Characterizing Ear2 Gene in Alternatively Activated Macrophages and Its Potential Anti-Helminth Property in Helminth Infections
By: Jiayi Lily Ma
INTRODUCTION
1.1 Background
A. Two general categories of macrophages
- Classically activated macrophages (M1 macrophages) and alternatively activated macrophages (AAMs or M2 macrophages) (1).
- M1 macrophages are activated by IFNy or LPS. M2 macrophages are activated by interleukins such as IL-4 or IL-13 (1).
B. Helminths elicit Th2 response and causes AAMs differentiation (8). •Recent evidence show that AAMs enhance Th2 response and mediate direct worm killing and expulsion (2).
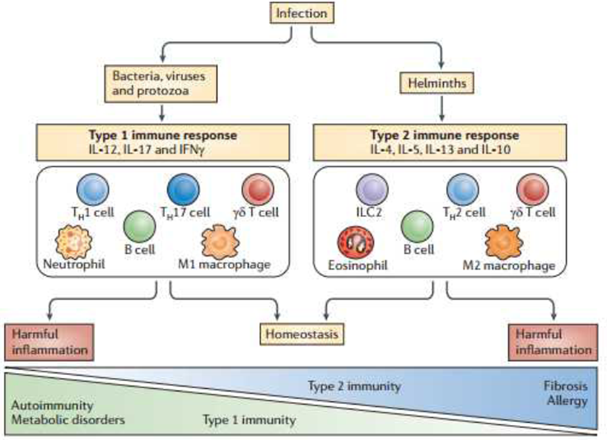
(3)
1.2 Significance
A. Helminth Infections: One of the most neglected tropical diseases that cause high morbidity worldwide (4)
•2007 study: helminth affect ~2.7 billion people earning <$2 a day in developing countries in the Sub-Saharan regions, Asia and Africa (4).
•Helminth infections adversely affect growth, physical fitness, memory and cognition development in children (5)
•Certain parasitic helminths cause neonatal prematurity, reduced neonatal birth weight, and increased maternal morbidity and mortality (5).
B. Limited drugs developed
•Between 1975 and 2004, only four drugs— albendazole, oxamniquine, praziquantel, and ivermectin — were developed (5).
•Current vaccines against helminth infections proved elusive in part because of incomplete understanding of components of Th2 immune response critical to host defense (2).
1.3 Literature Review:
A. Ear2, eosinophil-associated ribonuclease 2, is a member of the Ribonuclease A superfamily (10)
•EDN, eosinophil-derived neurotoxin, is the human homolog of Ear2 (6).
•EDN and Ear2 induce dendritic cell migration (7,8)
B. Human Ears (Eosinophil-associated ribonuclease) have antiviral properties
•Clear viruses such as the respiratory syncytial virus (RSV) and human-immunodeficiency virus (HIV) (8, 9).
C. Human Ears are involved in innate mechanisms
•Target specific pathogens (9)
•ECP, Eosinophil Cationic Protein is capable of killing different parasites and bacteria in vitro (10)
1.4 Research Problem
•What role does Ear2 play in AAMs during helminth infection?
MATERIALS AND METHODS
2.1 Prior methodology
A. Mouse treatments carried out by researchers in the laboratory
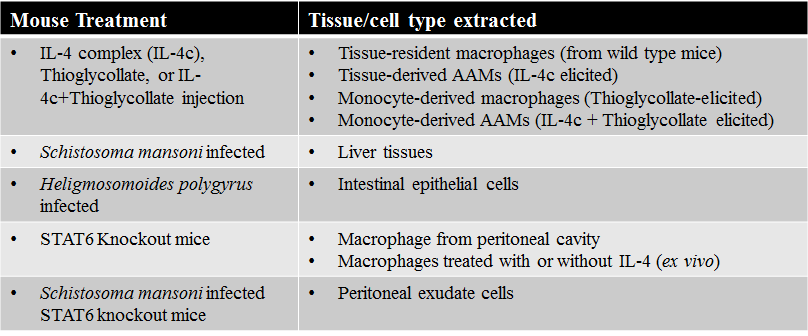
B. Ribonuclease activity assay
•Mice injected with Thioglycollate or Thioglycollate + IL-4c
–Peritoneal exudate cells harvested by peritoneal lavage
2.2 Methodology (All experiments carried out by student researcher)
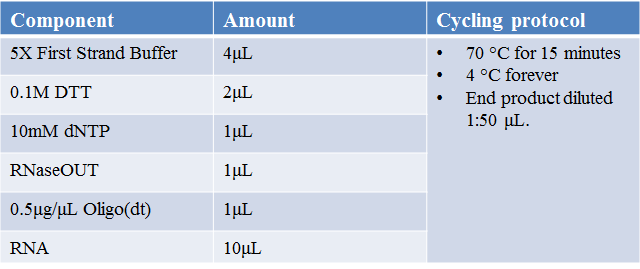
A. RNA Isolation
•RNeasy Mini Kit from Qiagen and following manufacturer’s instructions.
