Light Emission and Light Quanta (Photons)
M. Rocha
Physics 2B
Electromagnetic Waves
Oscillating charges create oscillating magnetic fields, which in turn create oscillating electric fields



The result are oscillating electric and magnetic fields that regenerate each other while traveling on space
Electromagnetic Wave Production (Classical)
Electromagnetic Waves are produced by accelerating or oscillating charges

Can you produce light with an antenna?
According to classical theory you could
In practice this is a challenge that no one has successfully achieved (still under active research)
In order to create EM waves with wavelength you would need an antenna of length ,
and an oscillating current with a frequency
So how is light produced?

We already know that objects emit light via black-body radiation if their temperatures are high enough
Does temperature accelerates charges like tiny antennas and produce light in a classical way?
The Ultraviolet Catastrophe
When trying to explain black-body radiation as just electromagnetic energy produced by accelerating charges due to temperature, Rayleigh ran into the ultraviolet catastrophe
Infinite energy at high frequencies (short wavelengths)!
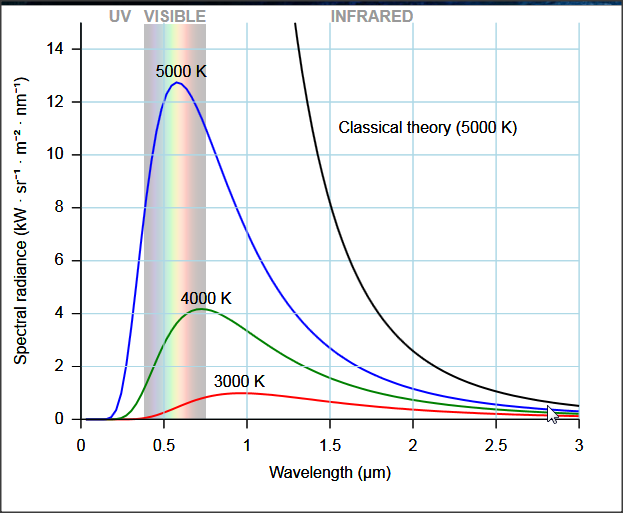
The ultraviolet catastrophe was solved by Max Planck. Assuming that EM radiation was emitted in discrete packets (quanta) Plank was able to explain black-body radiation
Light Quanta
Plank's found the emission of radiation is quantized, meaning that radiation energy can only be transferred in chunks of size:
The Photon
Not too long after Planck's discovery Einstein introduced the concept of the photon to explain the photoelectric effect


Text
Light is emitted and absorbed in packets called photons. The photon energy is proportional to the frequency of light and given by E = hf
Text
So, is light made out of particles or waves?
And let the weirdness of the quantum world begin!
The Wave-Particle Duality
In the quantum world (the universe at sub-atomic scales) not just light but matter and energy must be explained as both, particles and waves
In Einstein's Words:
It seems as though we must use sometimes the one theory and sometimes the other, while at times we may use either. We are faced with a new kind of difficulty. We have two contradictory pictures of reality; separately neither of them fully explains the phenomena of light, but together they do.
Checkpoint 1
What is the wavelength of a photon with ?
A violet photon!

Light Emission

Charged particle or Photon
Atoms emit light when their electrons change from higher energy levels to lower energy levels
A photon is emitted when an electron gets de-excited
The Atom
Early 20th-century model
Current model

Atomic Energy Levels
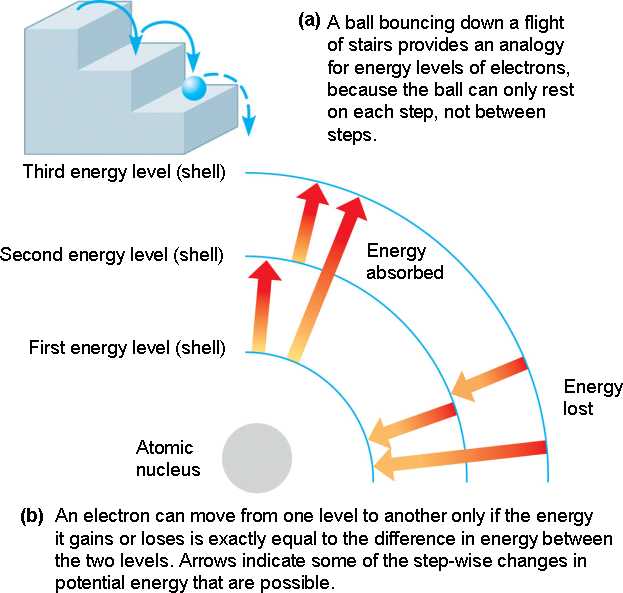
Explanation of Quantized Energy Levels
Electrons can only be at discrete energy levels (orbit radius), because the circumference of their orbit has to be an integer number of their wavelength in order to form standing waves, otherwise the experience destructive interference
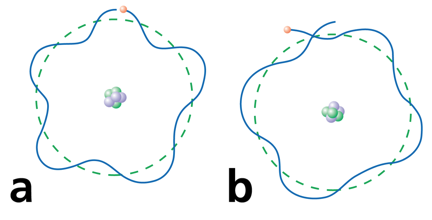
In 1924 Louis de Broglie's suggested that electrons have a wavelength. This explained the quantized energy levels of atoms
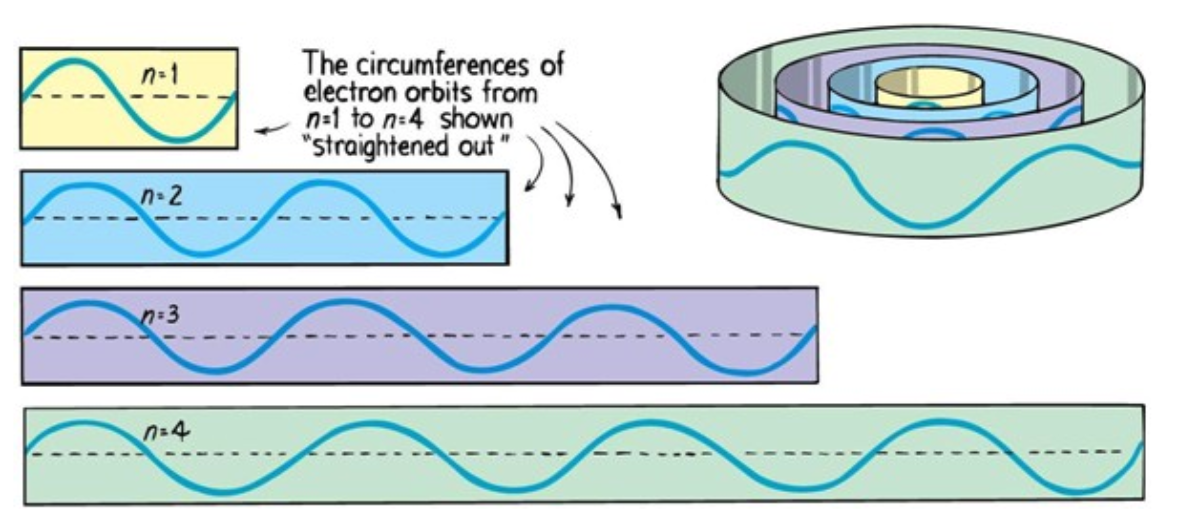

