Properties of Matter
M. Rocha
Physics 2A
What is matter made of?
Aristotles
Leucippus and Democritus
450 BCE


4 essential elements
Atoms
Brownian Motion
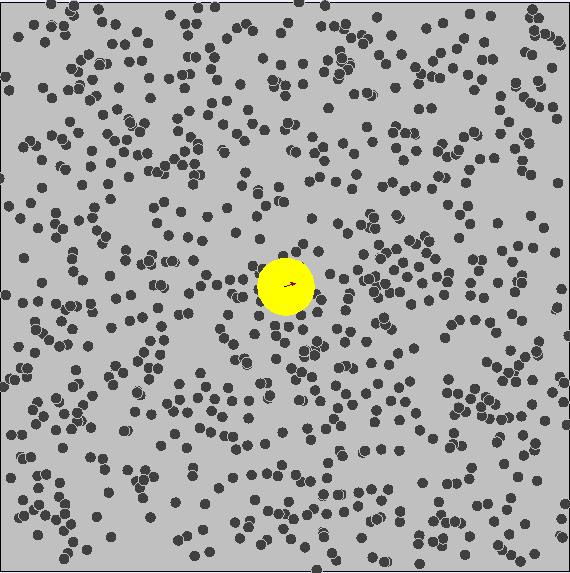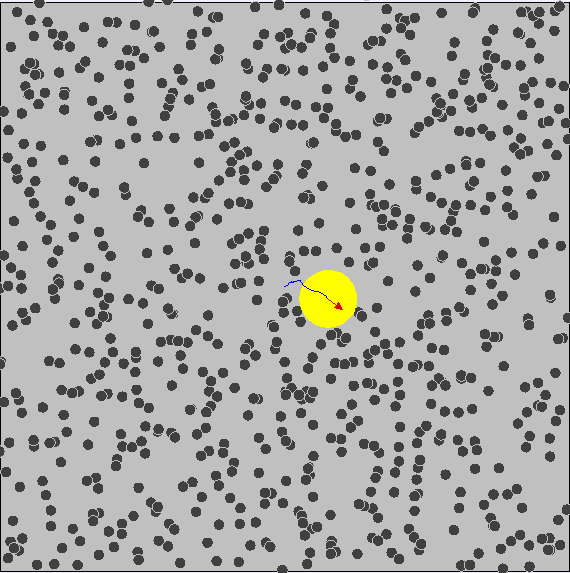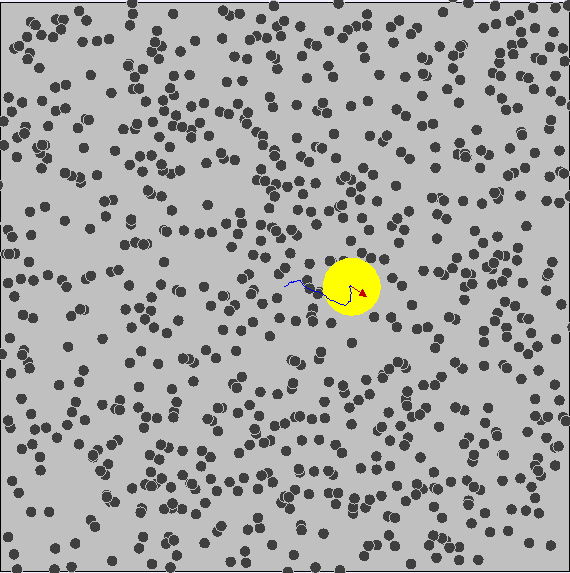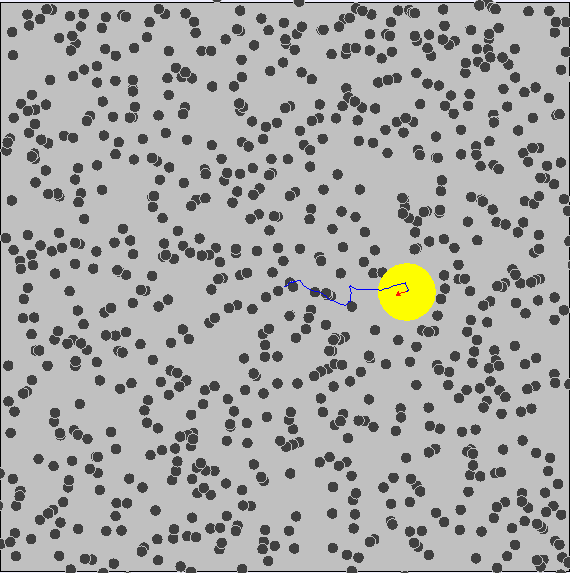
First evidence of the Atomic Model
First observed by Robert Brown (1827) First explained by Albert Einstein (1905)
Rutherford Scattering
The Rutherford scattering experiment (1911) demonstrated that atoms must have a core (Nucleus)
The Atom
Early 20th-century model
Current model
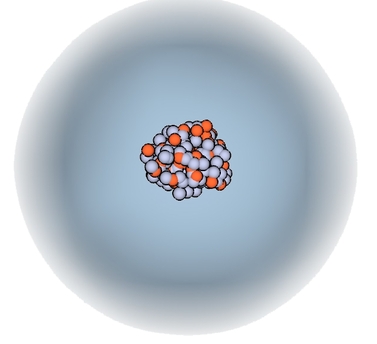