•Reverse transcription of the isolated RNA to cDNA:
B.Gene Expression
•Quantitative polymerase chain reaction (qPCR)
-SYBR Green qPCR Kit (Applied Biosystems)
-Each reaction: 0.25μL forward primer, 0.25μL reverse primer, 10μL SybrGreen PCR Mastermix, 8μL deionized water, and 1μL cDNA.
•Each sample tested in technical duplicates.
•Ear2 primer sequences and housekeeping gene GAPDH primer sequences:
Ear2 Forward: 5’-CCT GTA ACC CCA GAA CTC CA-3’
Ear2 Reverse: 5’-CAG ATG AGC AAA GGT GCA AA-3’
GAPDH Forward: 5’-GCC TTC CGT GTT CCT ACC C-3’
GAPDH Reverse: 5’-TGC CTG CTT CAC CAC CTT C-3’
•Gene expression calculated using :
ΔCt= [Ct]Target -[Ct]Housekeeping gene
Relative expression = 2^(-ΔCt),
where Ct= minimum number of cycles required for thermocycler to detect fluorescent signals of SYBR Green dye.
C. Ribonuclease Activity Assay
•Supernatant collection procedure:
1. Cells from peritoneal lavage, described earlier in “Prior Methodology” were centrifuged and supernatant discarded.
2. Cell pellets re-suspended in RPMI media and plated.
3. Cells left to adhere by incubation at 37° C for 2 hours; non-adherent cells removed.
4. Adhered cells incubated for another 3 hours; supernatant harvested for assay.
•Ribonuclease activity
1.Ribonuclease A Detection Kit (Sigma-Aldrich) following manufacturer's instructions.
2.Absorbance measured using Victor X Multilabel Plate Reader at 280nm.
RESULTS
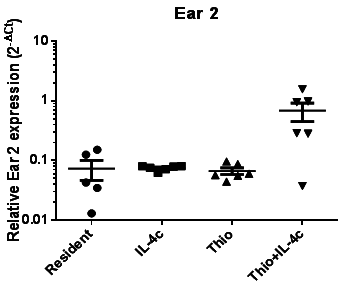
Fig. 1. High level of Ear2 expression in monocyte-derived AAMs (Thioglycollate+IL-4c induced) from mouse model.
Quantitative qPCR
Fig. 2. During the 7th week of infection, the host experiences a “switch” from pro-inflammatory to anti-inflammatory immune response. Ear2 expressions in uninfected and infected mice are significantly different during Week 7.
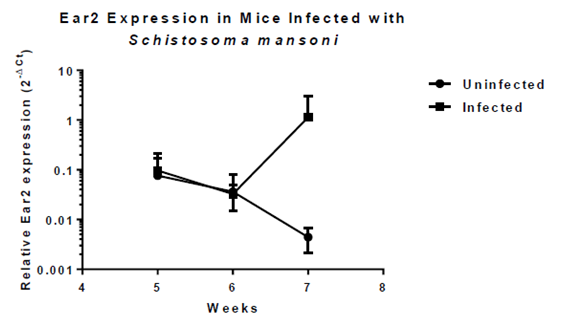
Fig. 3. During the secondary infection of the nematode H. Polygyrus, the host experiences a Th2 and AAM regulated immune response in attempt to expel the parasite; Ear2 is highly up-regulated during this stage of the infection.
