Computational modeling of cardiac arrhytmia


Nele Vandersickel


Normal function of the heart
Normal function of the heart
Normal function of the heart
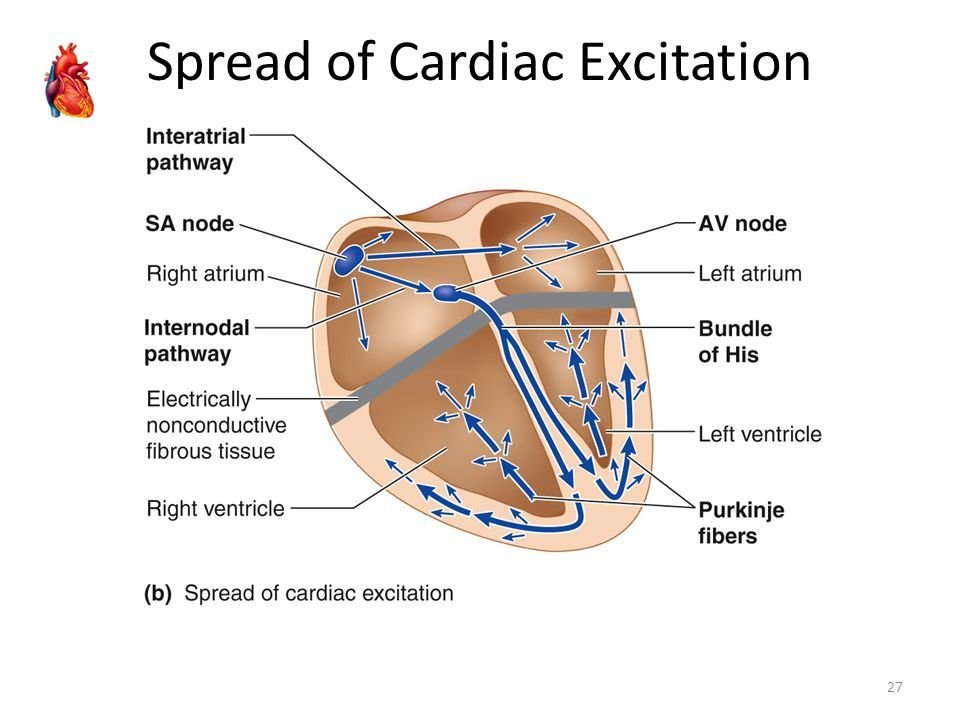
Normal rhythm 60 beats/min: determined by the SA node => 100.000 beats per day.
:delay of the signal
Normal function of the heart
-
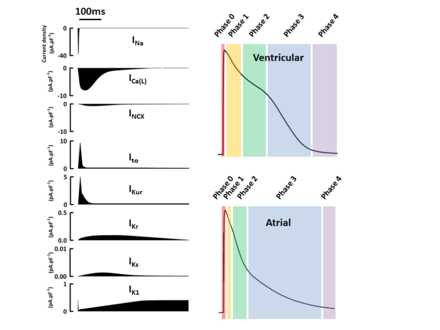
Ischaemic heart disease: Ventricular arrhythmias remain the leading cause of death from coronary artery disease
- Stroke can also be caused by cardiac arrhythmia: e.g. atrial fibrillation
Cardiac arrhythmia

Main questions
-
Mechanisms of the different types of arrhtyhmia
- Control of arrhythmia, how to remove it from the heart
Computer modeling can help with these questions!
Cardiac arrhythmia
Four major categories of cardiac arrhythmia
Regular
Irregular
Atrial Tachycardia
Atrial Fibrillation
Ventricular Fibrillation

Ventricular Tachycardia
Four major categories of cardiac arrhythmia
Regular
Irregular
Atrial Tachycardia
Atrial Fibrillation
Ventricular Tachycardia
Ventricular Fibrillation