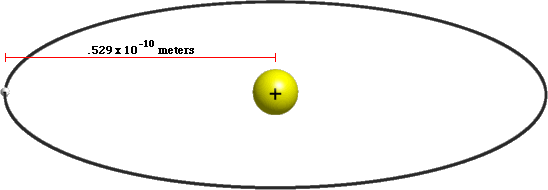
Atoms are tiny ~10-10 meters


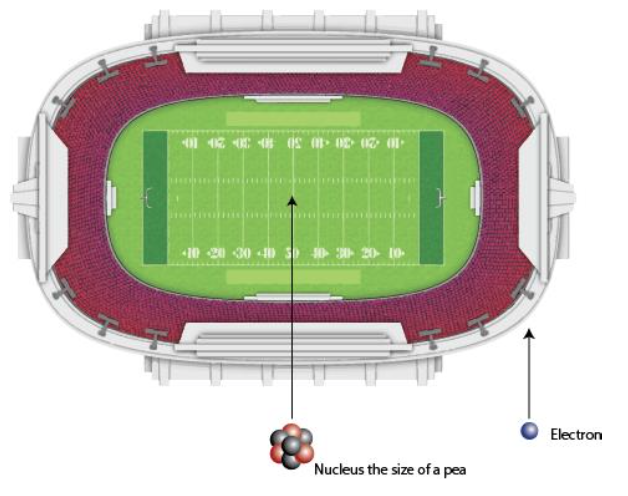
Atoms mostly empty space
Atoms are 99.9999999999996% empty space!
Atoms are numerous
There are 1023 atoms in a gram of water.
- That is 100x more than the number of grains of sand in all the beaches of the world!
- And as many as the number of stars in the observable Universe!


There are as many atoms in a grain of sand than stars in the Universe!
Atomic Imaging
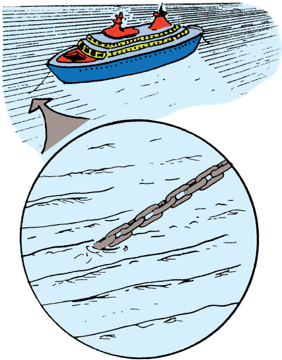
Atoms are too small to be seen with visible light
Light is made up of waves, and atoms are smaller than the the wavelengths of visible light
Atomic Imaging
You need electron beams, electron beams behave like waves
The wavelength of electron beams is small enough to detect atoms
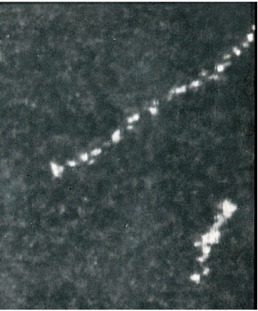
The Scanning Electron Microscope (SEM) produced the first picture of individual thorium atoms in 1970
Atomic Imaging
You need electron beams, electron beams behave like waves
Today we use Scanning Tunneling Microscopes (STM) for atomic imaging

Atomic Imaging
You need electron beams, electron beams behave like waves
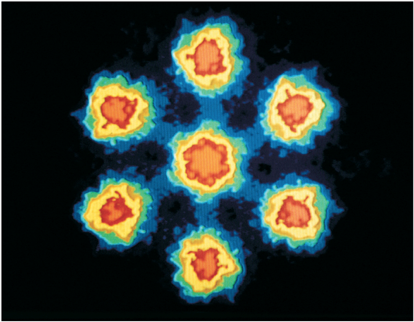
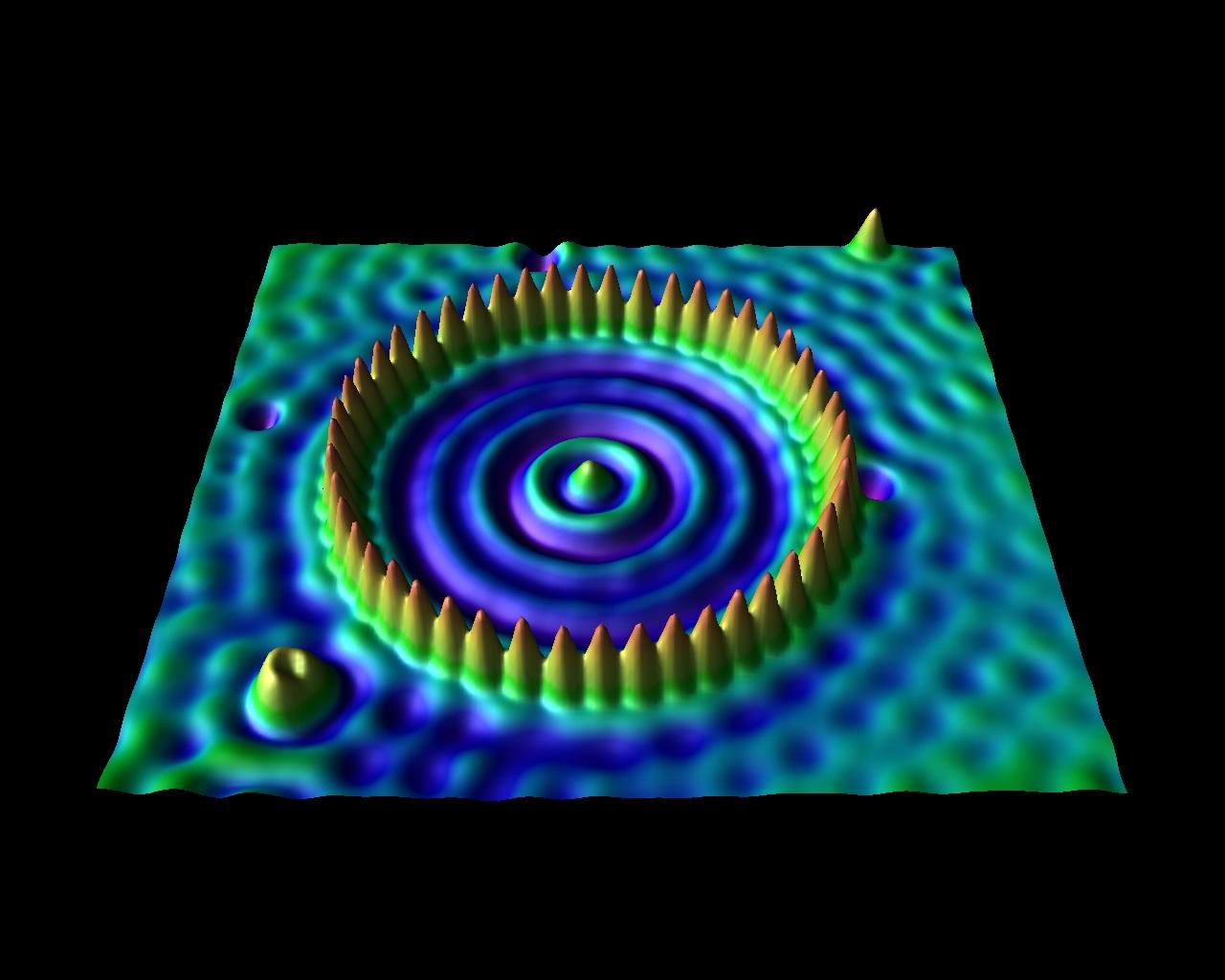
Ring of 48 iron atoms
Uranium atoms
Images produce with Scanning Tunneling Microscopes (STM)

