Voxelwise optimization of hemodynamic lags to improve regional CVR estimates in
breath-hold fMRI
Stefano Moia¹*, Rachael C. Stickland²*, Apoorva Ayyagari², Maite Termenon¹, César Caballero-Gaudes¹, and Molly G. Bright²
1. Basque Center on Cognition, Brain and Language, San Sebastian, Spain 2. Northwestern University, Chicago (IL), USA *co-first authors



| smoia | |
| @SteMoia | |
| s.moia.research@gmail.com |
Cerebrovascular reactivity (CVR)
CVR is the responsiveness of brain vessels to a vasodilatory or vasoconstrictive stimulus to maintain homeostasis of cerebral blood flow (CBF) and volume (CBV).
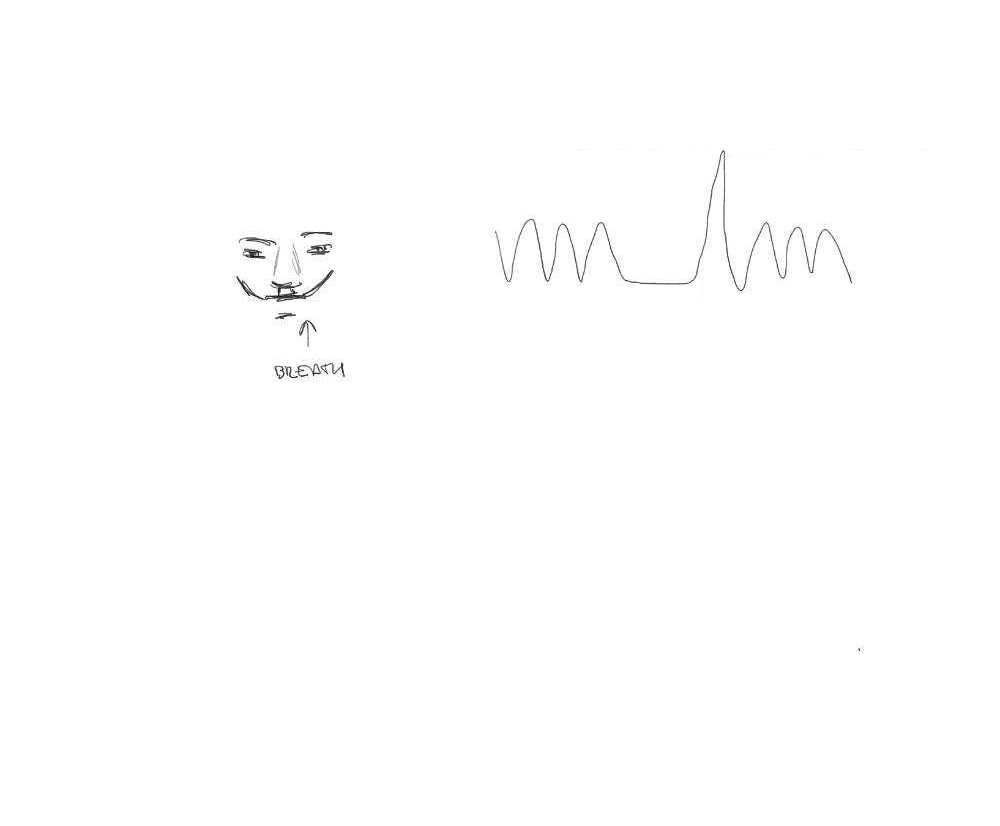
CVR can be measured during BOLD fMRI experiments with Breath-holds (BH) and other voluntary respiration challenges¹
BH tasks induce a state of hypercapnia in the subject
The vessels dilate → Increase of blood flow → Increase of %BOLD signal