1. Basic properties and computer modeling
1. Basic properties and computer modeling: single cell
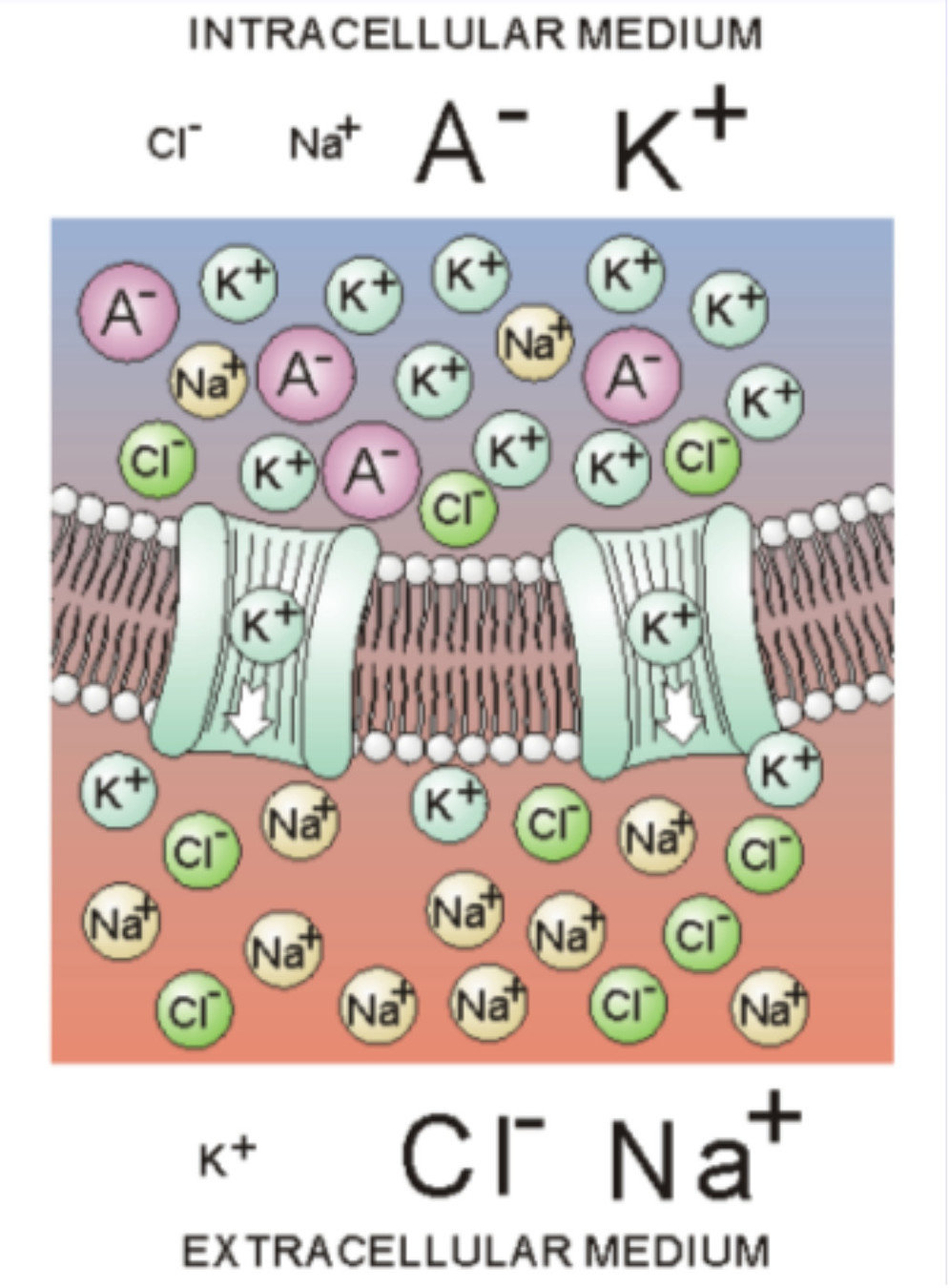
The membrane: contains channels which can open and close
1. Basic properties and computer modeling: single cell
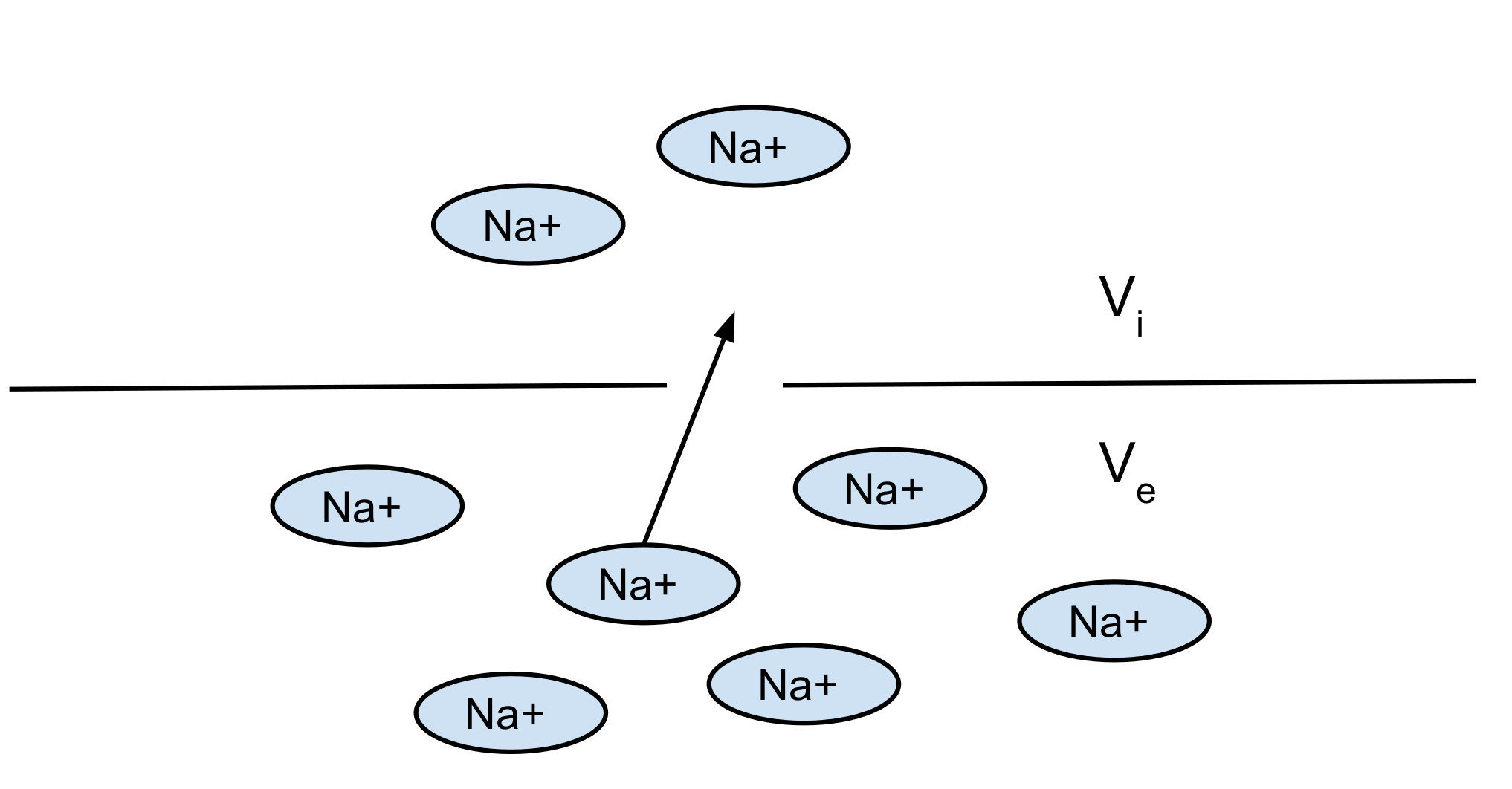
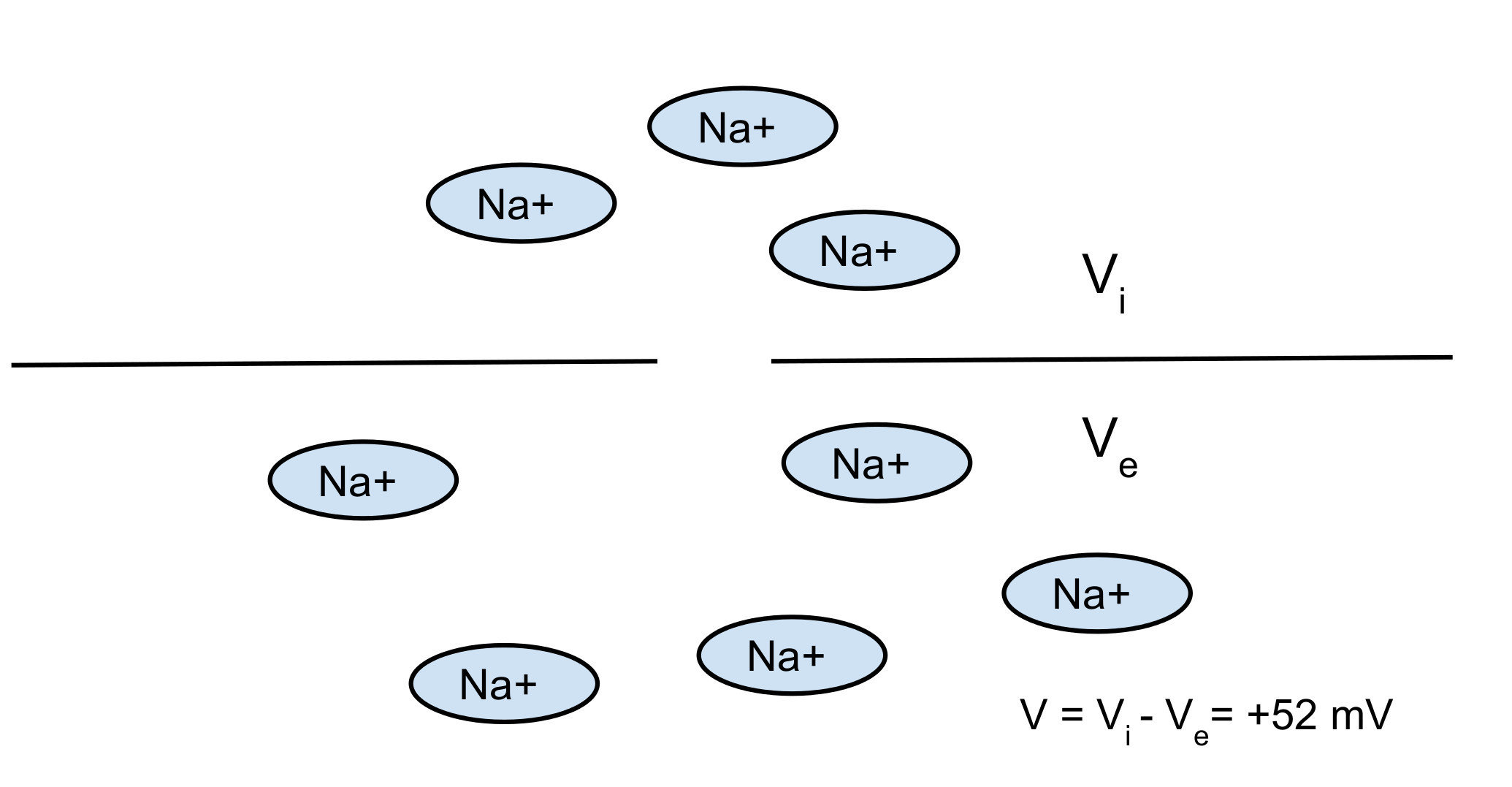
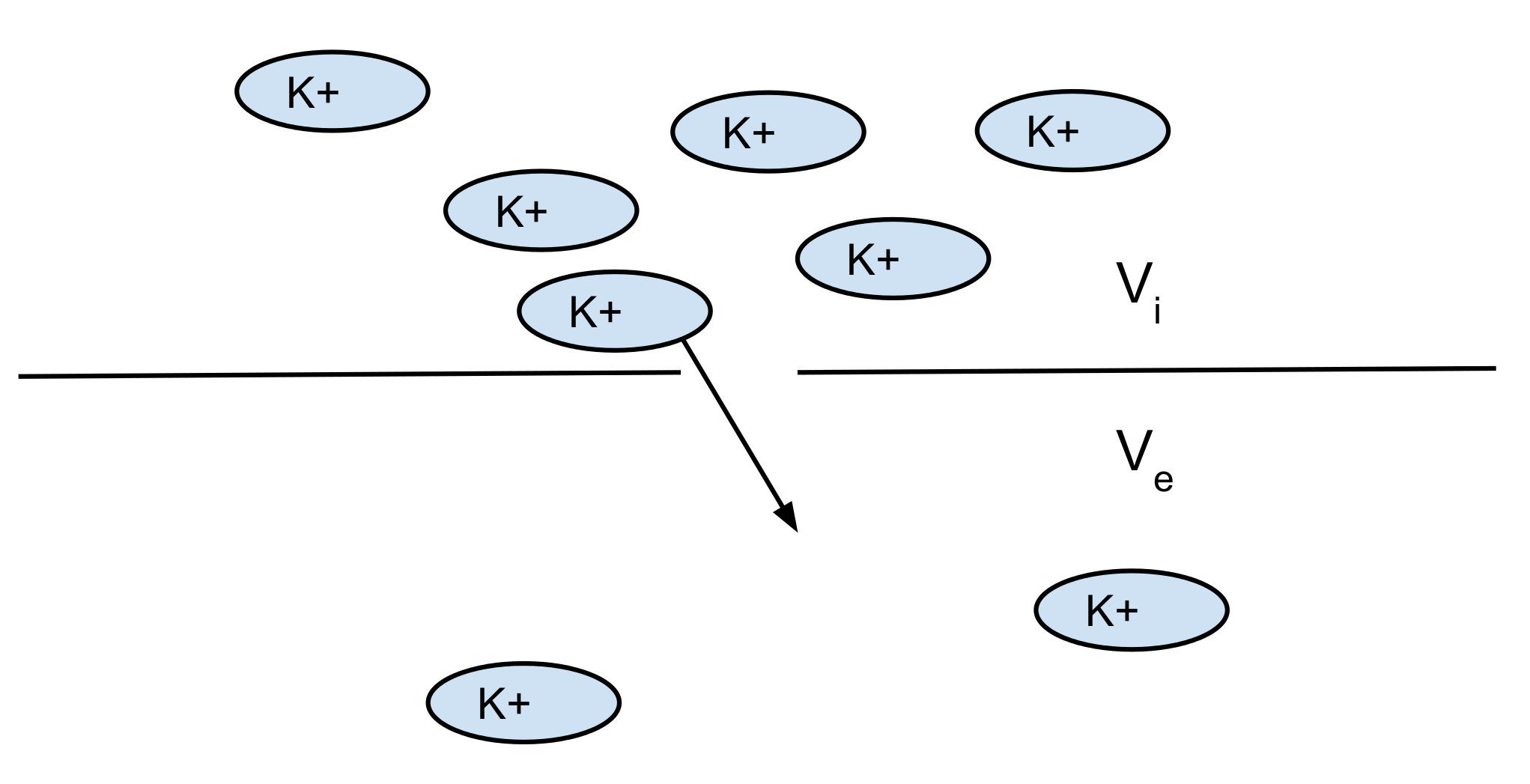
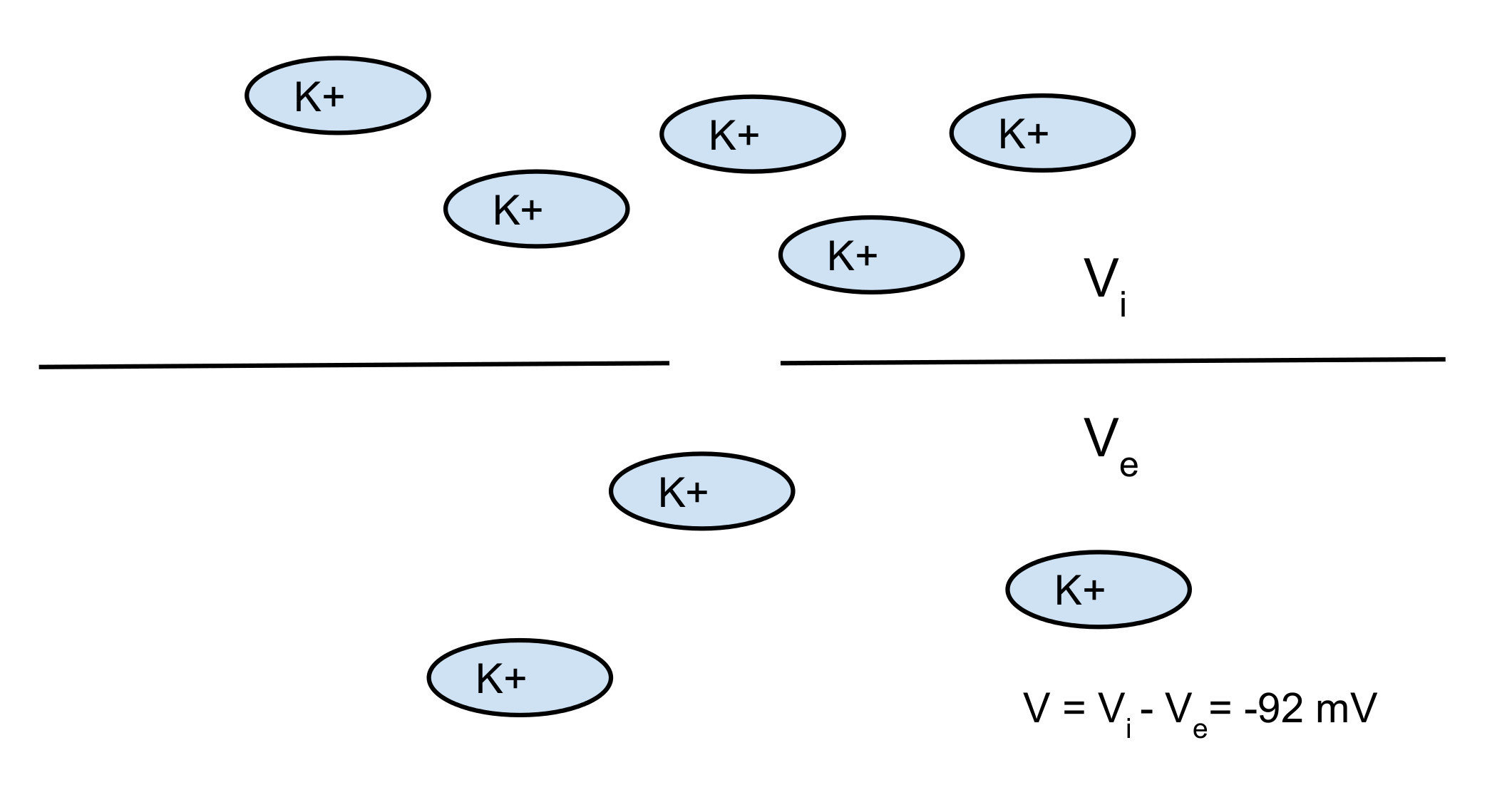
