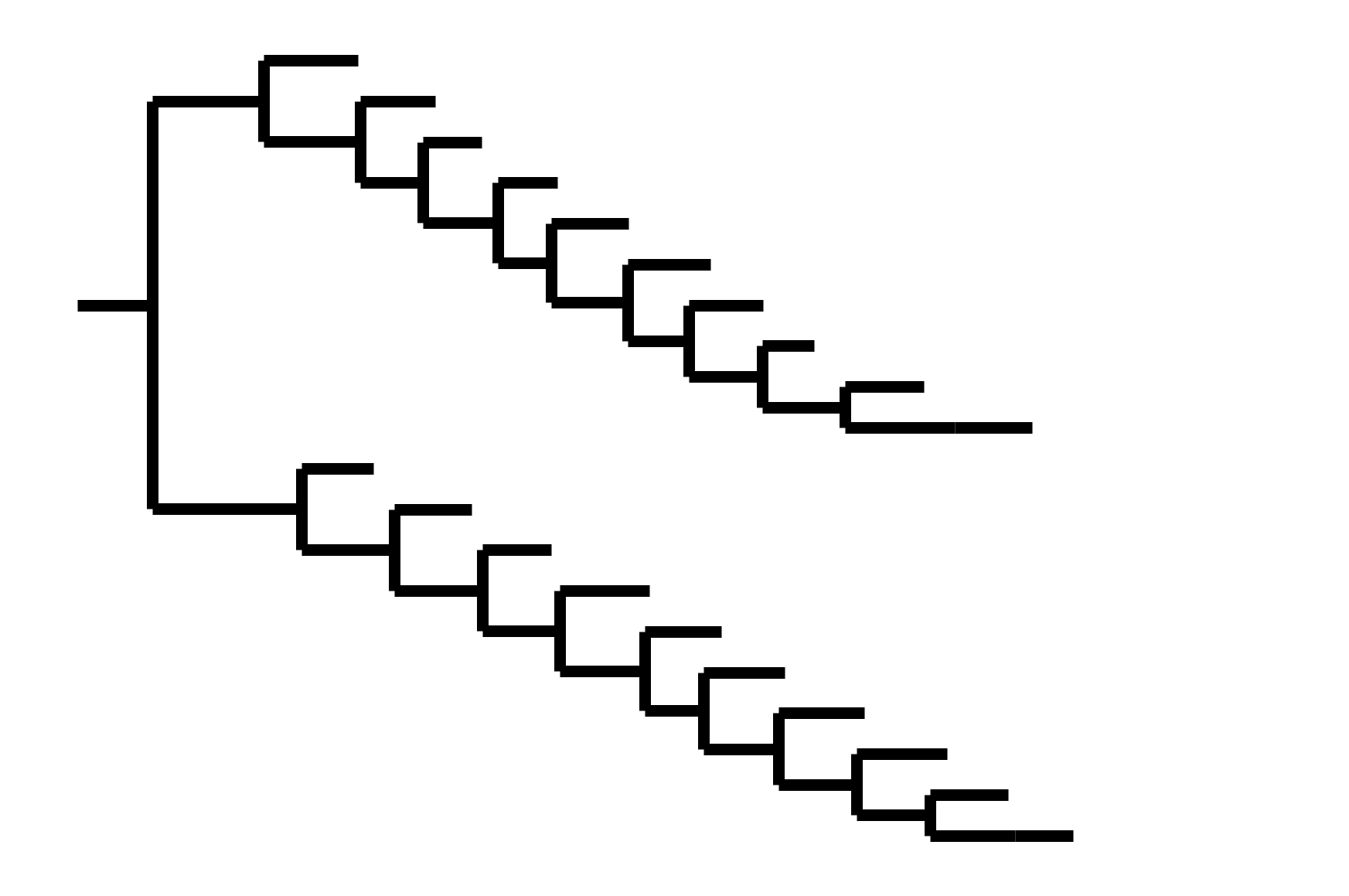

CoV-229E has ladder-like tree:
- new variants displace old ones
- new variants descend from recent successful ones
Human influenza A evolves this way too. It's theoretically possible to pick single well-matched vaccine strain.
CoV-OC43 split into two ladder-like lineages. Influenza B evolves this way too. It's theoretically possible to pick well-matched bivalent vaccine.
Phylogenetic tree shape & vaccine strategy

CoV-229E has ladder-like tree:
- new variants displace old ones
- new variants descend from recent successful ones
Human influenza A evolves this way too. It's theoretically possible to pick single well-matched vaccine strain.