Ant under an electron microscope
The elements
When a substance is composed of only one kind of atom, it is called an element
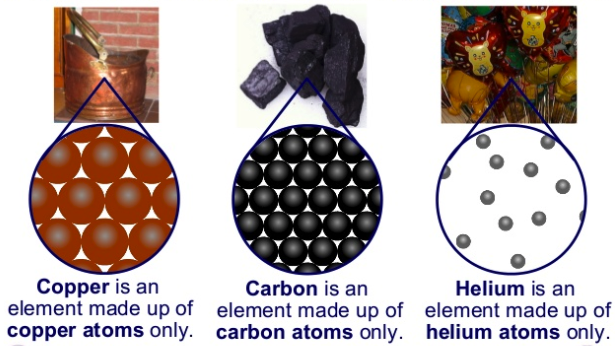
The elements
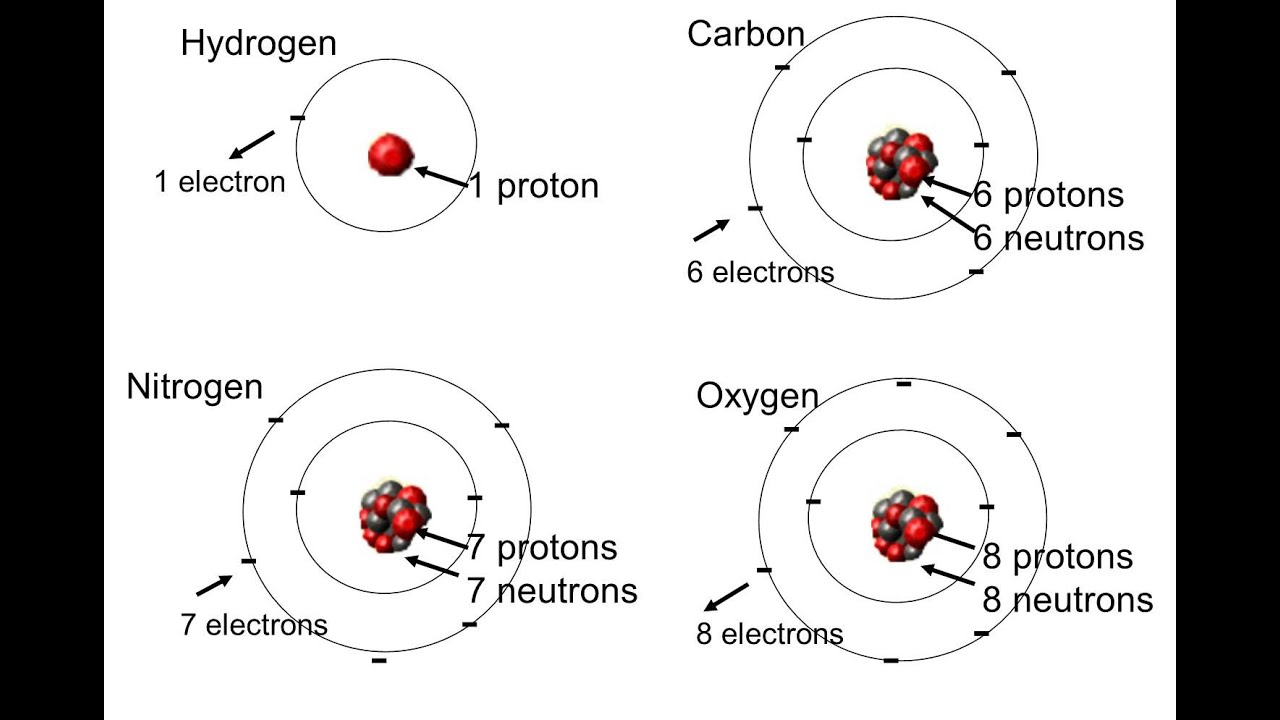
When a substance is composed of only one kind of atom, it is called an element
The number of protons determines the kind of atom (element)
Checkpoint 1
You observe two atoms, one with 6 protons and 7 electrons and the other one with 6 protons and 5 electrons, are they the same element?
Yes, same number of protons.
The elements
The periodic table categorizes all the known elements

