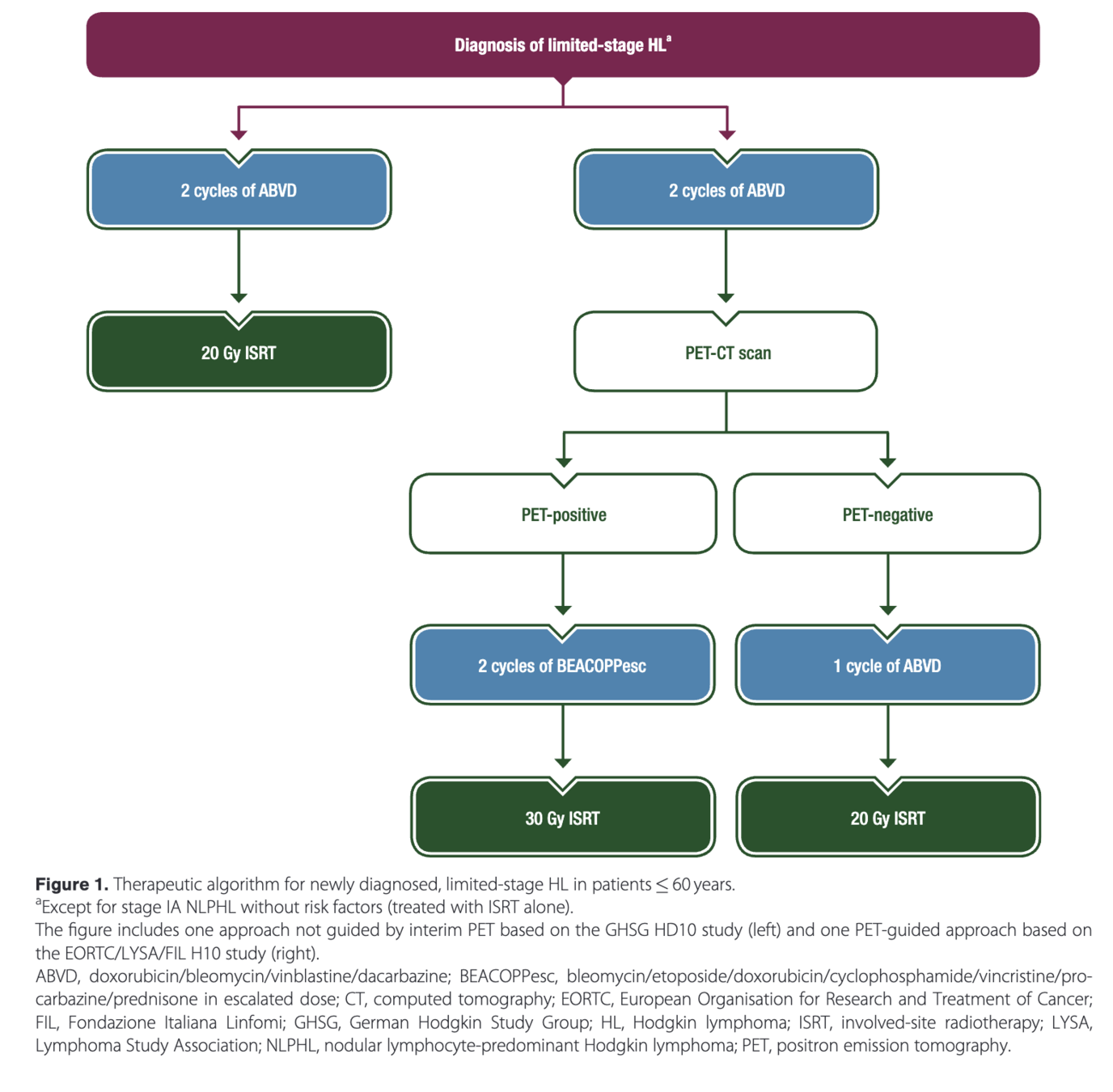
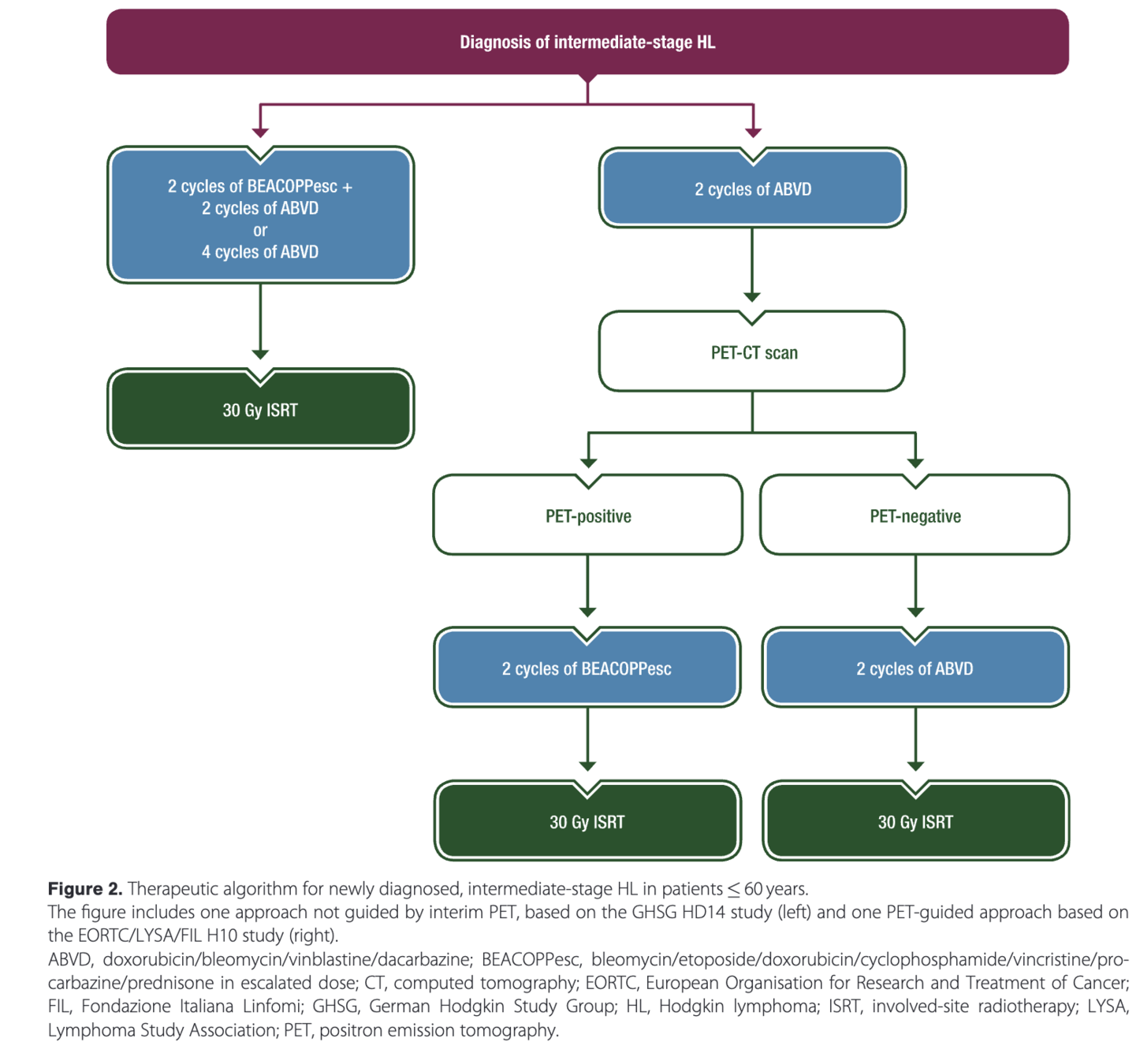