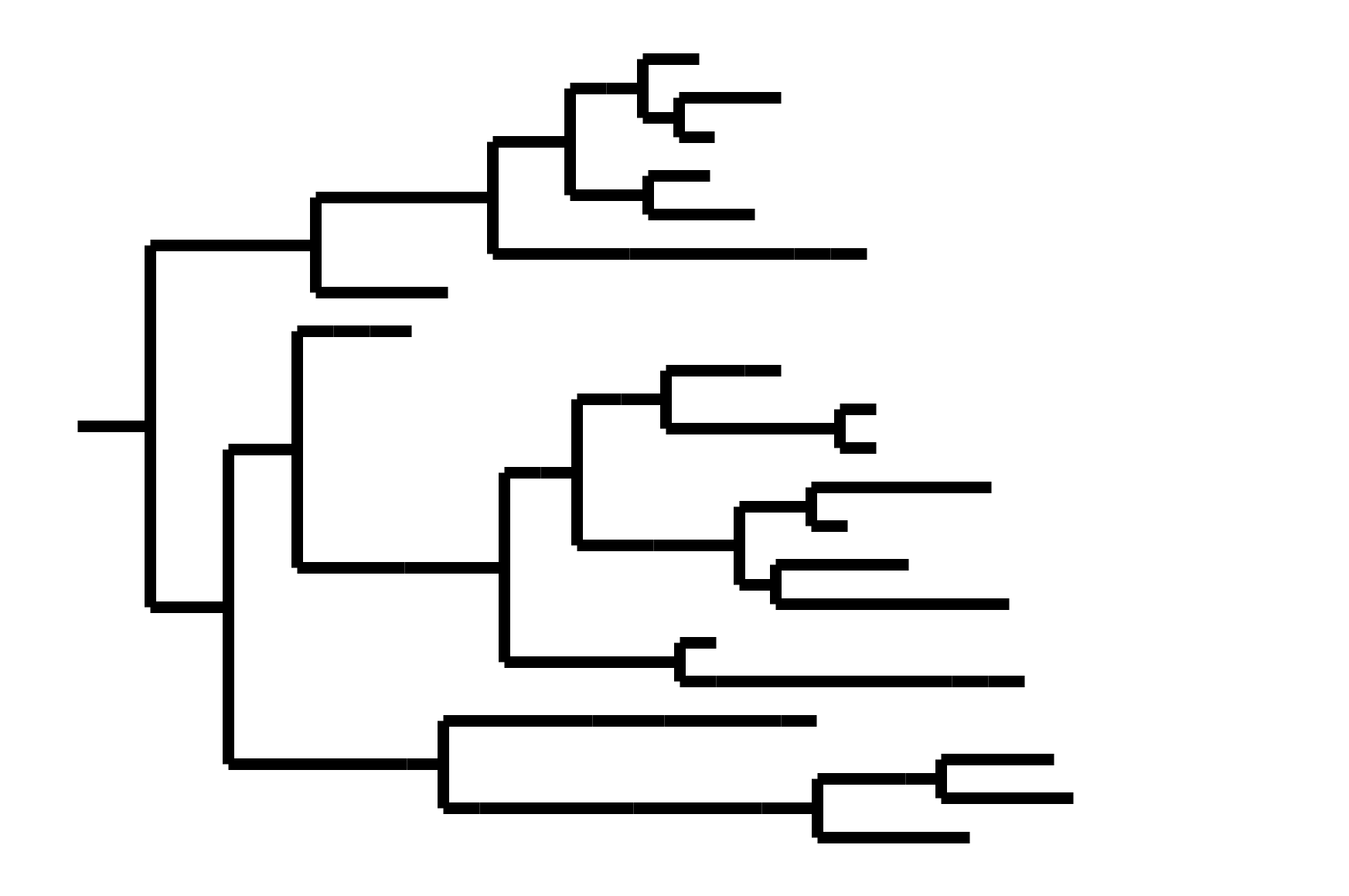
CoV-OC43 split into two ladder-like lineages. Influenza B evolves this way too. It's theoretically possible to pick well-matched bivalent vaccine.
In non-ladder-like tree, there can be high standing genetic variation. Makes picking vaccine strains difficult.
How did Omicron acquire so many mutations with no sampled evolutionary intermediates?
The molecular clock identifies unusual patterns in Omicron evolution