CVR mapping [%BOLD/mmHg] can be obtained through a linear regression analysis using the recordings of exhaled CO2
(PETCO2 is a reliable proxy of CO2 partial pressure in arterial blood)

1. Kastrup et al. 1998
BH-induced CVR: Issues
Two key problems:
- There's a measurement delay, and regional variations of the physiological delay
- The BH task presents motion collinear to signal of interest
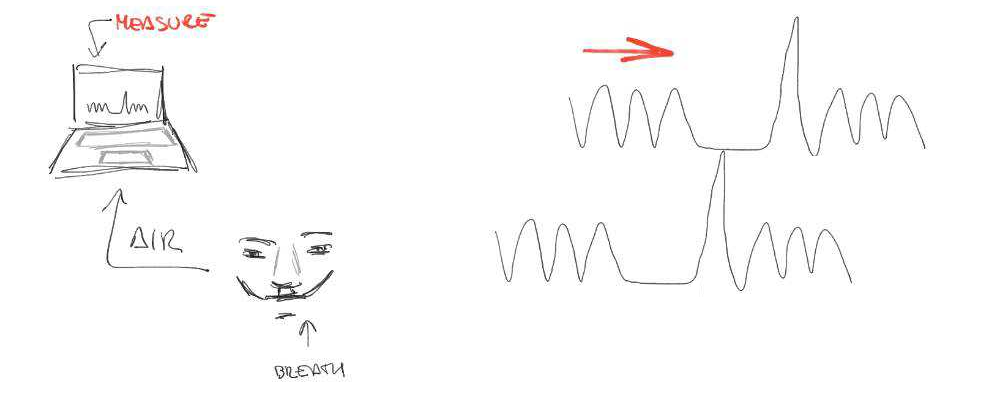
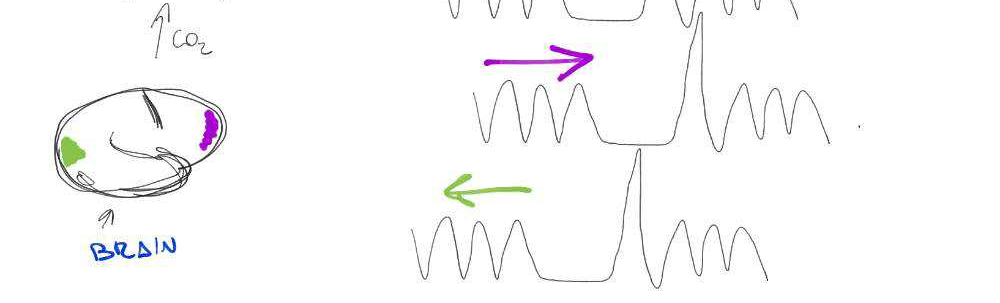
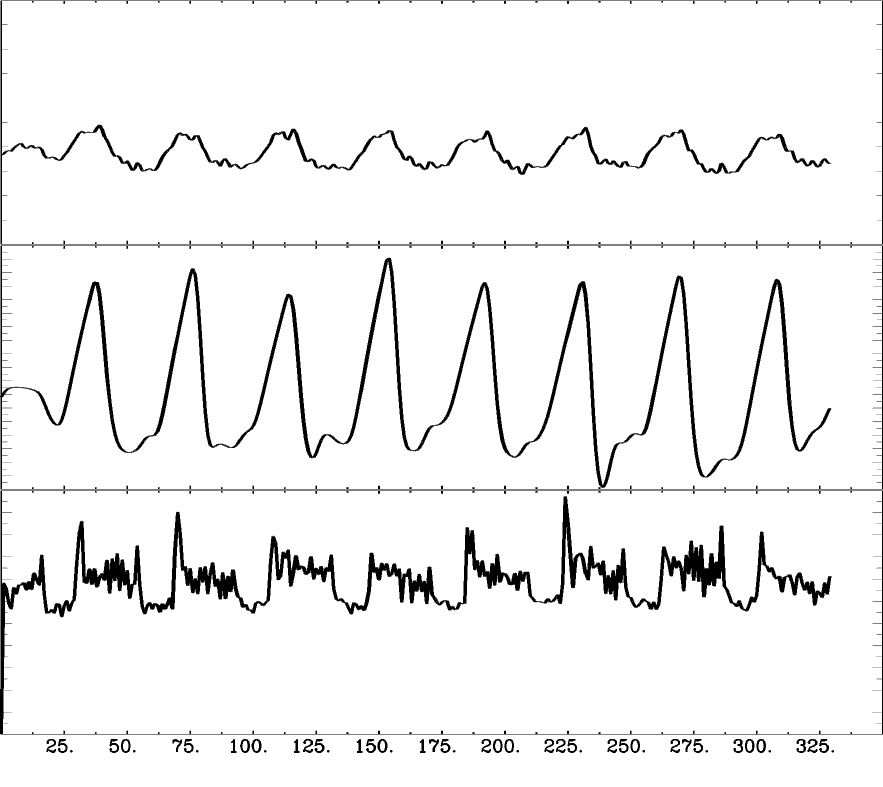
Motion
CO2
BOLD
CVR estimates optimisation
Different methods have been proposed to take into account
lag when modeling CVR:
- RIPTiDe: cross-correlation (X-corr)¹
- Lagged GLM after orthogonalising signal of interest to noise (e.g. motion)²
1. Frederick et al. 2012, Tong et al. 2011, 2. Sousa et al. 2014, 3. Lindquist et al. 2019

However:
- The signal of interest cannot be modified if quantitative measures of CVR are expected.