Nerst potential: 2 opposite forces:
- diffusion
- electrical force
You can find the equilibrium which gives you the Nerst potential
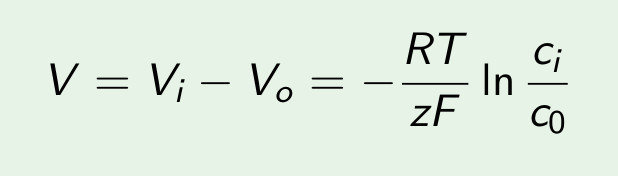
1. Basic properties and computer modeling: single cell
Nerst potential: 2 opposite forces:
- diffusion
- electrical force
You can find the equilibrium which gives you the Nerst potential
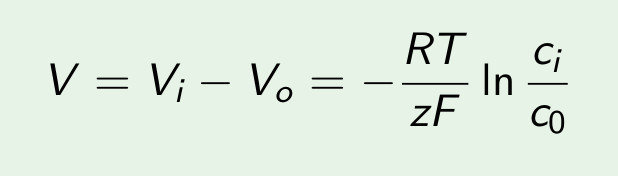
1. Basic properties and computer modeling: single cell
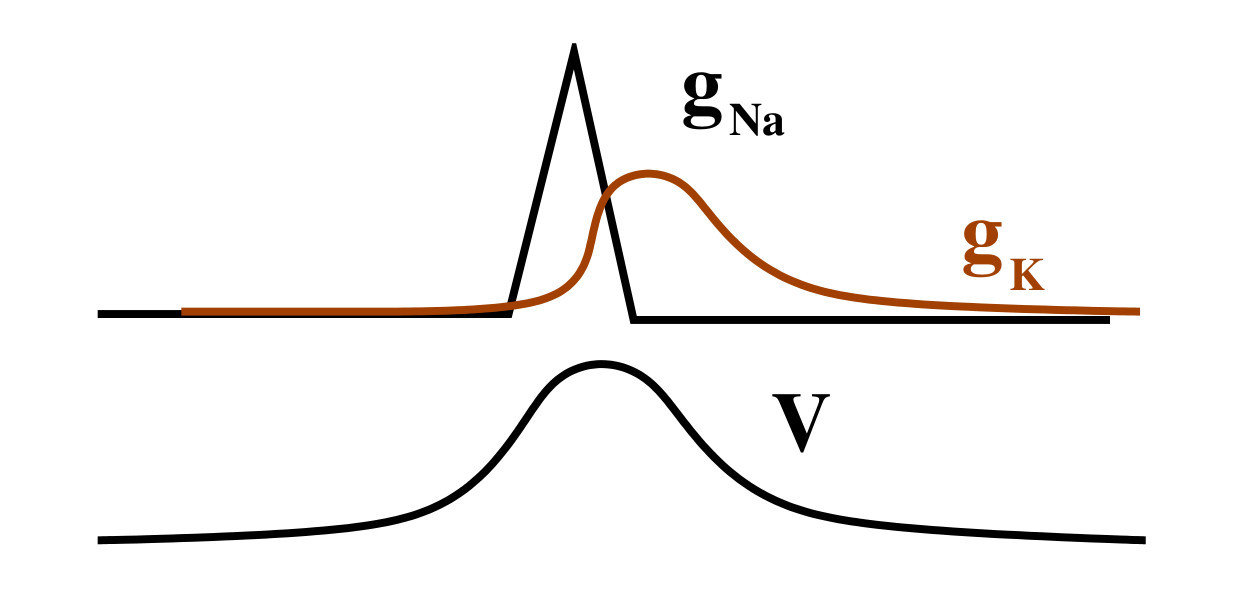
Most easy model: Hodgkin huxley (nerve cell)
1. Basic properties and computer modeling: single cell
Most easy model: Hodgkin huxley (nerve cell)
1. Basic properties and computer modeling: single cell
Most easy model: Hodgkin huxley (nerve cell)
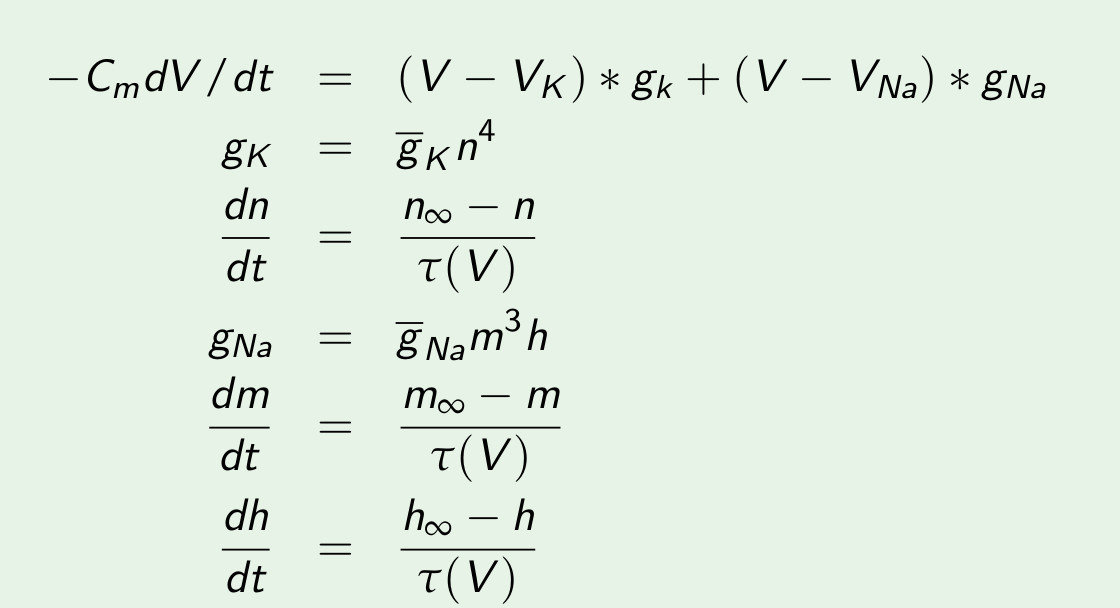
1. Basic properties and computer modeling: single cell
The whole cell: many more channels