Excess antibody-escape mutations in spike are characteristic of viruses that evolve in chronic human infections
During typical acute infections, random transmission bottlenecks disrupt selection
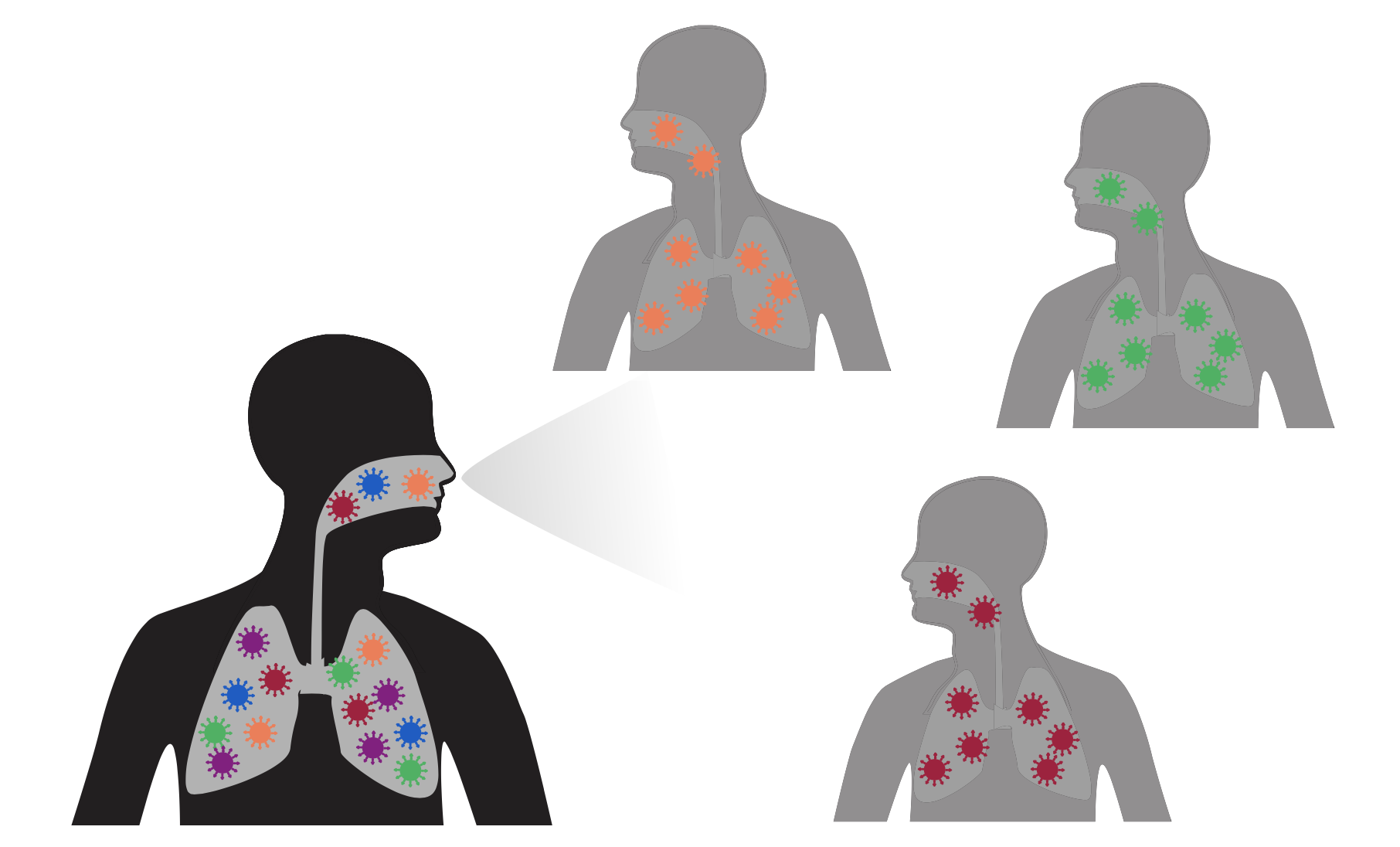
But in chronic infections of 100+ days, selection can fix antibody-escape mutations without bottlenecks
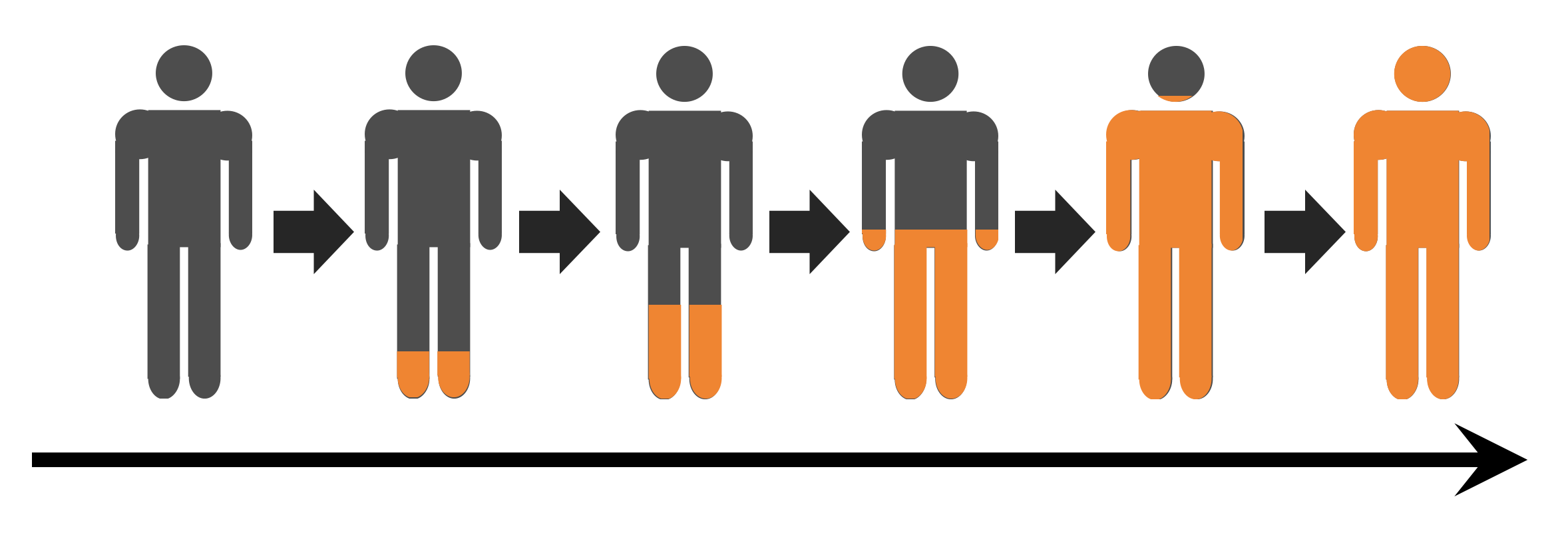
If evolution is being driven by neutralizing antibody escape, what might be next?