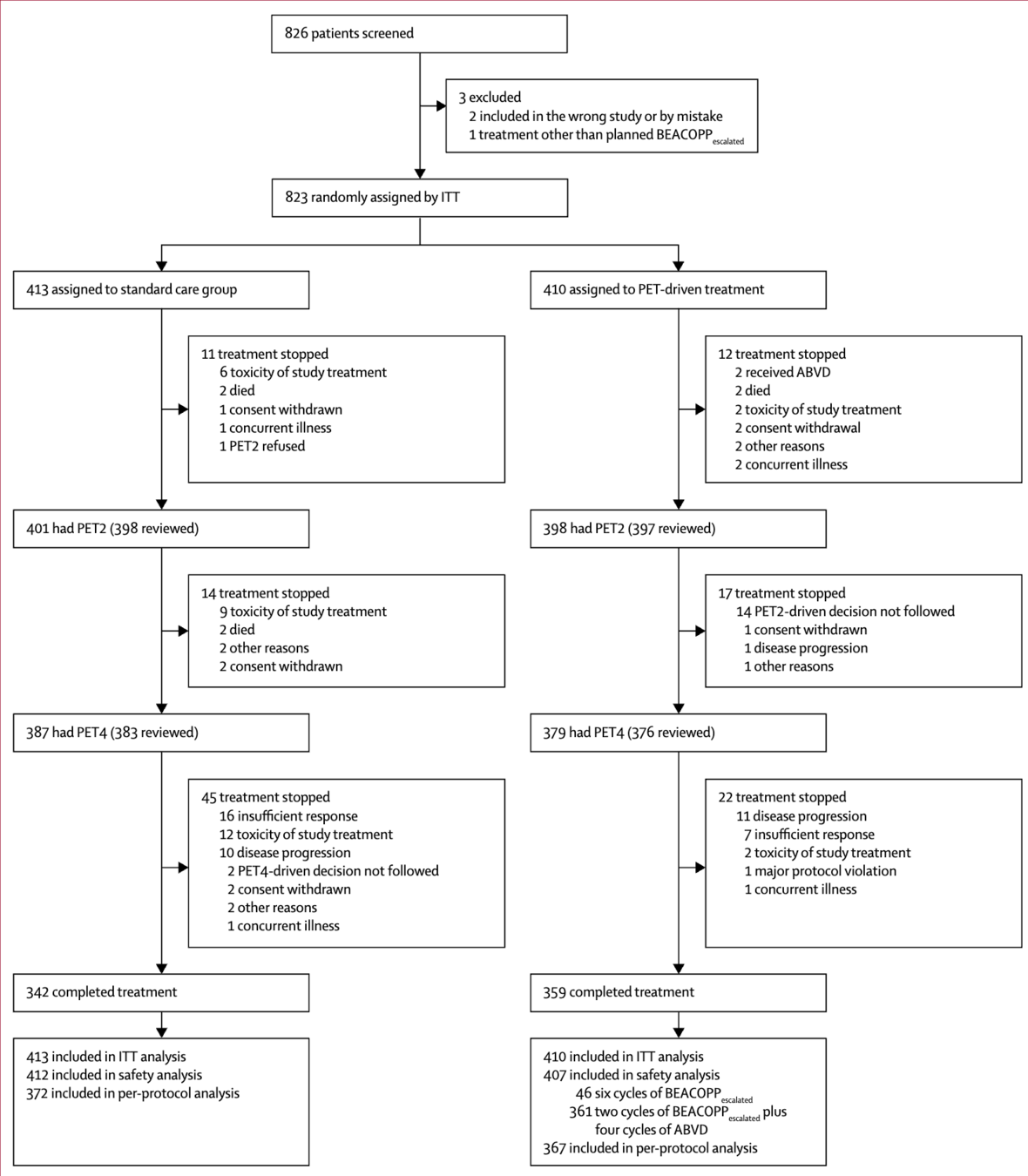
AHL2011: PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma
Casasnovas et al.