The elements
When a substance is composed of only one kind of atom, it is called an element
The number of protons and electrons in an atom determines the kind of atom (element) and its properties
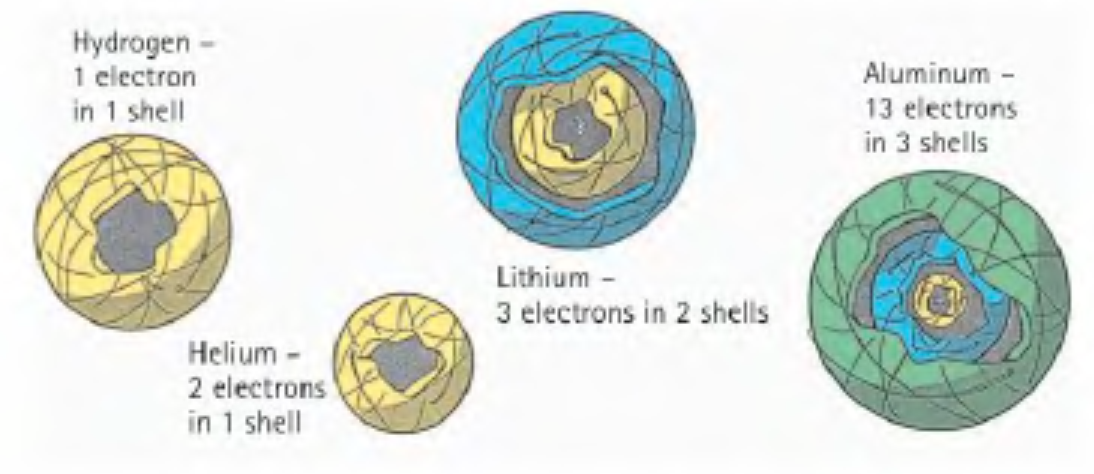
The elements
The periodic table categorizes all the known elements
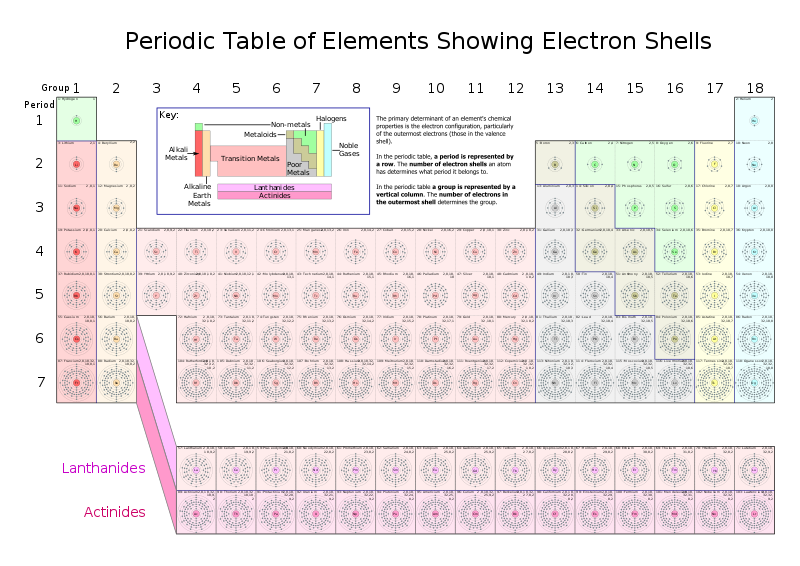
The elements
The periodic table categorizes all the known elements

Ions
Ions are charged atoms. They occur when the number of protons is not the same as the number of electrons
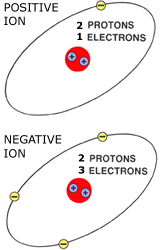
Helium (2 protons) Ions
Checkpoint 2
If you have a Carbon atom with 6 protons and 5 electrons what kind of Carbon ion is this, positive or negative?
It is a positive Carbon ion.
Isotopes of an element
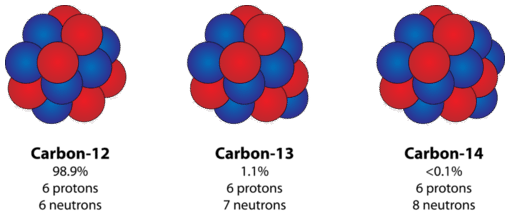
Carbon Isotopes
Atoms of the same element that have different numbers of neutrons are called isotopes
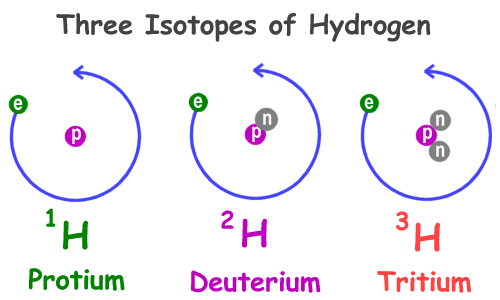
Hydrogen Isotopes
Checkpoint 3
How many protons and neutrons the Helium-3 isotope has?
2 protons and 1 neutron
Molecules
A molecule forms when two or more atoms bond together by sharing electrons

Caffeine Molecule
Simple Molecules
Compound Molecule
Water Molecule
Mixtures

Mixtures form when different elements mix together without forming chemical bonds (no electron sharing)
The atmosphere is an example of a mixture