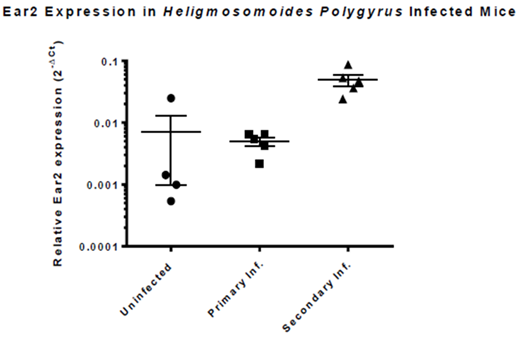
Fig. 4. Macrophages were isolated from WT and STAT6 KO mice and then treated with (+) or without (-) IL-4. Six hours after the IL-4 treatment, Ear2 is down-regulated in STAT6 KO mice as compared to WT mice. After 12 hours, there is a clear difference between Ear2 expression in WT mice and in STAT6 KO mice. Over time, the difference in expression begins to grow significantly.
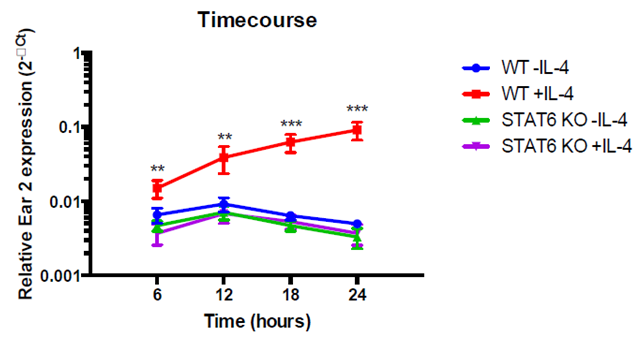
Fig.5. Expression of Ear2 is higher in PECs of WT mice than in PECs of infected STAT6 KO mice, thus confirming that Ear2 is STAT6-dependent.
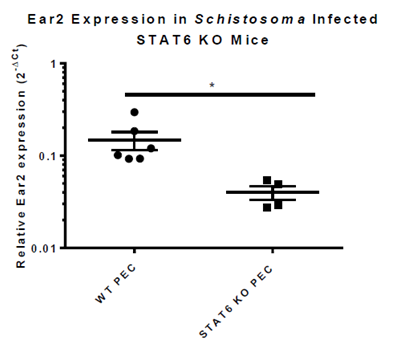
Fig. 6a, 6b. Ribonuclease activity can be calculated using the slope: Δln (E0-Ef)/Δt. However, since both graphs show non-linear relationships, there is an insignificant amount of Ear2 enzyme activity in both monocyte-derived macrophages and monocyte-derived AAMs.
*Note: Ribonuclease activity was assayed in biological and technical duplicates. However, since no significant amount of ribonuclease activity was detected, only some graphs are shown.
Enzyme Activity Assay
Fig. 7a, 7b. Ribonuclease activity is insignificant in the lavage fluid of monocyte-derived macrophages and monocyte-derived AAMs.
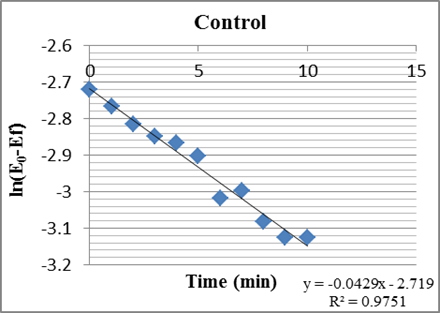
Fig. 8. The linear relationship of the control shows that the ribnonuclease activity assay was carried out correctly.