- Denoising is made in multiple steps - it should not³

Methods: data acquisition

1. Bright et al. 2013
CO2 and O2 sampling
↑
↓


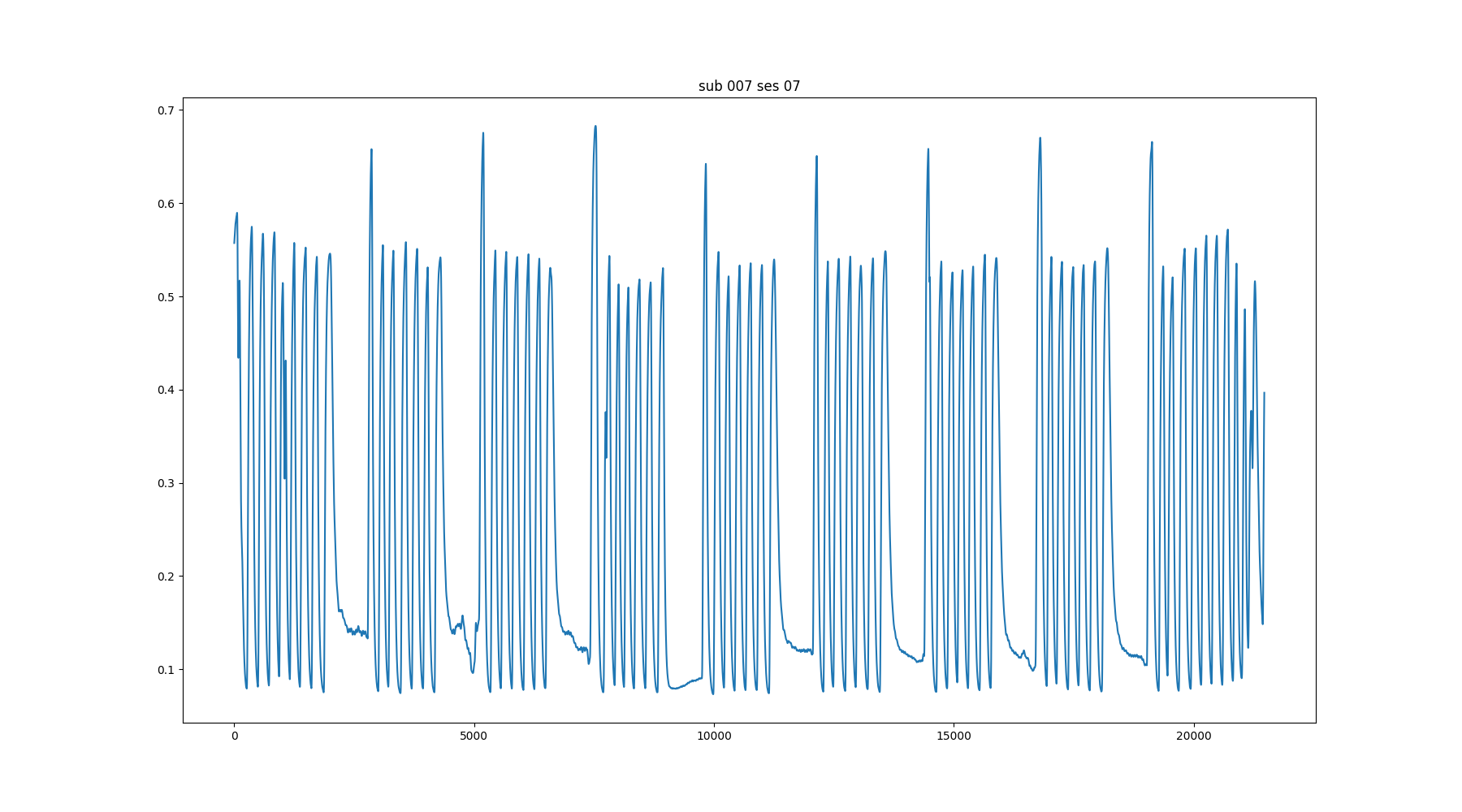
8 subjects (4F, 25-40y)
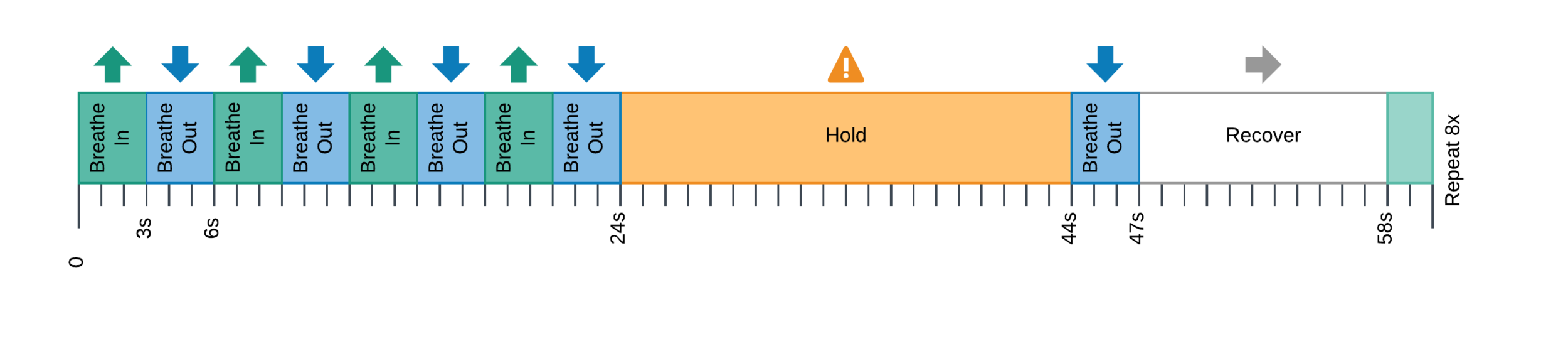
BreathHold task¹ (8 repetitions of a BreathHold trial)
Multi-Echo fMRI (340 volumes, TR=1.5 s,
TEs=10.6/28.69/46.78/64.87/82.96 ms,
FA=70°, MB=4, GRAPPA=2, 52 slices, Partial-Fourier=6/8, FoV=211x211 mm², voxel size=2.4x2.4x3 mm³).