The Standard Model

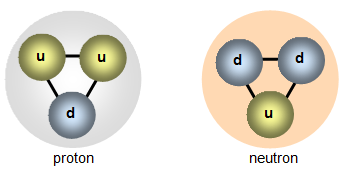
Protons and Neutrons are made of Quarks
Fundamental Forces:
Strong, Electromagnetic + Weak
Gravity?
All of the matter that we know is made of these 9 fundamental particles
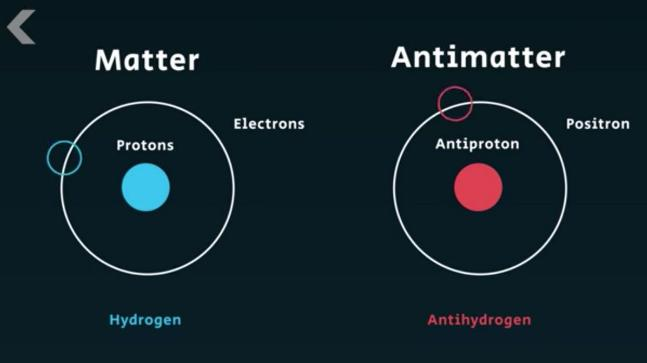
Antimatter
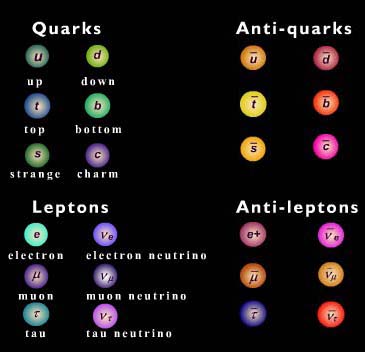
All particles have an anti-particle with the same properties but opposite charge. When matter and anti-matter combine they annihilate to produce pure energy
Dark Matter
~85% of matter in the universe is made of something that does not interact with light, thus we can not see. We call this dark matter

We know dark matter is there and how much is there purely by its gravitational effects. Dark matter holds the universe together.
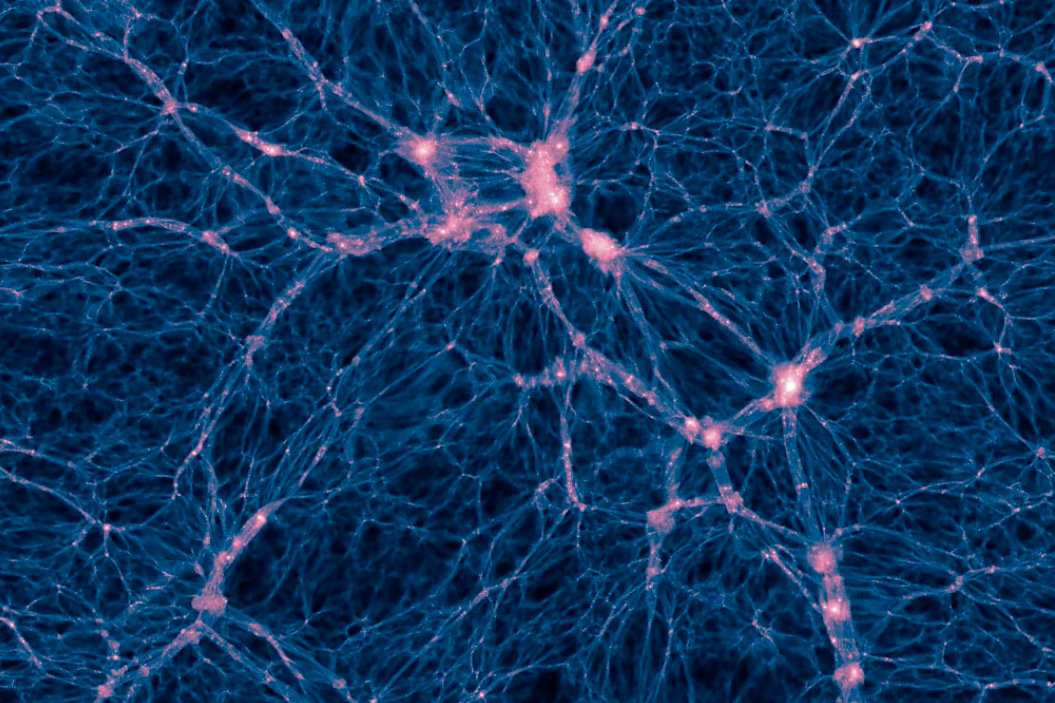
Dark Matter
Dark Matter


Galaxy Formation
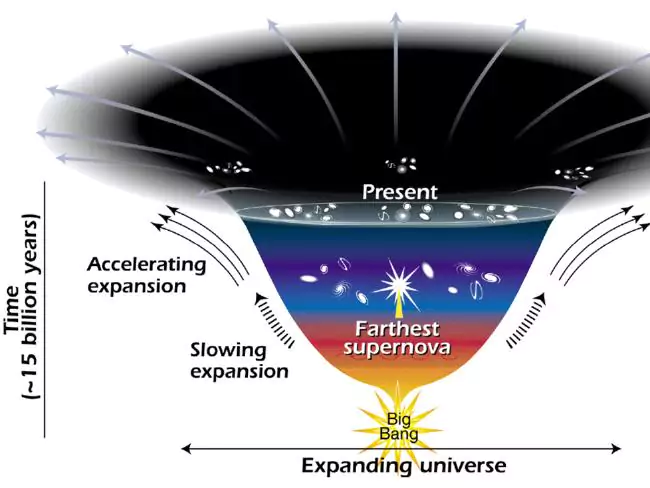
Dark Energy
We know the Universe is expanding at an accelerated rate. In order to explain this there must be an unknown form of energy that makes ~70% of the Universe and produces a expanding force (like antigravity)