Deep mutational scanning to map RBD mutations that escape antibody binding
RBD
fluorescently labeled antibody
yeast
fluorescent tag on RBD
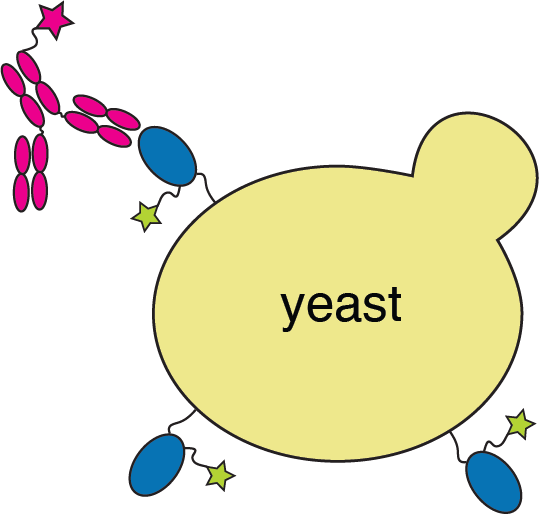
Experiments combine flow cytometry and deep sequencing of a library of yeast expressing all RBD mutants
These measurements can be made on ~10,000 to ~100,000 mutants all at once

Escape map from one antibody (LY-CoV555, ie bamlanivimab)
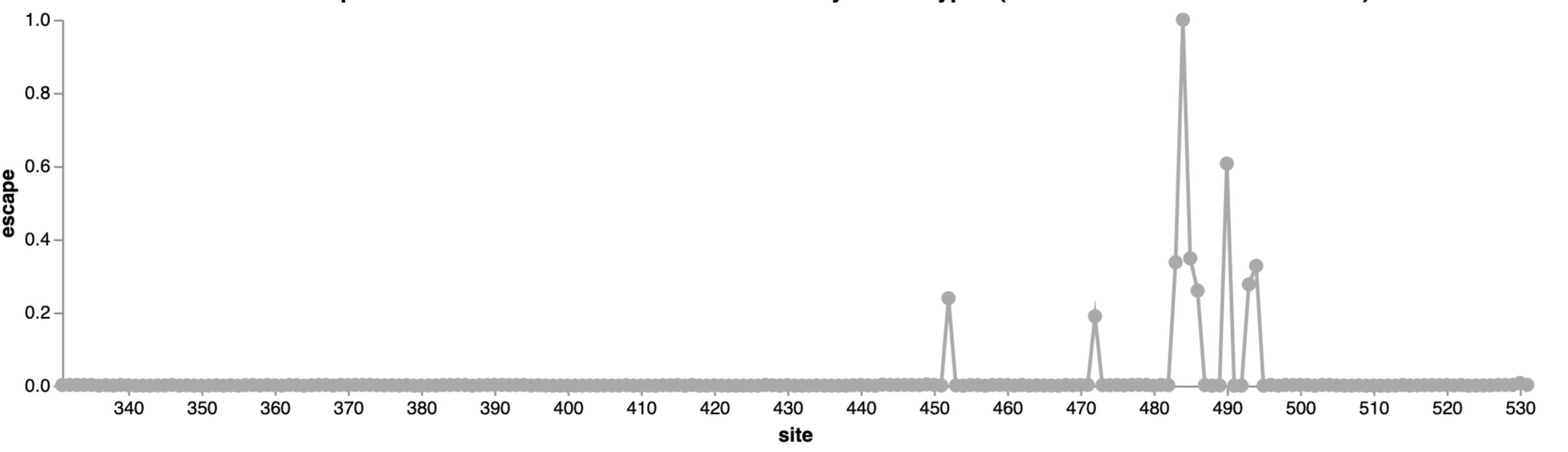
484
452
490
This turned out be an unfortunate choice of an antibody for Eli Lilly to develop as drug, as mutations at sites 484 and 452 were prevalent by early to mid 2021