Lancet Oncology 2019
Introduction
- Standard treatment for advanced Hodgkin lymphoma is 6-8 cycles of escalated BEACOPP
- Improves progression-free survival compared to ABVD, but has increased toxicity
- PET after 2 cycles (PET2) is highly prognostic
- Hypothesis: PET2 could guide treatment de-escalation in early responders
Methods
- Phase 3 randomized non-inferiority trial
- 823 patients with newly diagnosed advanced Hodgkin lymphoma
- Randomized 1:1 to:
- Standard arm: 6 cycles of escalated BEACOPP
- PET-driven arm: Treatment adapted based on PET2 results
- Primary endpoint: Progression-free survival
Eligibility criteria
- Age 16-60 years
- Newly diagnosed classical Hodgkin lymphoma
- Ann Arbor stage III-IV or stage II with B symptoms or mediastinal bulk
- ECOG performance status 0-2
- No prior treatment for Hodgkin lymphoma
- Adequate organ function
Patient characteristics
- Median age: 30 years
- Ann Arbor stage III-IV: 89%
- B symptoms: 68%
- IPS ≥3: 58%
- Bulky disease (≥10 cm): 34%
Methods
- Phase 3 randomized non-inferiority trial
- 823 patients with newly diagnosed advanced Hodgkin lymphoma
- Randomized 1:1 to:
- Standard arm: 6 cycles of escalated BEACOPP
- PET-driven arm: Treatment adapted based on PET2 results
- Primary endpoint: Progression-free survival
escBEACOPP
BEACOPP (regular): Bleomycin, Etoposide, Adriamycin (doxorubicin), Cyclophosphamide, Oncovin (vincristine), Procarbazine, and Prednisone.
Escalated BEACOPP is an intensified version
-
Higher doses of etoposide, doxorubicin, and cyclophosphamide.
-