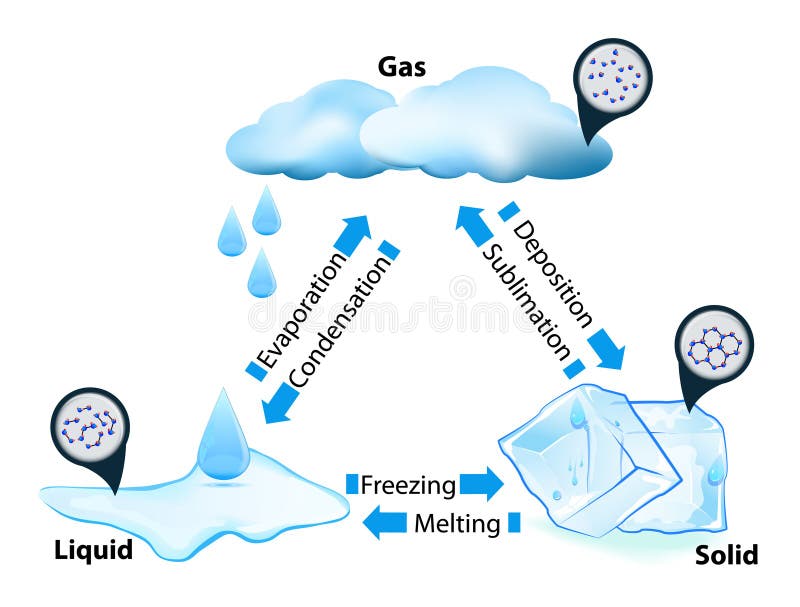
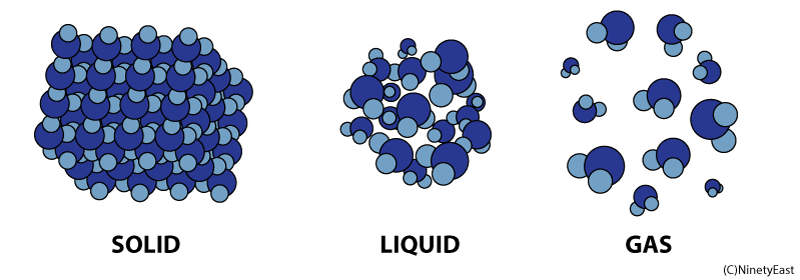
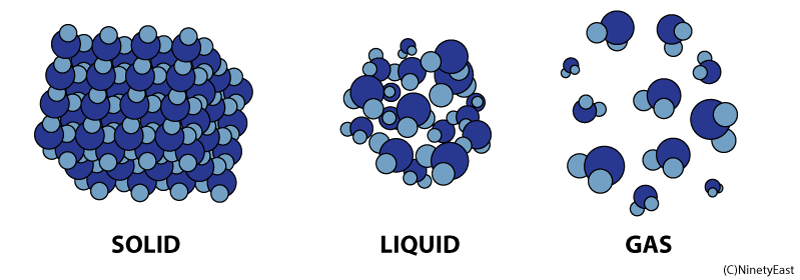
Properties of Matter in Different States:
Solids, Liquids and Gases
Solids
Crystals
Amorphous
Rubber, plastic, wood
Metals, minerals, salts



NaCl Sodium Chloride (Salt)
Frozen H2O (Ice)


Neoprene
Wood
Properties of Solids
Density - Amount of mass per unit volume
Elasticity - How much a solid's shape changes when a deforming force acts, and how well it returns to its original shape when the force is removed.
Checkpoint 1
1 liter of water weights 1 kg. A liter is a measure of volume, 1 liter = 1000 cm^3. What is the density of water?
1000 g/1000 cm^3 = 1 g/cm^3
or
1 kg/L = 1g/ml
Checkpoint 2
When water freezes, it expands. What does this say about the density of ice compared to the density of water?
Water is more dense than ice.
Hooke's Law
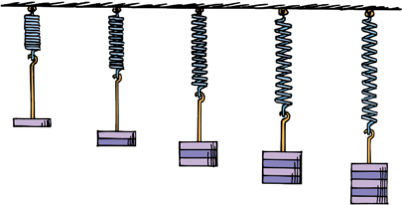
For elastic materials, the stretch is directly proportional to the applied force.
Hooke's law holds as long as the material is not stretched or compressed beyond the elastic limit (when permanent deformation occurs).
Checkpoint 3
A tree branch is found to obey Hooke’s law. When a 20 kg load is hung from the end of it, the branch sags 10 cm. If a 60 kg load is hung from the same place, how much will the branch sag? (Assume none of these loads makes the branch sag beyond its elastic limit).
A 60 kg load is 3x greater than the 20 kg load, so the branch sags 10cm x 3 = 30 cm
Liquids
In the liquid phase, molecules can flow freely from position to position by sliding over one another. A liquid takes the shape of its container
Properties of Fluids
Density - Amount of mass per unit volume
Pressure - Force per unit area
Pressure
Pressure is force per unit area
Metric unit of pressure is Pascal.
1 Pascal = 1 Newtons per square meter
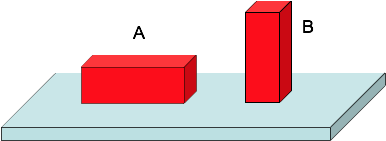
A pressure < B pressure
A pressure < B pressure
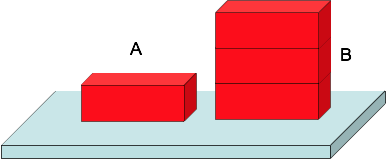
Checkpoint 4
How does the pressure in A) compares to the pressure in B)?
A) has 3x more force over 3x more area, so The pressure in A) is the same as in B)