1. Basic properties and computer modeling: single cell
The whole cell: also internal dynamics

1. Basic properties and computer modeling: single cell

The whole cell: many more channels
1. Basic properties and computer modeling: single cell
1. Basic properties and computer modeling: single cell
The whole cell: many more channels
The whole cell: many more channels

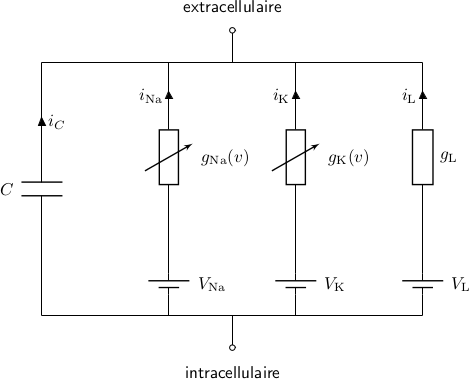
All the channels can be modeled as:
1. Basic properties and computer modeling: single cell
The whole cell: many more channels
- Only the most simple model (HH) can be solved analytically
- All other models have to be solved numerically with the computer
- At each timestep you can output any variable that you want: voltage, currents, concentration...
1. Basic properties and computer modeling: single cell
The whole cell: many more channels
-
There exist many different different models: for the ventricles 3 major models, more than 10 other models
-
The ORD model is being used to test the effect of drugs: ’Comprehensive in-vitro Pro-arrhythmia Assay (CiPA) initiative www.cipaproject.org. CiPA aims to replace the existing pro-arrhythmic safety testing and Thorough QT Study with a combination of ion channel screening, mathematical modelling, stem-cell derived myocyte experiments and more lightweight Phase I ECG monitoring’
1. Basic properties and computer modeling: single cell
Different cells are coupled

1. Basic properties and computer modeling: 2D modeling

-
conduction of a wavefront
-
refraction: during the wavefront: no new wave can be excited
-
resting state: new wave can arrive

Single cell
2D tissue
1. Basic properties and computer modeling: 2D modeling

Colliding waves annihilate
1. Basic properties and computer modeling: 2D modeling
Wave can rotate in a ring, rotation is possible if the length of a ring (L) is longer than the product of the refractory period and the velocity of the wave: L > Rv

1. Basic properties and computer modeling: 2D modeling
Wave can rotate around an obstacle/Wave can rotate around itself
Anatomical reentry
Functional reentry
Period of rotation is usually close to refractoriness time
1. Basic properties and computer modeling: 2D modeling
In a rabbit heart (Allessie 1973):
- first to record it
- not big enough preparation, called it a leading circle
- wrong idea still exists up today

