Bohr Models
Step 1
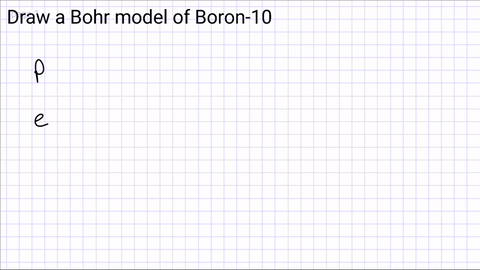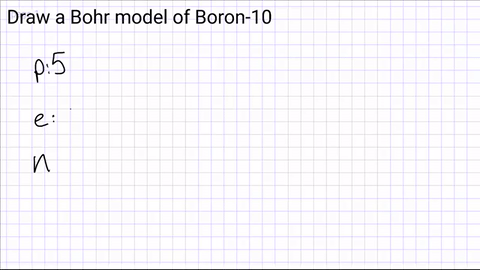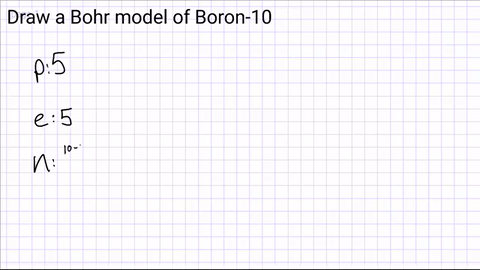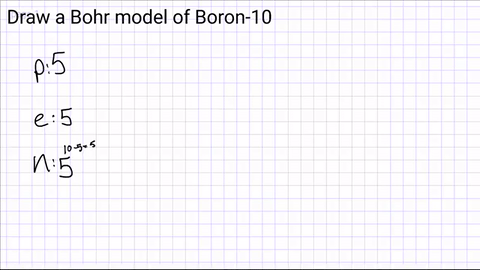
Write down how many protons, neutrons and electrons are in the atom?
Step 2
Draw a nucleus and include the number of protons and neutrons
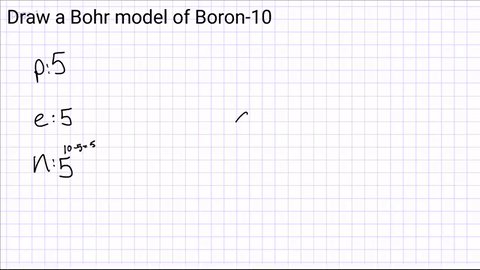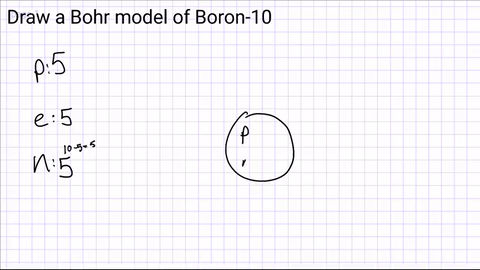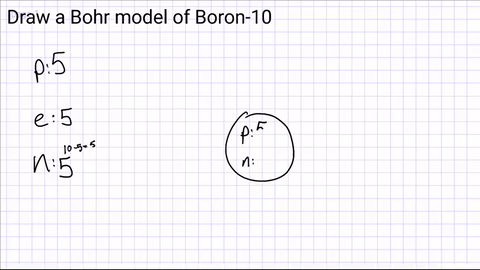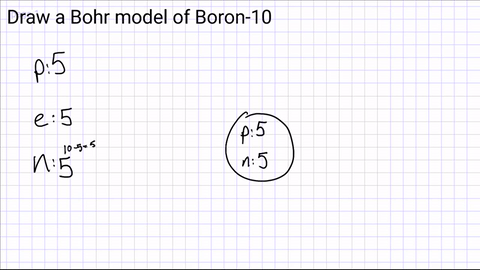
Step 3
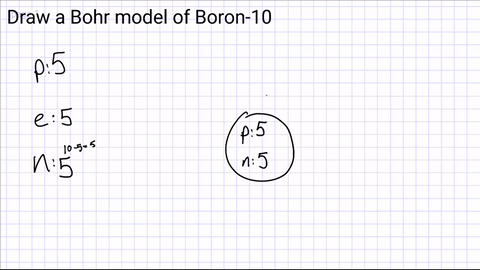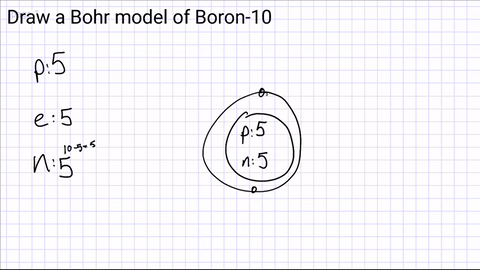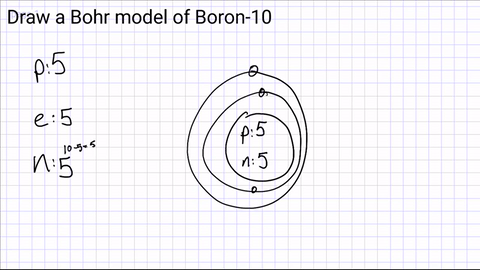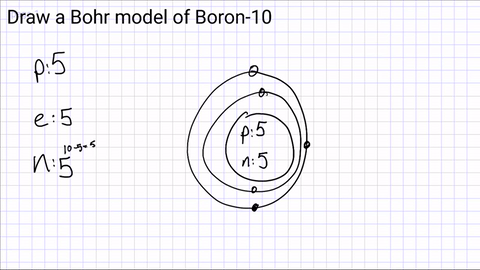
Draw your first energy level and start adding electrons.
The first energy level can hold a maximum of 2 electrons
(if you have more than 2 electrons, you will need another energy level!)
Note: Maximum electron count:
1st Level- 2 electrons
2nd Level- 8 electrons
3rd Level- 8 electrons
4th Level- 8 electrons
This is a tiny lie, but it works for the elements we will use
practice
Makes
Perfect