Weight is distributed over hundreds of nails, so the pressure at the point of each nail safely small

Pressure in Liquids
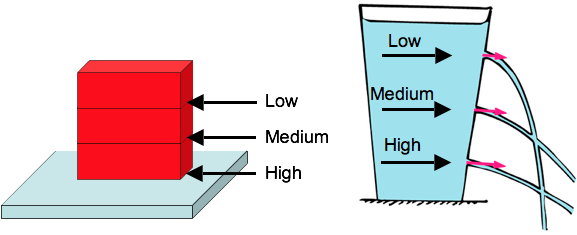
Pressure in a liquid depends on depth.
As with bricks, weight of what’s above determines pressure
Pressure in Liquids
Pressure in a liquid depends on depth.
The weight of what’s directly above determines pressure
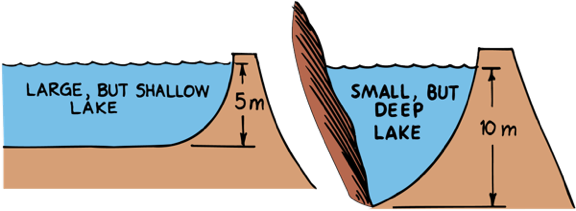
Less Pressure
More Pressure
Buoyancy
If the weight exceeds the buoyancy force then the object sinks, otherwise it floats
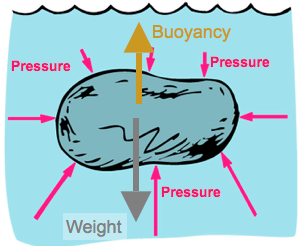
Since pressure depends on depth, a submerged object has more force due to pressure below it than above it.
Net effect is to have a net upward force, which we call buoyancy.
Buoyancy
When an object is submerged, it displaces a volume of water equal to the volume of the object itself
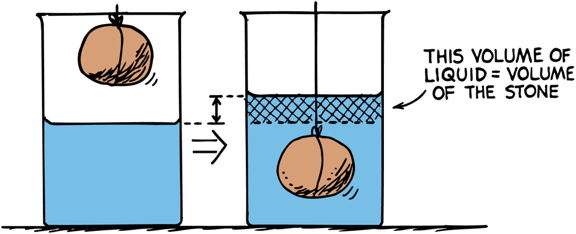
Arquimedes' principle
Weight of liquid displaced by floating or submerged object equals the buoyant force on the object
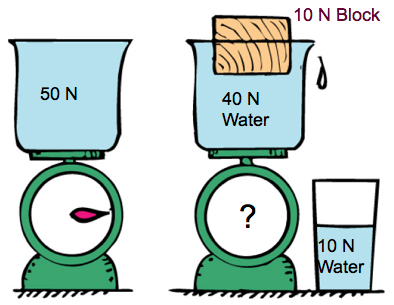
Checkpoint 5
If you place a block of wood in the water, does scale reading goes up, down, or stays the same?
Stays the same
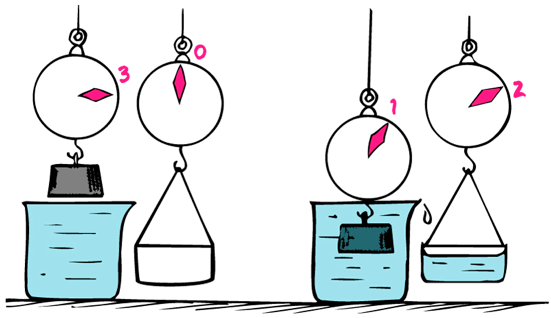
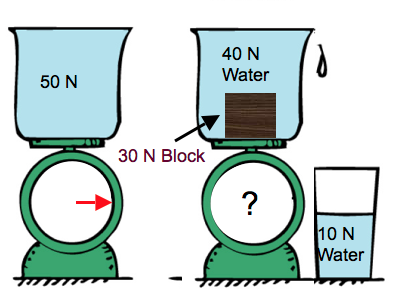
Checkpoint 6
If you place a block of petrified wood in the water, does scale reading goes up, down, or stays the same?
Goes up
Density & Floating

A billiard ball (density = 4.0 g/cm3) floats in a cup of mercury
(density = 13.6 g/cm3)
By Archimede’s principle, a solid object will float if the density of the object is less than the density of the liquid
Density & Floating