Infection / vaccination elicit polyclonal antibodies
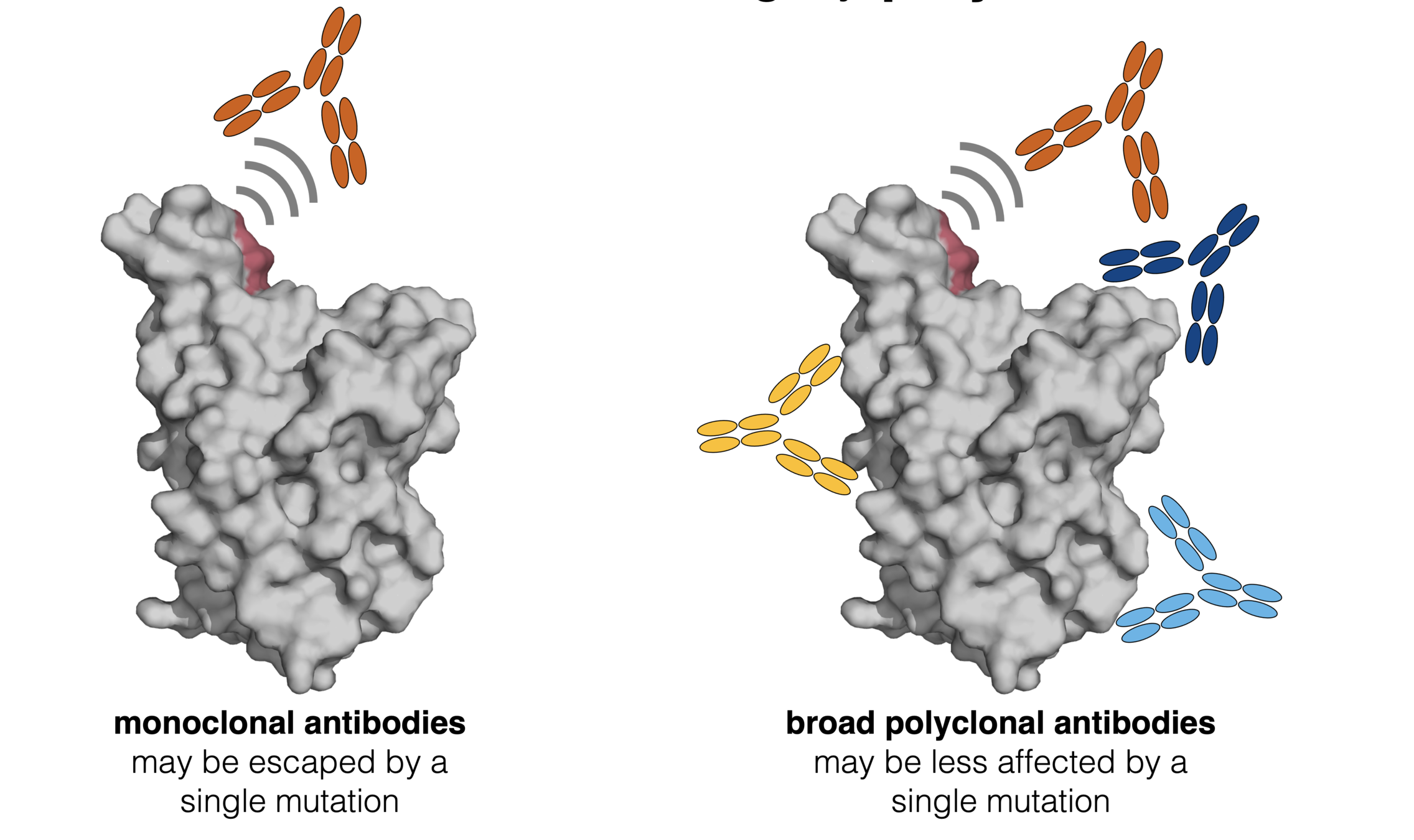
Deep mutational scanning has now been applied to many antibodies
36 antibodies mapped by Tyler Starr & Allie Greaney in Bloom lab.
36 antibodies mapped by Tyler Starr & Allie Greaney in Bloom lab.
>4,000 (!) antibodies mapped by Yunlong Cao et al at Peking University. See here and here.
Deep mutational scanning has now been applied to many antibodies
Sites of likely future escape in XBB
Interactive escape calculator: https://jbloomlab.github.io/SARS2-RBD-escape-calc/