Atomic Energy Levels
Electrons can only be at discrete energy levels (orbit radius), so that the circumference of their orbit is an integer number of their wavelength



Atomic Energy Levels
Different atoms have different radius (potential energy) and thus different energy levels for their electrons
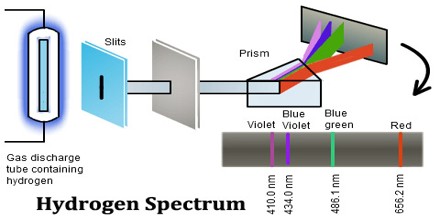
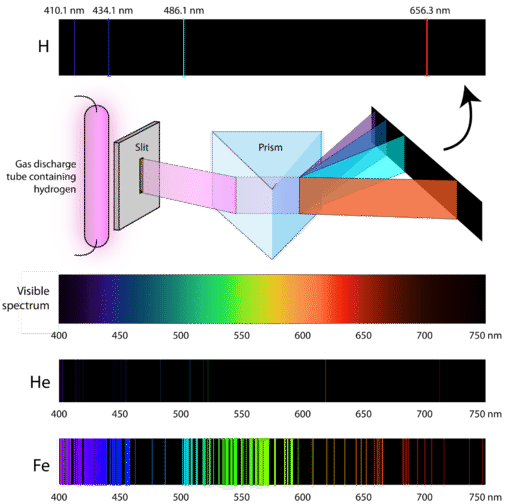
Emission Spectrum
If you disperse the light emitted by specific elements, you would get a characteristic spectrum for each element
Each element has a different emission spectrum, which can be thought as the signature of that element
Emission Spectrum
Each element has a different emission spectrum, which can be thought as the signature of that element
Checkpoint 2
On the emission spectrum of Hydrogen there is visible line at 660 nm, what is the energy of the photons producing this line ?

660 nm

Mostly hydrogen (Pinkish Color)

Hydrogen Spectrum
Planetary nebula are metal rich (Many Colors)


Hourglass Nebula
Ring Nebula
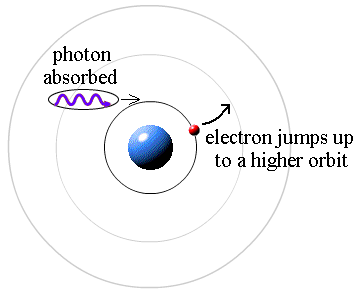
Absorption Spectrum
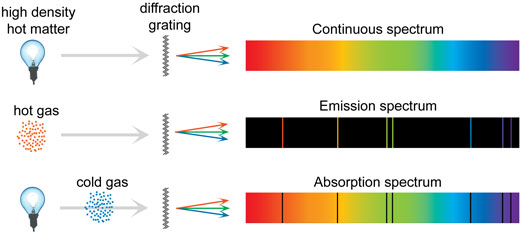
If electrons in an atom get excited by light, they absorb photons at the same specific frequencies as they would emit when de-excited
Fluorescence
Fluorescence happens when electrons de-excite in energy steps smaller than when they got excited


Fluorescence Lamps

In fluorescence lamps first mercury atoms get excited and emit mostly UV photons when de-excited. Then those UV photons excite phosphor atoms in the coating. Phosphor atoms de-excite in smaller steps (fluorescence) producing lower frequency light. The combination of high frequency photons from the mercury and low frequency photons from the phosphor is white light

Phosphorescence

Phosphorescence happens when electrons are boosted into higher energy levels and become "stuck". As a result, there is a time delay between the processes of excitation and de-excitation
The element phosphorus is used in a variety glow in the dark materials
Light Emitting Diode (LED)
An LED Chip consists of two semiconductors, one with loose electrons and the other with "holes"
When a voltage is applied, electrons cross the barrier, fall in the holes and emit light