1. Basic properties and computer modeling: 2D modeling - experimental evidence
In a sheep and dog epicardial muscle (Davidenko, Jalife 1973):
--> First to record a real spiral wave

1. Basic properties and computer modeling: 2D modeling - experimental evidence
Cell cultures
(lab of L. Glass, neonatal rat cells)
1. Basic properties and computer modeling: 2D modeling - experimental evidence
Whole heart of guinea pig (lab of J. Jalife, 2002)
1. Basic properties and computer modeling: 2D modeling - experimental evidence
Many other systems also have spiral waves!
1. Basic properties and computer modeling: 2D modeling - experimental evidence
1. Basic properties and computer modeling: 2D modeling - experimental evidence
Many other systems also have spiral waves!
1. Basic properties and computer modeling: 2D modeling - experimental evidence
Many other systems also have spiral waves!
Cable equation



1. Basic properties and computer modeling: 2D modeling


Cable equation
1. Basic properties and computer modeling: 2D modeling
A spiral wave becomes a scroll wave: it can be open or closed


1. Basic properties and computer modeling: whole heart modelling

Whole heart simulation: mesh of the heart + fiber orientation
1. Basic properties and computer modeling: whole heart modelling
Spiral waves have many different names
1. Basic properties and computer modeling: whole heart modelling
Anatomical reentry
Functional reentry = rotors
2. Types of arrhythmia and treatment
Four major categories of cardiac arrhythmia
Torsade de Pointes
Regular
Irregular
Atrial Tachycardia
Atrial Fibrillation
Ventricular Fibrillation



Ventricular Tachycardia

Regular
Irregular
Atrial Tachycardia
Atrial Fibrillation
Ventricular Fibrillation



Ventricular Tachycardia


Ventricular Tachycardia


Ventricular Tachycardia

2. Types of arrhythmia and treatmen: atrial tachycardia
- In atria
- Regular
- Mechanism: anatomical reentry
- No immediate risk of dying
Regular
Atrial Tachycardia

Multiple Hypothesis
1. Persistant AF is maintained by rotors
2. Multiple wavelets maintain AF
3. Double layer hypothesis
4. Mother rotor fibrillation
5. Atrial fibrillation driven by micro-anatomic intramural re-entry
2. Types of arrhythmia and treatment: atrial fibrillation
- In atria
- Irregular
- Mechanism: unknown
- No immediate risk of dying. high risk of stroke
Atrial Fibrillation

Atrial Fibrillation

2. Types of arrhythmia and treatment: ventricular tachycardia
- In ventricles
- Regular
- Mechanism: anatomical reentry in 3D
- Risk of dying
Ventricular Tachycardia

Ventricular Tachycardia

2. Types of arrhythmia and treatment: ventricular fibrillation
- In ventricles
- Irregular
- Mechanism: still no consensus
- Dying within minutes
Ventricular Fibrillation

Ventricular Fibrillation

How to stop a reentry circuit?
Ablation: burning of tissue
2. Types of arrhythmia and treatment
3. More on Atrial Tachycardia
3. Atrial Tachycardia: a real case
Looks like typical case of flutter?
The atria form a closed surface
We can simplify the left atrium by deforming it to a sphere with 3 holes. While maintaining its topological properties!
MV
3. Atrial Tachycardia: Anatomy of the left atrium: 3 natural holes
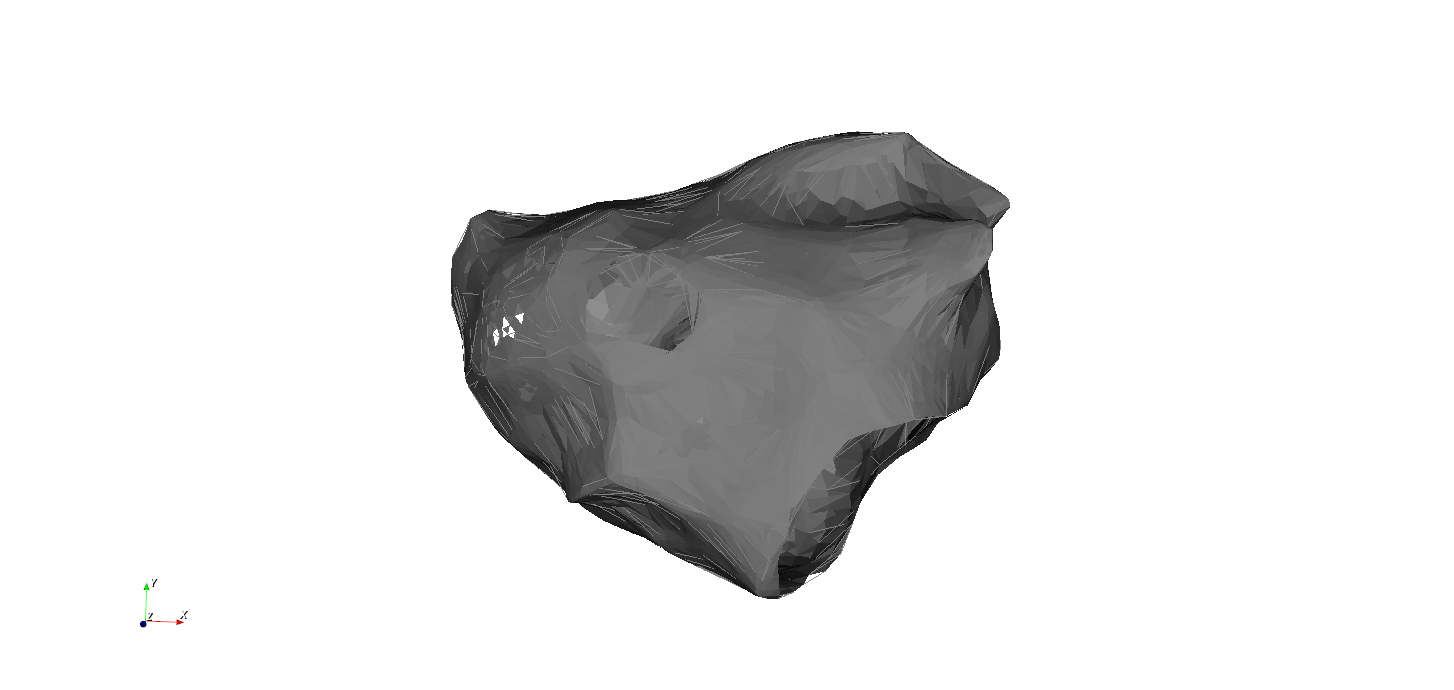
MV
LPV
RPV