ACE2 affinity from: https://jbloomlab.github.io/SARS-CoV-2-RBD_DMS_Omicron/RBD-heatmaps/
Fitness effects from: https://jbloomlab.github.io/SARS2-mut-fitness/S.html
Generalizing deep mutational scanning to other viral entry proteins
Lentiviral pseudotyping
- Many viruses have entry proteins amenable to lentiviral pseudotyping.
- However, traditional pseudotyping does not create genotype-phenotype link.
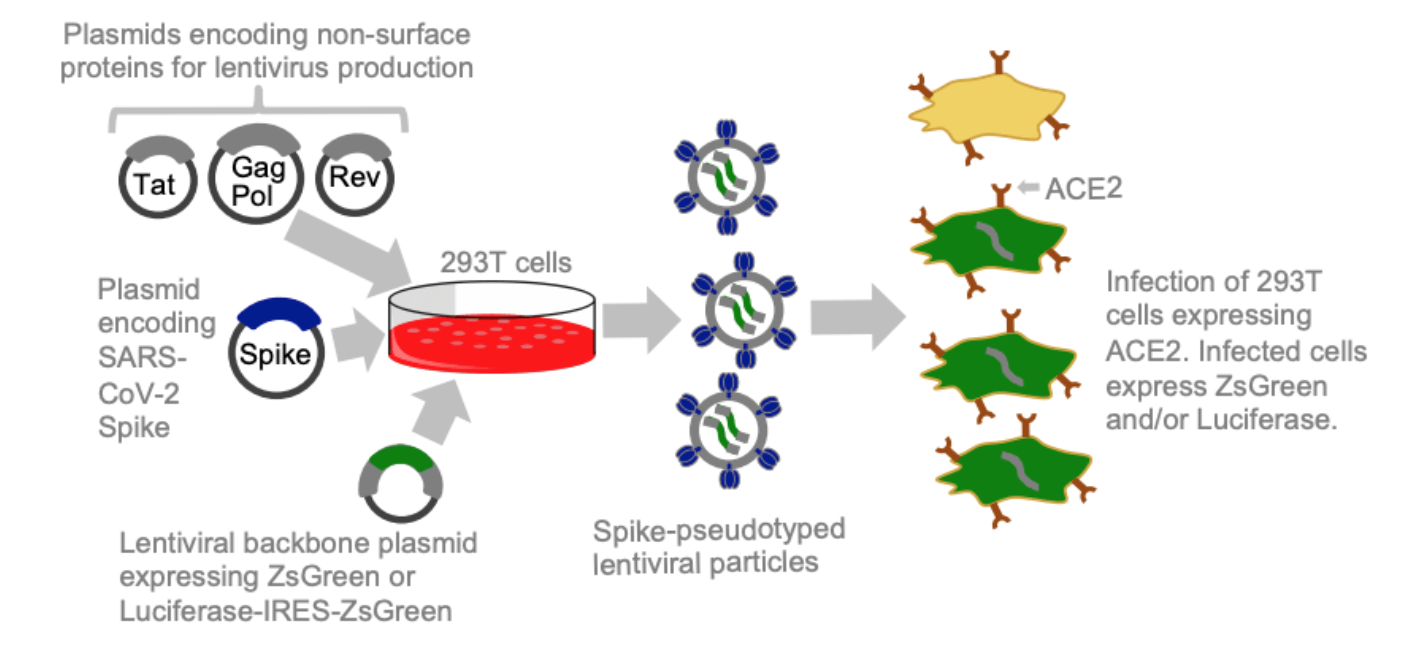
Two-step method to create genotype-phenotype linked spike-pseudotypes
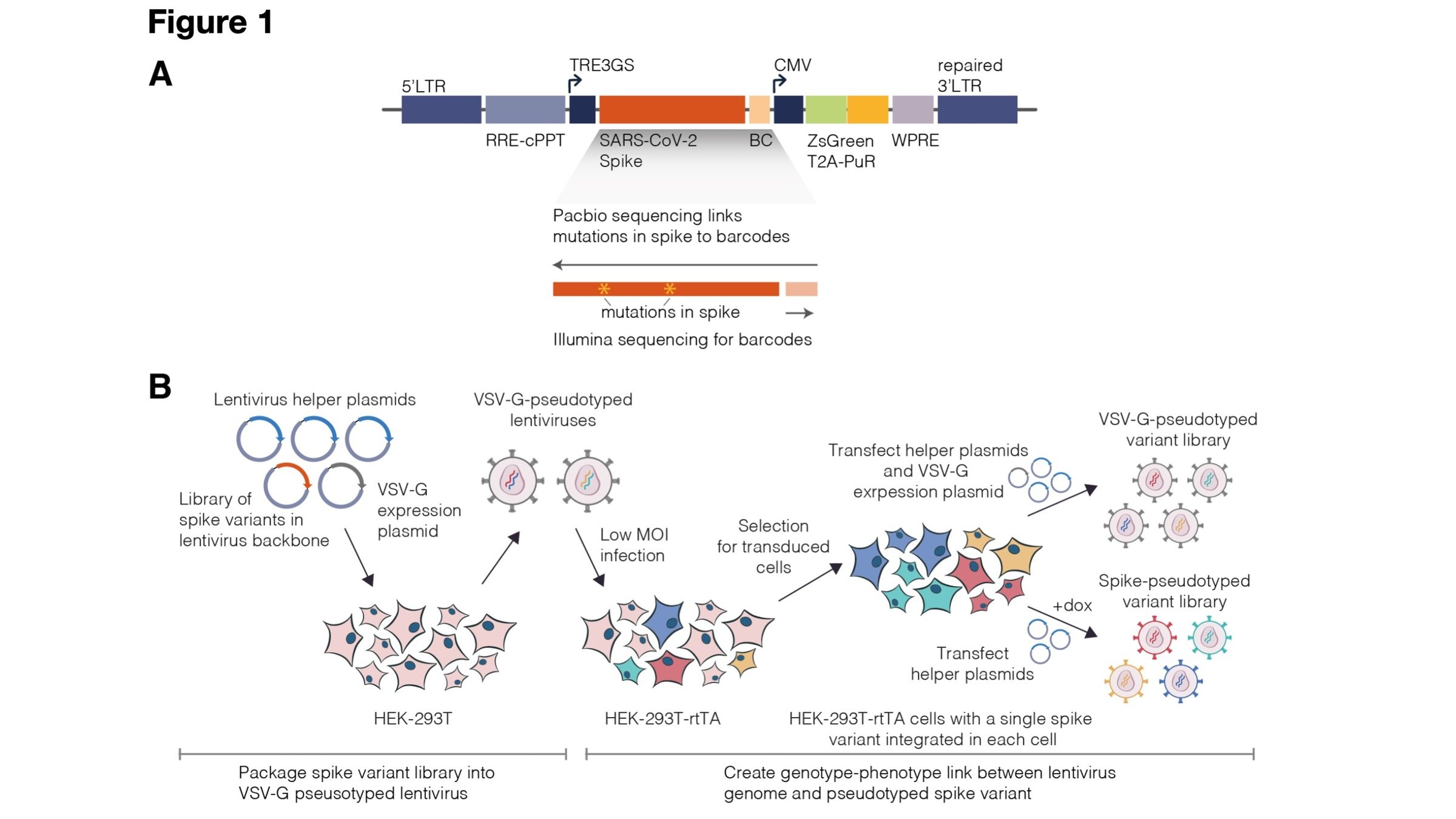
Sequencing measures relative amounts, so include neutralization standard