Density of wood is about 0.5 to 0.8 g/cm^3. It is not surprising that wooden ships float
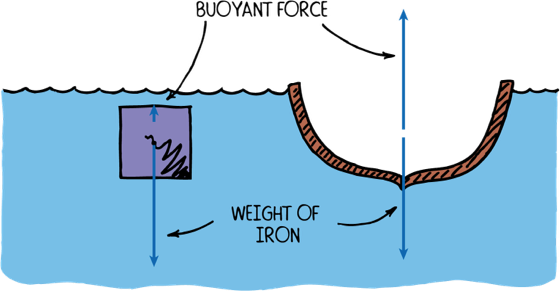
Density of iron 7.9 g/cm^3. How is it that a battleship can float?
Density & Floating
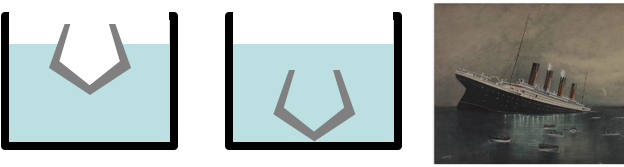
An iron ship floats since it is hollow inside. If water floods the inside then it sinks
When an object is not solid then it floats if the average density, (total mass)/(total volume), is less than the density of the liquid.
Density & Floating
An iron ship floats since it is hollow inside. If water floods the inside then it sinks
When an object is not solid then it floats if the average density, (total mass)/(total volume), is less than the density of the liquid.
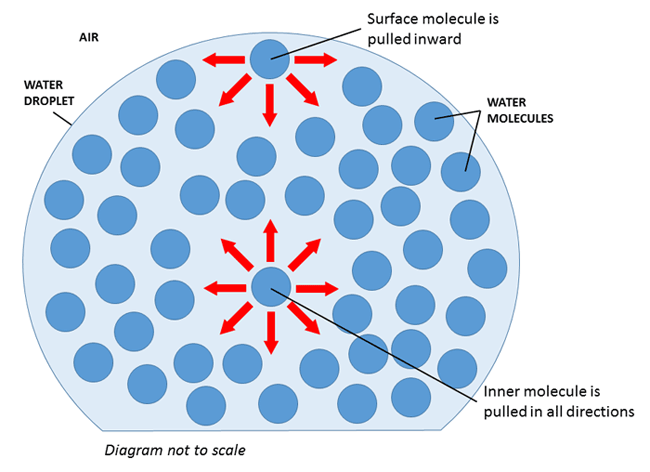
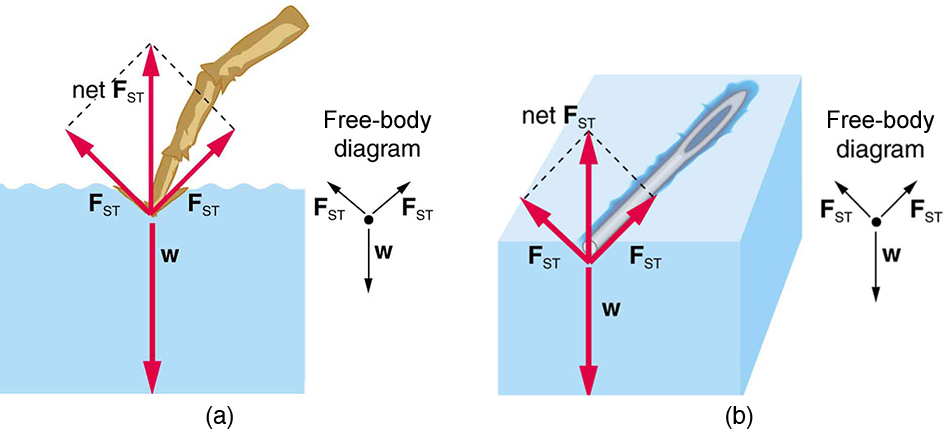
Surface Tension

Gases
Molecules in gases are free to expand indefinitely and fill all the space available. Gases flow just like liquids, so gases are also called fluids.
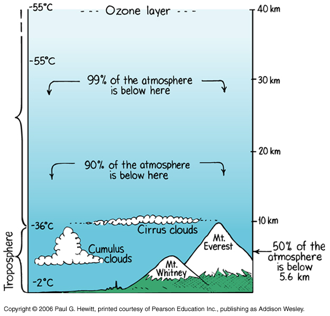
Atmospheric Pressure
We live at the bottom of a fluid "ocean" of air — the atmosphere.
The density of air in the atmosphere decreases with increasing altitude
Atmospheric Pressure
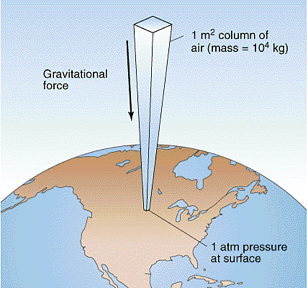
Atmospheric column of air
Base: 1 square meter
Height: 10 km = 10000 m
Volume: 10,000 m^3
Mass: 10,000 kg
Weight: 100,000 N = 10 tons
Pressure: 100,000 Pascals
Atmospheric pressure is really strong!
~15 pounds per square inch
We live at the bottom of a fluid "ocean" of air — the atmosphere.

Atmospheric Pressure is Really Strong
The interior of the tank car was washed out & cleaned with steam. Then all the outlet valves were shut and the tank car was sealed.
All the workers went home for the evening and when they returned, this is what they found.
Apparently as the tank car cooled, it collapsed. The shell on these tank cars is 1/2 inch thick steel.
Buoyancy on Air
Objects can float in air, just as they float in water, if the objects’ average density is less than the density of air

Boyle's Law
When the volume of a gas is decreased, the density increases and therefore pressure increases
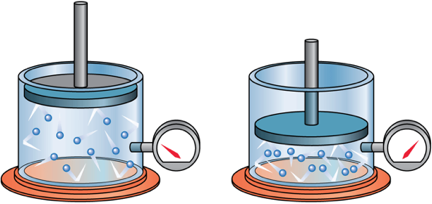
Boyle's Law
When the volume of a gas is decreased, the density increases and therfore pressure increases


Boyle's Law
Checkpoint
If you were to dive 10 m deep you would experience 2 atmospheres of pressure. If you breathe compressed air at that depth the air you breath would have to be at 2 atmospheres of pressure. By how much would the volume of your lungs expand if you then shoot to the surface while holding your breath?
Your lungs would expand by 2 times, i.e. twice the volume.