More examples below! Press 'down'
Example
Draw a Bohr Model for Oxygen-16
P:8
N:8
2. Draw the nucleus
3. Draw the first energy level
4. Begin adding your electrons to the first energy level (max of 2)
1. Figure out p, e, n
5. Continue adding electrons until you reached your total
p: 8
n: 8 (16-8=8)
e: 8 (same as protons for a neutral atom)
More examples below! Press 'down'
Example
Draw a Bohr Model for Magnesium-25
P:12
N:13
2. Draw the nucleus
3. Draw the first energy level
4. Begin adding your electrons to the first energy level (max of 2)
1. Figure out p, e, n
5. Continue adding electrons until you reached your total (second level max is 8)
p: 12
n: 13 (25-12=13)
e: 12 (same as protons for a neutral atom)
More examples below! Press 'down'
Example
Draw a Bohr Model for
P:6
N:8
2. Draw the nucleus
3. Draw the first energy level
4. Begin adding your electrons to the first energy level (max of 2)
1. Figure out p, e, n
5. Continue adding electrons until you reached your total (second level max is 8)
p: 6
n: 8 (14-6=8)
e: 6 (same as protons for a neutral atom)
More examples below! Press 'down'
Example
Draw a Bohr Model for
P:10
N:11
2. Draw the nucleus
3. Draw the first energy level
4. Begin adding your electrons to the first energy level (max of 2)
1. Figure out p, e, n
5. Continue adding electrons until you reached your total (second level max is 8)
p: 10
n: 11 (21-11=11)
e: 10 (same as protons for a neutral atom)
More examples below! Press 'down'
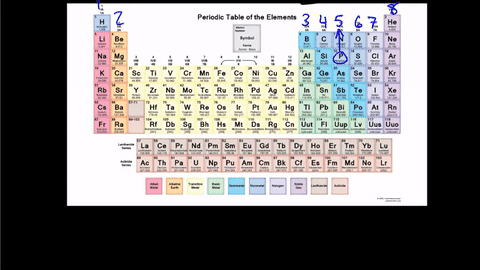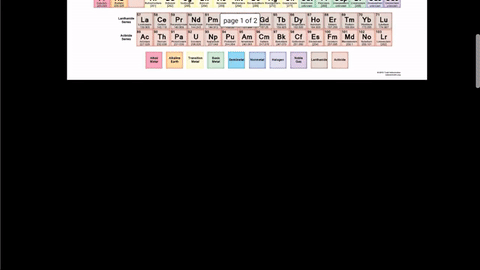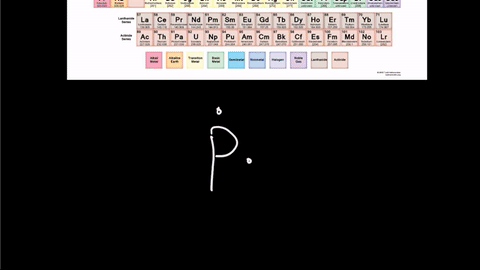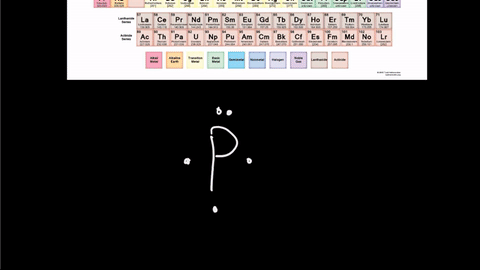
Lewis Dot structures
Step 1
Figure out the number of VALENCE ELECTRONS in the atom

The electrons on the outermost energy level
Draw the Lewis Dot Structure for Phosphorus-30
Step 2
Write the element symbol in the middle.
Then add the valence electrons around the outside
Draw the Lewis Dot Structure for Phosphorus-30
practice
Makes
Perfect

More examples below! Press 'down'
Example
Draw a Lewis Dot Structure for Oxygen-16
2. Write the element symbol
3. Going clockwise, add valence electrons to each side
NOTE: The maximum number of valence electrons anything can have is...
1. Figure how many valence electrons
8!
Oxygen is in the 6th column, so it has 6 electrons.
More examples below! Press 'down'
We can confirm this by drawing a Bohr Model of it
O
Example
Draw a Lewis Dot Structure for Chlorine-35
2. Write the element symbol
3. Going clockwise, add valence electrons to each side
NOTE: The maximum number of valence electrons anything can have is...
1. Figure how many valence electrons
8!
Chlorine is in the 7th column, so it has 7 electrons.
More examples below! Press 'down'
We can confirm this by drawing a Bohr Model of it
Cl
Example
Draw a Lewis Dot Structure for Magnesium-25
2. Write the element symbol
3. Going clockwise, add valence electrons to each side
NOTE: The maximum number of valence electrons anything can have is...
1. Figure how many valence electrons
8!
Magnesium is in the 2nd column, so it has 2 electrons.
We can confirm this by drawing a Bohr Model of it
Mg