MP2RAGE and T2w (176 slices, FoV=256mm, voxel=1mm³ isotropic)
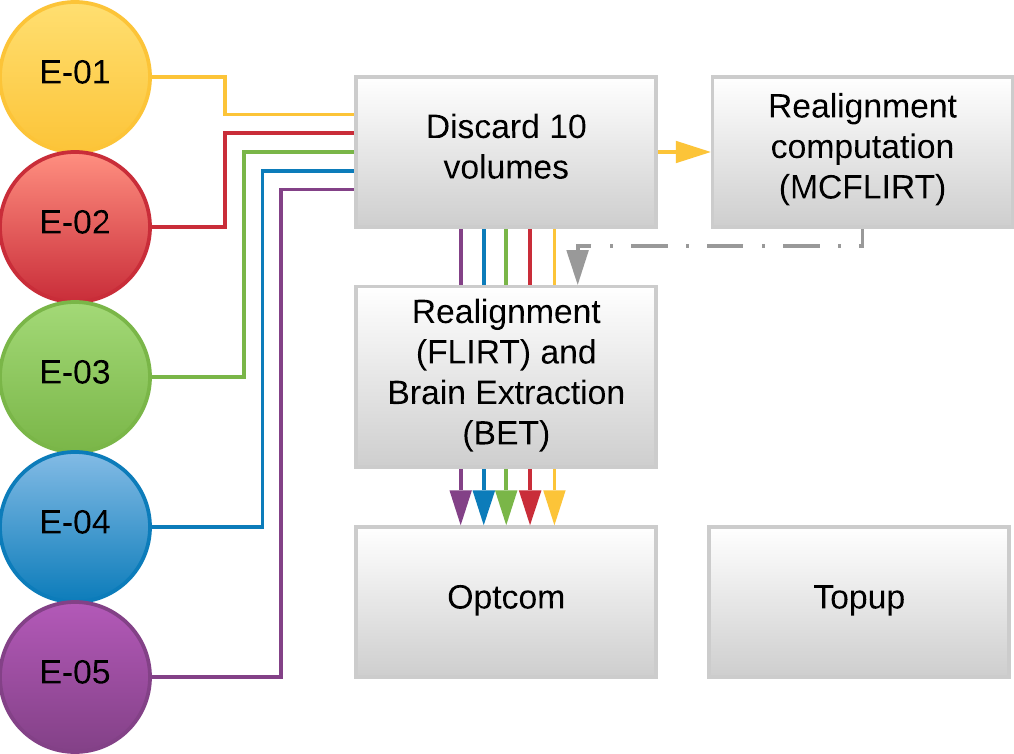
Methods: Preprocessing
- MP2RAGE and T2w skullstripped (AFNI)
- MP2RAGE normalised to MNI152 template (ANTs)
- T2w registered to MP2RAGE and to SBREF (FSL)
- MP2RAGE segmented and GM registered to SBREF (ANTs)
- GM eroded (FSL)
- EPI realigned to SBREF, saved the motion parameters (ANTs)
- EPI skullstripped (FSL)
- EPI distortion corrections (FSL)



Methods: Analysis
- Peaks detection in exhaled CO2 traces
- Amplitude envelope (PETCO2) convolved with HRF
- Find max correlation of PETCO2 and avg GM (bulk shift)
- PETCO2 signal shifted between -9 and +9 sec
- Shifted regressors interpolated at the TR
- Compute GLM with each lagged CO2 regressor and nuisance regression (12 motion parameters and low frequency trends)
- For each voxel, select the lagged model with highest explained variance (R²) (fine shift)
For comparison:
- Non-optimised CVR map (bulk shift) (Non Opt)
- GLM without motion parameters included (NoMot)
- GLM after motion regression (SeqMot)
↓
optimised CVR map and lag map (SimMot)


Lag
R²
Results
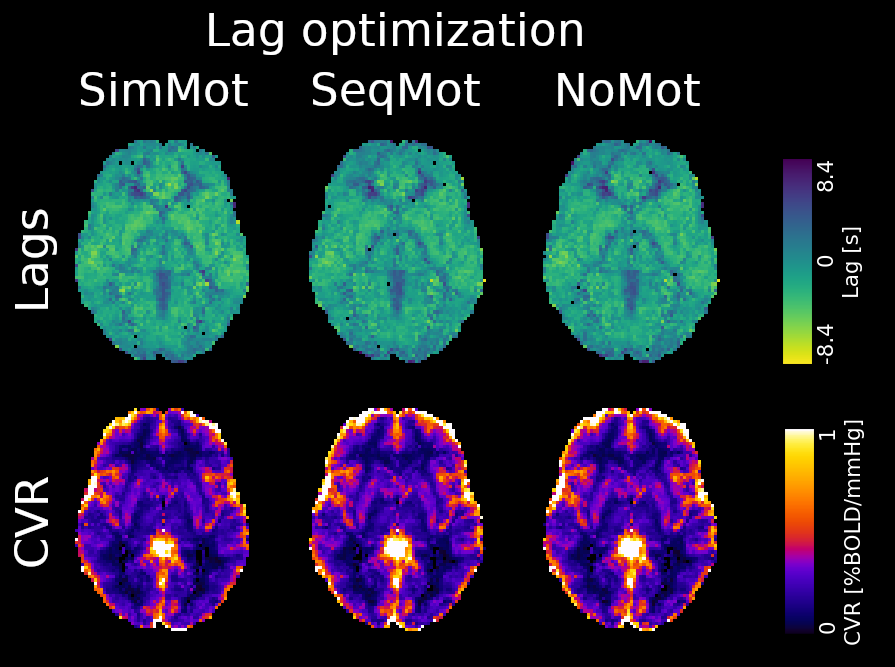
| CNR of lag maps | SimMot | SeqMot | NoMot |
|---|---|---|---|
| GM-WM | 0.52 ±0.21 | 0.46 ±0.26 | 0.49 ±0.25 |
| GM-Putamen | 0.47 ±0.22 | 0.44 ±0.21 | 0.44 ±0.21 |
| GM-Cerebellum | 0.82 ±0.15 | 0.69 ±0.17* | 0.69 ±0.16* |
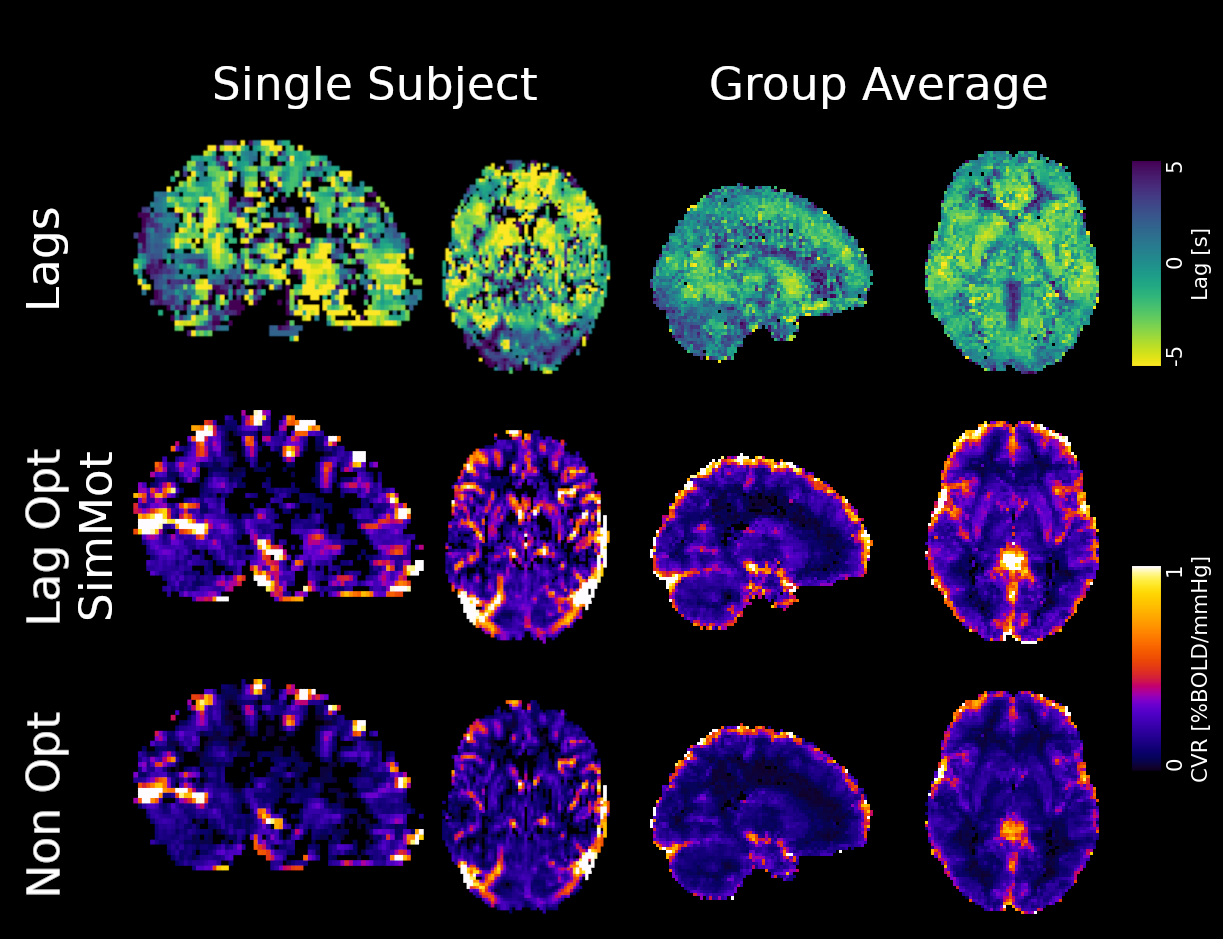
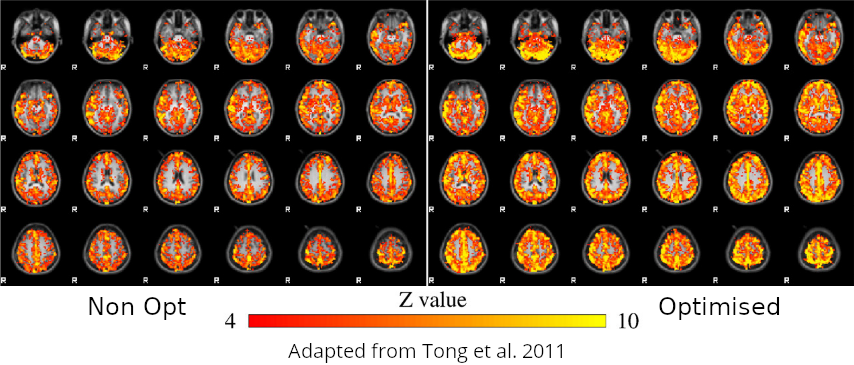
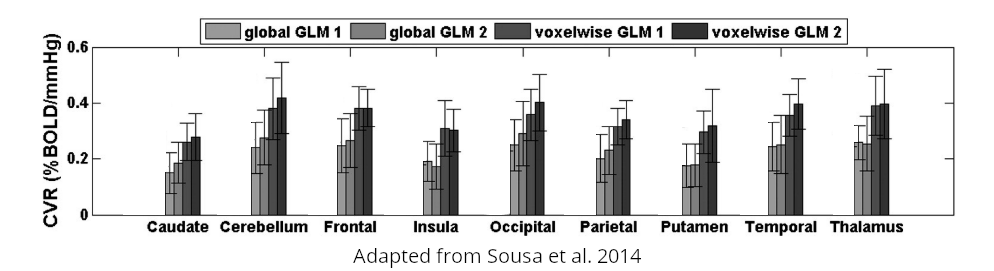
Non optimising leads to underestimate the CVR, especially in subcortical areas.
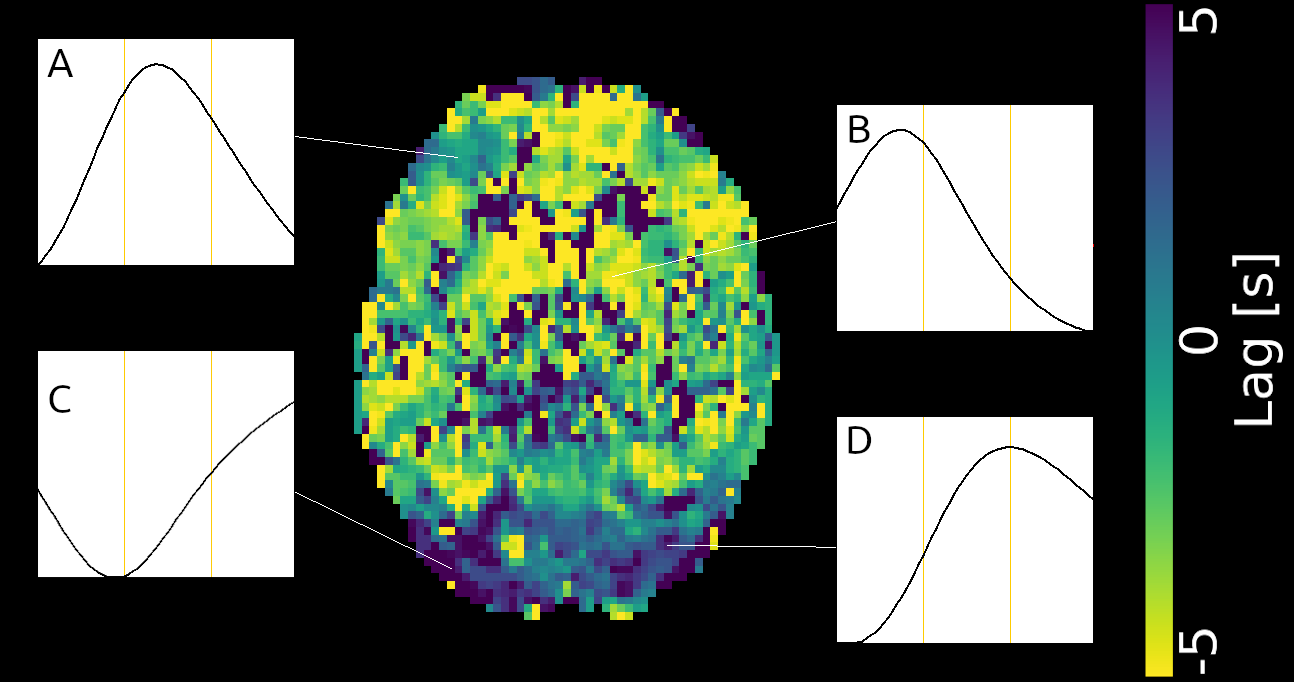
Lag maps show anatomical consistency
→
→
→
→
→
→
→
→
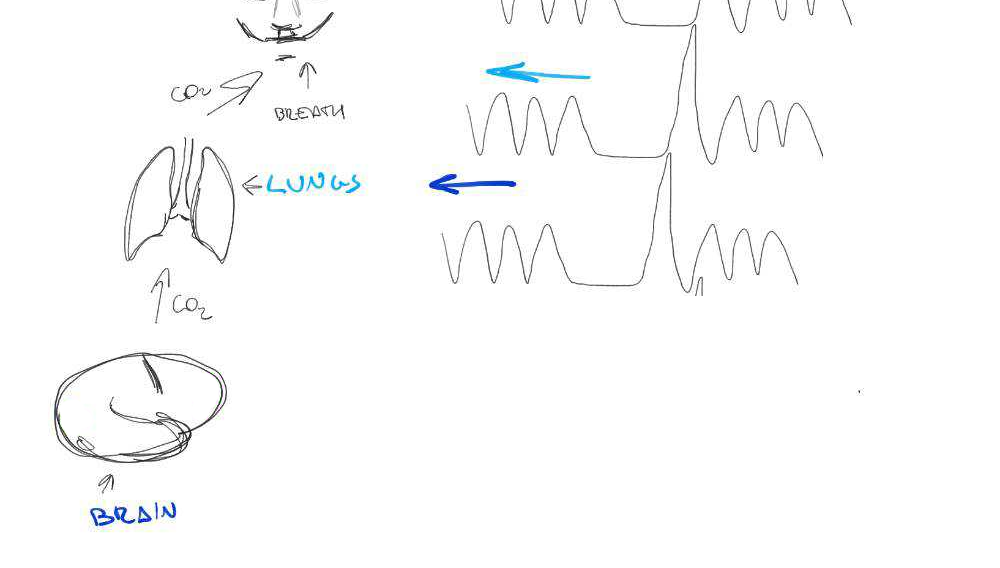
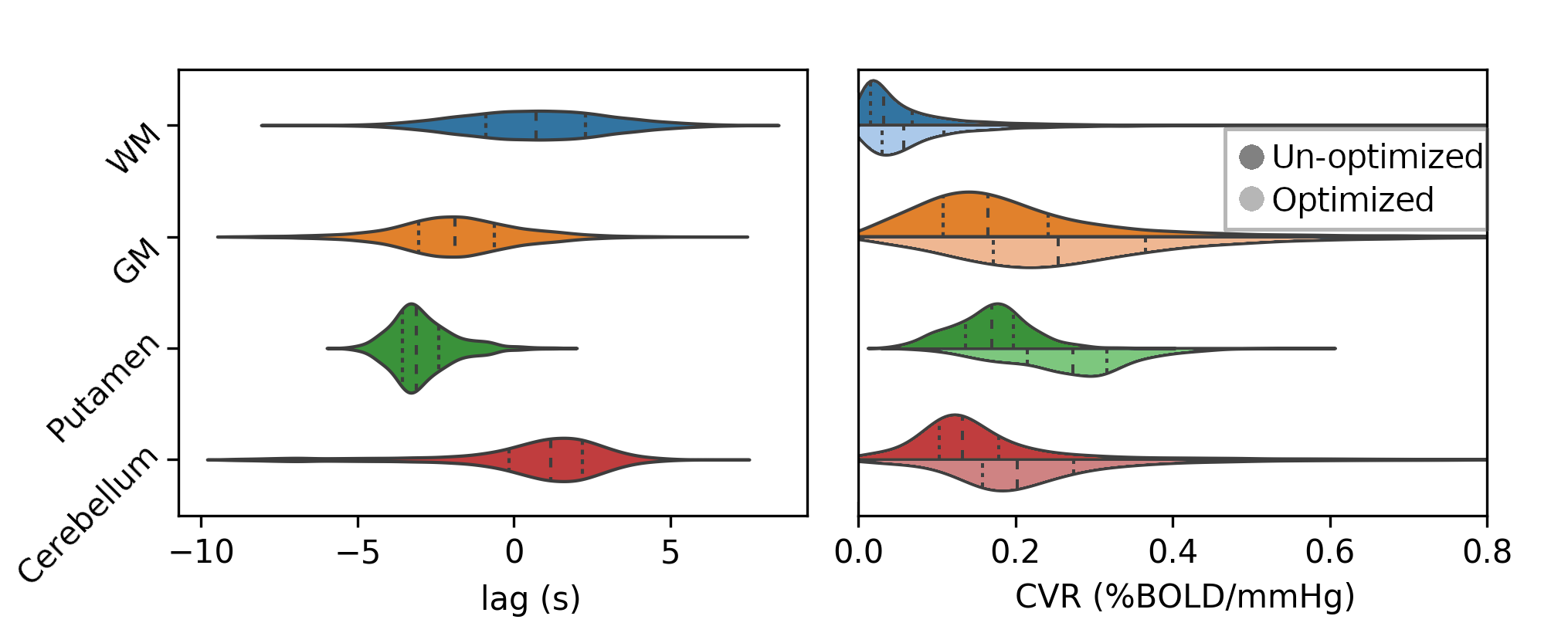
Regional variation in lag responses consistent with previous evidence¹ (e.g. Putamen has earlier response than GM)