SVC
TV

200 different simulations
All possible virtual ablation lines: 600 simulation
3. Atrial Tachycardia: Simulations
3. Atrial Tachycardia - 3 possible patterns
3 Patterns
Complete rotation
Incomplete rotation
Parallel activation
Good entrainment
Bad entrainment
Bad entrainment
3. Atrial Tachycardia - 3 possible patterns

3 Patterns
Complete rotation
Incomplete rotation
Parallel activation
3. Atrial Tachycardia - Ablation

Incomplete rotation becomes complete rotation, resulting in a slower AT
100% of simulations!
Index theorem
On a closed surface, the total number of clockwise and counterclockwise rotations must be equal.
- 1977, Glass. Science (limp generation)
- 1988, Winfree and Tyson. Physics Today (cardiac arrhythmia)
- 2004, Davidsen, Glass, Kapral. Physical Review E (cardiac arrhythmia)
Reentries can't be isolated entities. They must come in pairs!
Key insight 1: Reentries can't be isolated entities. They must come in pairs!
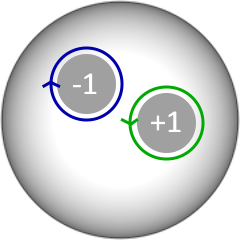
3. Atrial Tachycardia - Index Theorem > 20 years old!


3. Atrial Tachycardia - Index Theorem > 20 years old!


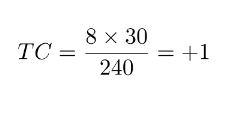
Complete rotation

Parallel activation



Incomplete rotation
Critical Boundary
Critical Boundary
Non-Critical Boundary
CB: Santucci et al. JACC EP 2023

CB:
CB:
NCB: 0
Incomplete rotation becomes complete rotation, resulting in a slower AT
Loops come in pairs of 2: currently second loop is always missed
3. Atrial Tachycardia - Ablation: connect the critical boundaries!



3. Atrial Tachycardia - Ablation: Clinical cases
200 simulation with 2 boundaries


Clinical cases

3. Atrial Tachycardia - Ablation: Clinical cases

CB:
CB:
NCB: 0
Incomplete rotation becomes complete rotation, resulting in a slower AT
3. Atrial Tachycardia - Ablation: Clinical cases

MV
LPV
RPV
3. Atrial Tachycardia: Scar creates additional holes


200 simulations with 4 boundaries
Clinical cases


3. Atrial Tachycardia: Scar creates additional holes
131 MRAT cases



3. Atrial Tachycardia: 131 clinical cases together!
3. Atrial Tachycardia: 20 detailed cases
20 detailed cases with slowing after ablation



Macro reentry
Localized reentry
Micro reentry
Focal
Rotor
Around anatomic obstacle, like valve or vessels.
Around non conducting area > 2-1.5cm, e.g. scar or functional block
-
Atypical Flutter (LA involving valves or vessels)
-
Atypical Flutter (RA involving valves or vessels)
-
Typical Flutter (clockwise and counter clockwise)
-
WPW
-
Atypical Flutter (LA with scar or previous ablation lines with gaps)
-
AVNRT (considering slow and fast pathway)
Around non conducting area < 1cm, e.g. scar or functional block
-
Atypical Flutter (LA with scar)
-
Atypical Flutter (RA crista terminalis region)
-
Ectopic AT
Spiral activation pattern with no scar in the core.
Focal activation from one single spot with centrifugal activation pattern
CL tends to be longer >350ms due to larger path
CL range varies and CL can shift around 20-40 ms during arrhythmia due to slight path variations
CL tends to be shorter due to short path, whole CL is covered by signals found in a small area like e.g. 4cm2 often a combination of double potentials in the „middle“ and very long fractionated signals surrounding
CL often not presented completely in one chamber
Focal activation patterns could also hint to epicardial entries – look for potential conducting structure like vein of Marshall, Bachmann´s etc. and exits that would fit a macro reentry with epicardial parts
We think non-existent in AT, especially as a stable configuration leading to a stable AT.
Maybe something close to a spiral pattern can exist in AF
-
AF
3. Atrial Tachycardia: Current Classification...
3. Atrial Tachycardia: Current Classification...
Macro reentry
Localized reentry
Micro reentry
Focal
Rotor
Around anatomic obstacle, like valve or vessels.
Around non conducting area > 2-1.5cm, e.g. scar or functional block
-
Atypical Flutter (LA involving valves or vessels)
-
Atypical Flutter (RA involving valves or vessels)
-
Typical Flutter (clockwise and counter clockwise)
-
WPW
-
Atypical Flutter (LA with scar or previous ablation lines with gaps)
-
AVNRT (considering slow and fast pathway)
Around non conducting area < 1cm, e.g. scar or functional block
-
Atypical Flutter (LA with scar)
-
Atypical Flutter (RA crista terminalis region)
-
Ectopic AT
Spiral activation pattern with no scar in the core.
Focal activation from one single spot with centrifugal activation pattern
CL tends to be longer >350ms due to larger path
CL range varies and CL can shift around 20-40 ms during arrhythmia due to slight path variations
CL tends to be shorter due to short path, whole CL is covered by signals found in a small area like e.g. 4cm2 often a combination of double potentials in the „middle“ and very long fractionated signals surrounding
CL often not presented completely in one chamber
Focal activation patterns could also hint to epicardial entries – look for potential conducting structure like vein of Marshall, Bachmann´s etc. and exits that would fit a macro reentry with epicardial parts
We think non-existent in AT, especially as a stable configuration leading to a stable AT.
Maybe something close to a spiral pattern can exist in AF
-
AF
All of this is replaced by our simple classification!
Upcoming treatment guidelines (EHRA-ESC, HRS), 2026
Novel therapy
Changed clinical practice, 2024

Dr. Mattias Duytschaever
Dr. Sebastian Knecht
Hospital Sint-Jan Brugge
Retrospectively and prospective published study
> 2024, 6000 downloads last 6 months

3. Real impact


3. Atrial Tachycardia: a real case
3. Atrial Tachycardia: a real case

4. Study of spiral waves
Excitability important for creation of a spiral wave: properties of the single cell!

very low excitability
low excitability
INa
INa

4. Spiral waves: dynamics

low excitability
- circular rotation
- the period is longer to the refractory period
- Substantial excitable gap
INa
4. Spiral waves: dynamics
high excitability
- instantaneous rotation
- the period is close to the refractory period
- There is a small excitable gap