CONCLUSION
3.1 Summary of study findings:
A.Ear2 is highly expressed in AAMs from different mouse models:
•Induced AAMs in vivo (Fig. 1)
•Schistosoma mansoni infection (Fig. 2)
•Heligmosomoides polygyrus infection (Fig. 3)
B.Ear2 expression is dependent upon STAT6 (Signal Transducer and Activator of Transcription 6)
•Ear2 expression is significantly lower in STAT6 KO (knockout) mice and helminth-infected STAT6 KO mice (Fig. 4 and Fig. 5)
C. Ear2’s ribonuclease activity is insignificant
• In both monocyte-derived macrophages and monocyte-derived AAMs (Fig. 6a, 6b, 7a, 7b)
3.2 Implications and significance:
A. AAMs and helminth infections
•AAMs are crucial for host defense: In S. mansoni infection, mice that cannot polarize macrophages into AAMs die during acute infection (11, 12, 13).
•Unclear whether AAMs directly kill helminthic parasites or enhance protective Th2 immune responses (2).
•Recent evidence: AAMs directly mediate worm killing, enhance Th2 immune response, and facilitate worm expulsion (2).
B. STAT6’s importance in Th2 response
•Signal Transducer and Activator of Transcription 6
•Regulate differentiation of Th2 cells, including AAMs (14)
C. Expression of Ear2 by AAMs may protect host from helminth infections via several possible mechanisms:
•Ear2 could be important for AAM differentiation.
-Ear2 found to be highly expressed in IL-4c elicited AAMs and in AAMs of mice with helminth infections.
•Ear2 could have direct anti-helminth activity
-During secondary infection of H. Polygyrus, Ear2 expression is highly upregulated.
-During this stage, immune system expels the parasite by recruiting AAMs.
-Ear2 may be directly involved in worm expulsion process.
•Ear2 could enhance protective Th2 response
-Ear2 expression is up-regulated during Week 7 of Schistosomiasis, when immune response switches from Th1 to Th2.
-Ear2 could play aiding role in granuloma formation crucial to host survival in S. mansoni infection.
D. Ear2 and STAT6
•Ear2 expression is STAT6-dependent
-Strengthens association between up-regulation of Ear2 and AAMs differentiation.
E. Ear2’s potential anti-helminth property is independent of its ribonuclease activity
•Enzyme activity assay showed no significant ribonuclease actitity in lavage fluid and supernantant of monocyte-derived AAMs
3.3 Limitations and future research
A. This study lacked Ear2 knockout mice to confirm proposals presented.
B. Future research
•The focus of my next research project would be to use Ear2 knockout mouse models to verify the proposals above regarding Ear2’s role in Th2 response triggered by helminth infections.
References
1.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews Immunology. 2008;8(12):958-69.
2.Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N, et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol. 2014; 15(10);938-46
3. Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement and adaptive immunity by helminthes. Nature Reviews Immunology. 2013;doi:10.1038/nri3476.
4. Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. The New England journal of medicine. 2007;357(10):1018-27.
5. Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. JCI. 2008(4):1311-1321.
6. McDevitt AL, Deming MS, Rosenberg HF, Dyer KD. Gene structure and enzymatic activity of mouse eosinophil-associated ribonuclease 2. Gene. 2001;267(1):23-30.
7.Marathe CM, University of California LA. Regulation of Inflammation by PPARs and LXRs: University of California, Los Angeles; 2007
References (continue)
8. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. The Journal of experimental medicine. 2008;205(1):79-90.
9. Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. Journal of leukocyte biology. 2001;70(5):691-8.
10. Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. Journal of immunology (Baltimore, Md : 1950). 1989;142(12):4428-34.20
11. Pearce EJ, C MK, Sun J, J JT, McKee AS, Cervi L. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunological reviews. 2004;201:117-26.
12. Schwartz C, Oeser K, Prazeres da Costa C, Layland LE, Voehringer D. T Cell-Derived IL-4/IL-13 Protects Mice against Fatal Schistosoma mansoni Infection Independently of Basophils. Journal of immunology (Baltimore, Md : 1950). 2014;193(7):3590-9.
13. Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and down modulates T helper 1 responses and immunopathology. Immunity. 2004;20(5):623-35.
14. Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunologic research. 2011;50(1):87-96.