→
→
→
1. Blockley et al. 2011, Bright et al. 2009
Area mostly
affected by
motion!
←
To improve CVR estimates,
compute lagged-regression of the signal of interest, with noise modelled simultaneously.
Take home message


- A. Kastrup, T.Q. Li, A. Takahashi, G. H. Glover, and M. E. Moseley, “Functional Magnetic Resonance Imaging of Regional Cerebral Blood Oxygenation Changes During Breath Holding“, Stroke, vol. 29, no. 12, pp. 2641-2645, Dec. 1998
- Frederick L. D. Nickerson, Y. Tong, “Physiological denoising of BOLD fMRI data using Regressor Interpolation at Progressive Time Delays (RIPTiDe) processing of concurrent fMRI and near-infrared spectroscopy (NIRS)“, Neuroimage, vol. 60, no. 3, pp. 1913-1923, Feb. 2012
- Y. Tong, P. Bergethon, B. Frederick “An improved method for mapping cerebrovascular reserve using concurrent fMRI and near-infrared spectroscopy with Regressor Interpolation at Progressive Time Delays (RIPTiDe)“, Neuroimage, vol. 56, no. 4, pp. 2047-2057, Apr. 2011
- I. Sousa, P. Vilela, P. Figueiredo, “Reproducibility of hypocapnic cerebrovascular reactivity measurements using BOLD fMRI in combination with a paced deep breathing task”, NeuroImage, vol. 98, pp. 31-41, Apr. 2014
- M. A. Lindquist, S. Geuter, T. D. Wager, and B. S. Caffo, “Modular preprocessing pipelines can reintroduce artifacts into fMRI data”, Hum. Brain Mapp., vol. 40, no. 8, pp. 2358–2376, Jun. 2019
- M. G. Bright and K. Murphy, “Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance”, Neuroimage, vol. 83, pp. 559–568, Dec. 2013
- N. P. Blockley, I. D. Driver, S. T. Francis, P. A. Gowland, “An improved method for acquiring cerebrovascular reactivity maps”, MRM, vol. 65, no. 5, pp. 1278-1286, May 2011.
- M. G. Bright, D. P. Bulte, P. Jezzard, and J. H. Duyn, “Characterization of regional heterogeneity in cerebrovascular reactivity dynamics using novel hypocapnia task and BOLD fMRI”, Neuroimage, vol. 48, no. 1, pp. 166–175, Oct. 2009.
References


That's all folks!
Thanks to...



...the SPiN-lab group @ BCBL
... the Brightlab - ANVIL group@ Northwestern University
...you for the (sustained) attention!
Research supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number K12HD073945, the European Union’s Horizon 2020 research and innovation program (Marie Skłodowska-Curie grant agreement No. 713673), a fellowship from La Caixa Foundation (ID 100010434, fellowship code LCF/BQ/IN17/11620063), the Spanish Ministry of Economy and Competitiveness (Ramon y Cajal Fellowship, RYC-2017- 21845), the Spanish State Research Agency (BCBL “Severo Ochoa” excellence accreditation, SEV- 2015-490), the Basque Government (BERC 2018-2021 and PIBA_2019_104), the Spanish Ministry of Science, Innovation and Universities (MICINN; FJCI-2017-31814)