INa
4. Spiral waves: dynamics
very high excitability
INa

If wavefront cannot make a complete turn without interaction with the tail of the wave, there will be interaction and thus meandering.
long refractory tail
Ik

4. Spiral waves: dynamics
Stable spiral
Meandering spiral
4. Spiral waves: dynamics
Hypothesis:
different types of spirals give rise to different arrhythmias

4. Spiral waves: dynamics

4. Spiral waves: restitution curves

4. Spiral waves: restitution curves
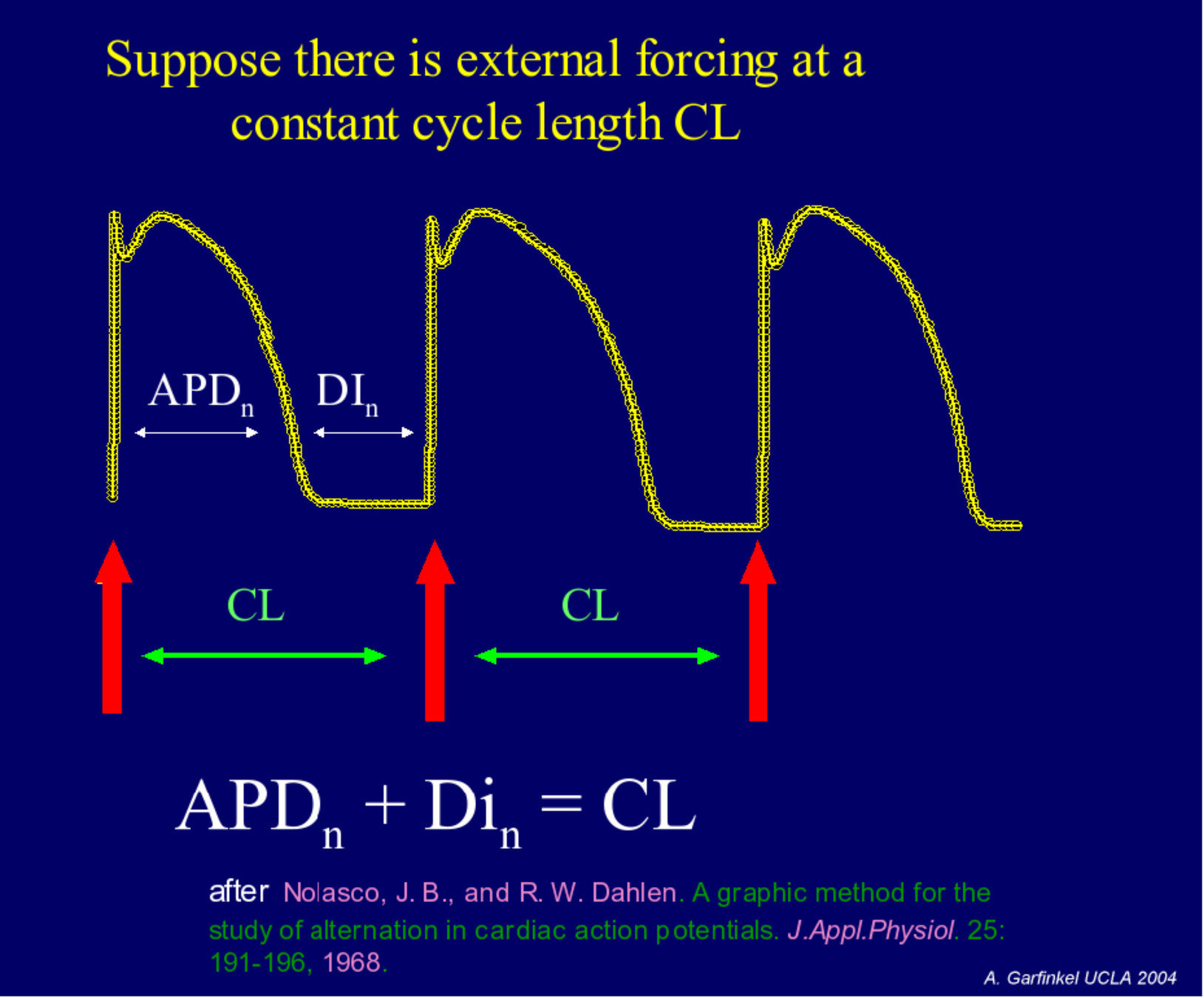
4. Spiral waves: restitution curves

4. Spiral waves: restitution curves
4. Spiral waves: restitution curves and link to breakup of spiral wave
Very dangerous!!!!
Hypothesis:
different types of spirals give rise to different arrhythmias

4. Spiral waves: dynamics

Typical in aging heart: more myofibroblast = fibrotic tissue
4. Spiral waves: influence fibrosis
Spiral waves are attracted by fibrotic tissue
4. Spiral waves: influence fibrosis
Spiral waves are attracted by fibrotic tissue
4. Spiral waves: influence fibrosis
In clinic: spiral waves are often found close to fibrotic tissue
Currently, electrophysiologists are already ablating based on computer modeling

4. Spiral waves: influence fibrosis
5. Clinical research with computer models
Computer modeling is useful to understand arrhythmias, however, only one group in the world (Baltimore) uses modeling to actually treat patients. Still a long way to go...
Computer modeling can however also be used to test new techniques
5. Clinical research with computer models
2. Anatomical reentry
1. Rotors or functional reentry
3. Focal sources
5. Cardiac arrhythmias: 3 main different types of mechanisms
We developed a methodology to determine a sources automatically: we describe the electrical propagation in the heart as a directed network:
DG-mapping

5. Tool to analyze arrhythmia: DGM




Huge field of applications
Brain
Search algorithm
Network theory
5. Tool to analyze arrhythmia: DGM
Network theory on the excitation of the heart: concept

5. Tool to analyze arrhythmia: DGM
Functional reentry

Anatomical reentry
5. Tool to analyze arrhythmia: DGM

Focal sources
5. Tool to analyze arrhythmia: DGM
DG-mapping on clinical AT cases

Optimization protocols
5. Tool to analyze arrhythmia: DGM
5. Tool to analyze arrhythmia: DGM
New version almost ready open source/source available with restrictions for commercial use

You can create your own pipeline!
www.dgmapping.com
5. Tool to analyze arrhythmia: DGM
www.dgmapping.com

Modeling can cover even more scales...