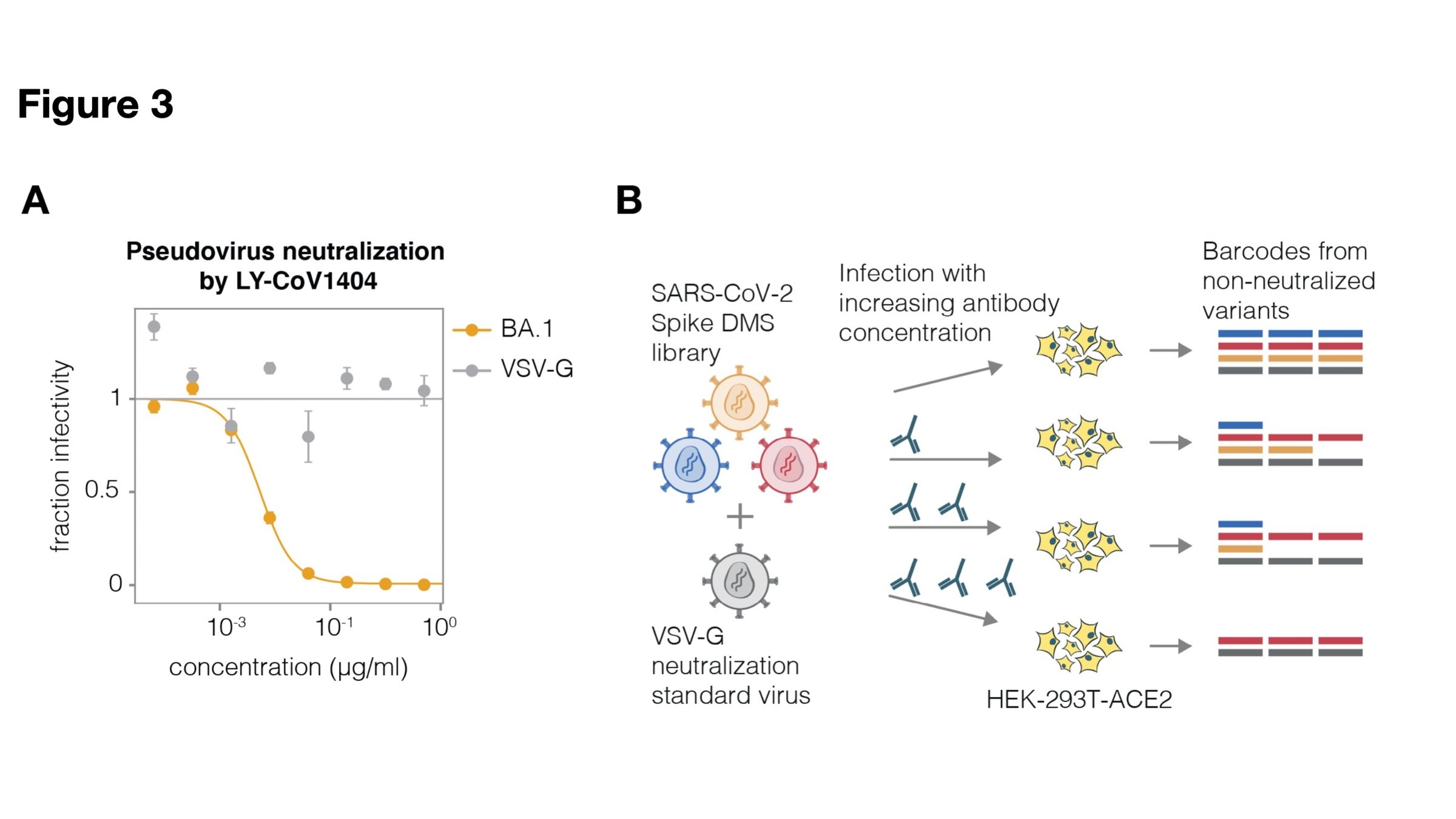
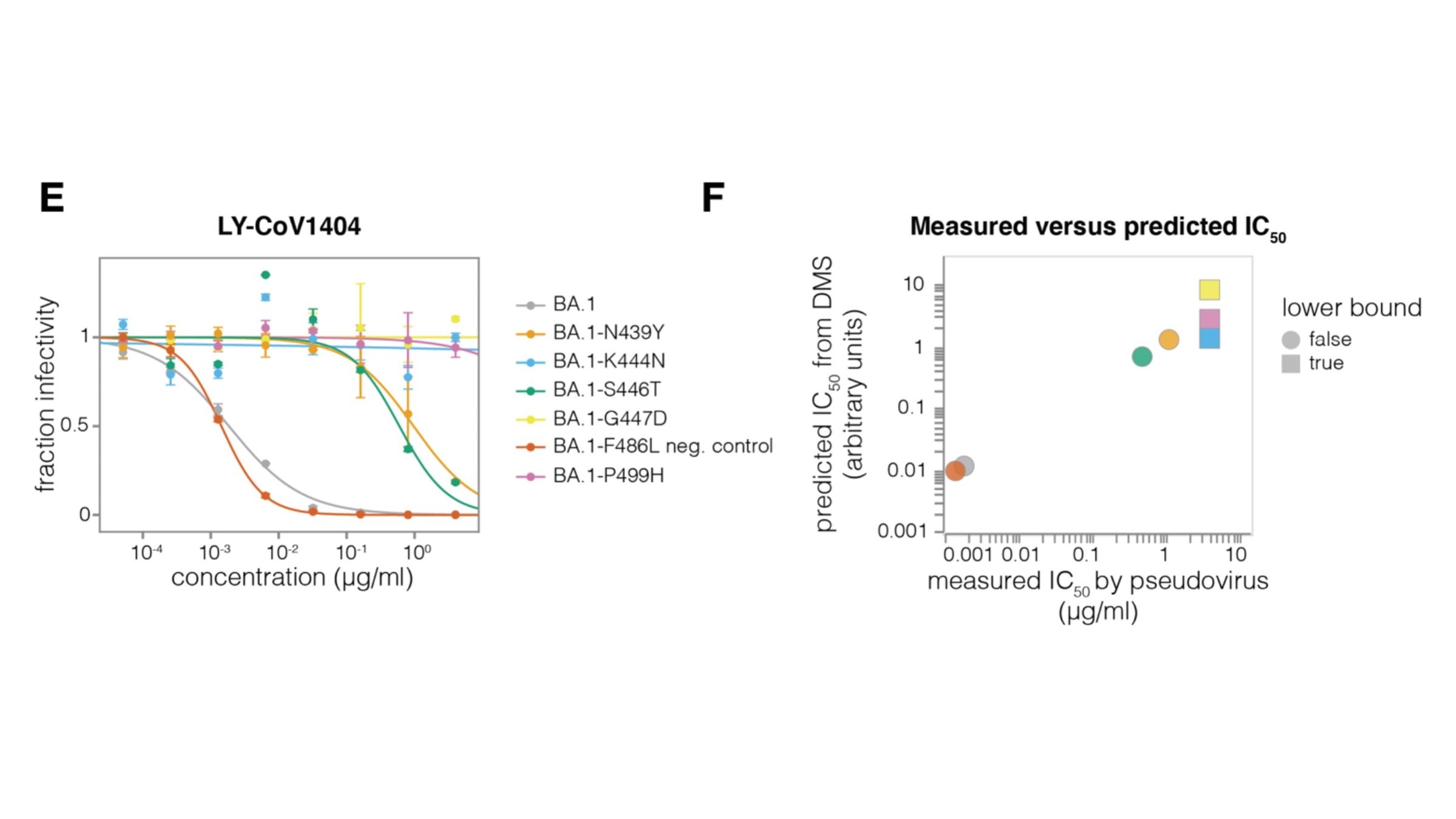
Example: RBD antibody LY-CoV1404
Validates very well
Example: S2 antibody CC67.105
Using this system, we mapped how all XBB.1.5 spike mutations affect serum antibody escape, spike-mediated infection, and ACE2 affinity
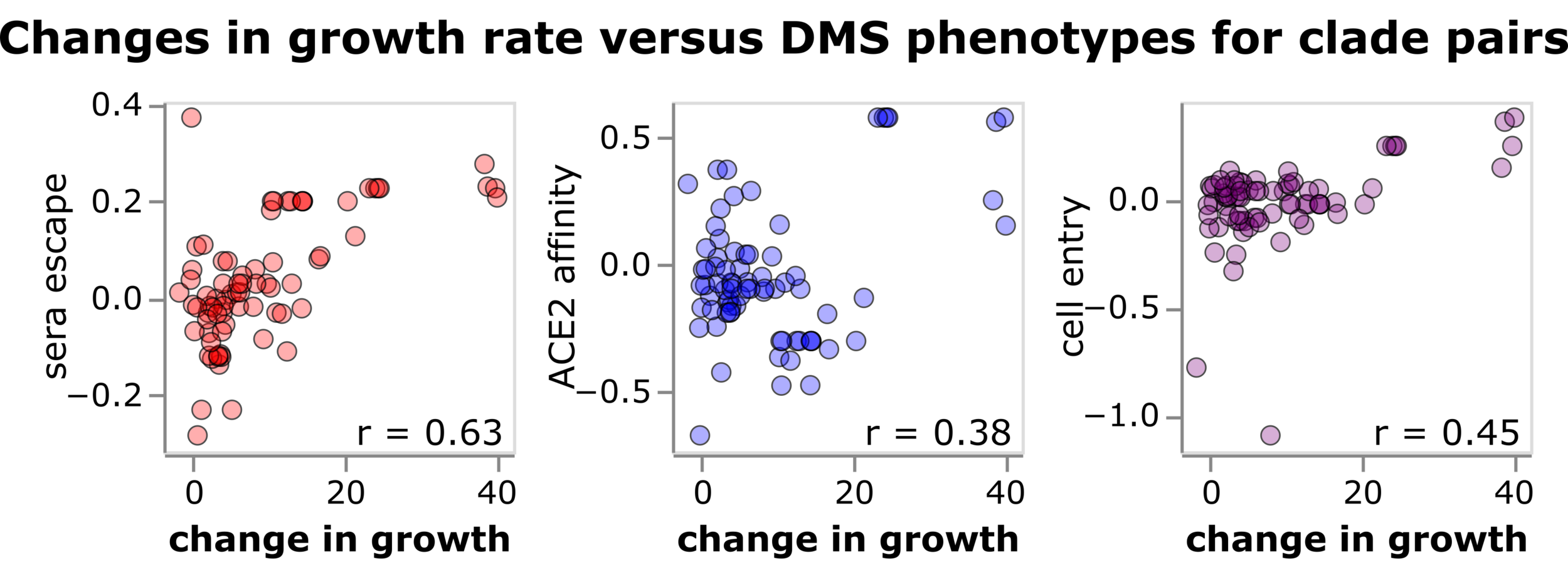
Spike molecular phenotypes measured in deep mutational scanning correlate with clade growth in humans
See here for details
See here for details
Model of clade growth from measured phenotypes

Conclusions
Some viruses evolve to escape antibodies, and unfortunately SARS-CoV-2 is one of those viruses.
Patterns of substitution in the SARS-CoV-2 spike are similar to those in other human coronaviruses (eg, CoV-229E).
We can use deep mutational scanning to measure molecular phenotypes of spike, including antibody escape and ACE2 affinity.
These molecular phenotypes correlate with the evolutionary success of these viruses in nature.
Bloom lab
Bernadeta Dadonaite
Kate Crawford
Caelan Radford
Tyler Starr
Allie Greaney
Rachel Eguia
rest of lab
University of Washington
Helen Chu and HAARVI cohort
David Veesler
Alex Greninger and UW Lab Medicine
Ben Murrell




Thanks