Granulocyte colony-stimulating factor (G-CSF) support is mandatory to manage the increased myelosuppression
-
Etoposide dose is increased from 100 mg/m² to 200 mg/m² on days 1-3
-
Doxorubicin is increased from 25 mg/m² to 35 mg/m² on day 1
-
Cyclophosphamide is increased from 650 mg/m² to 1250 mg/m² on day 1
ABVD
ABVD: Adriamycin (doxorubicin), Bleomycin, Vinblastine, and Dacarbazine.
typically:
- Doxorubicin: 25 mg/m² IV on days 1 and 15
- Bleomycin: 10 units/m² IV on days 1 and 15
- Vinblastine: 6 mg/m² IV on days 1 and 15
- Dacarbazine: 375 mg/m² IV on days 1 and 15
Each cycle of ABVD is typically 28 days long.
AHL2011 Trial Schema
Standard Arm
2 cycles BEACOPPesc
Day 1 - Day 42 (21 days per cycle)
↓
PET2 (no impact on treatment)
~Day 42
↓
2 cycles BEACOPPesc
Day 43 - Day 84 (21 days per cycle)
↓
PET4 (no impact on treatment)
~Day 84
↓
2 cycles BEACOPPesc
Day 85 - Day 126 (21 days per cycle)
PET-driven Arm
2 cycles BEACOPPesc
Day 1 - Day 42 (21 days per cycle)
↓
PET2
~Day 42
↓
PET2 Negative
↓
4 cycles ABVD
Day 43 - Day 154 (28 days per cycle)
PET2 Positive
↓
4 cycles BEACOPPesc
Day 43 - Day 126 (21 days per cycle)
↓
PET4 (for response assessment)
ABVD arm: ~Day 154
BEACOPPesc arm: ~Day 126
BEACOPPesc arm: ~Day 126
GITIL/FIL HD 0607 Trial Schema
2 cycles ABVD
Baseline - ~2 months
↓
PET2
~2 months
↓
PET2 Negative
↓
4 cycles ABVD
~2 - 6 months
↓
PET6
~6 months
↓
Randomization (if LNM ≥ 5cm and PET6 negative)
RT to LNM
No Further Treatment
PET2 Positive
↓
Randomization
4 cycles BEACOPPesc + 4 cycles BEACOPPbase
4 cycles BEACOPPesc + 4 cycles BEACOPPbase + Rituximab
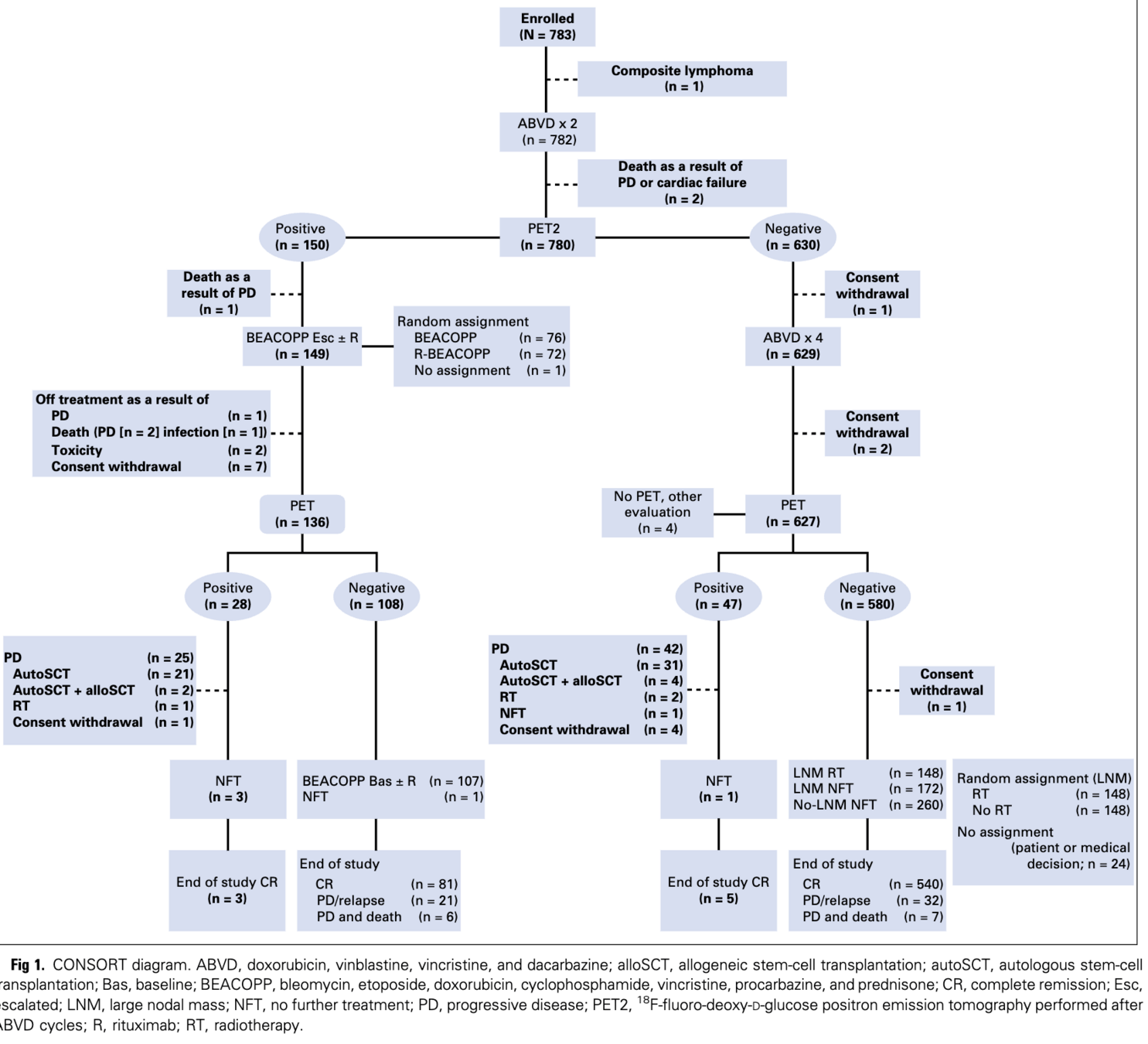
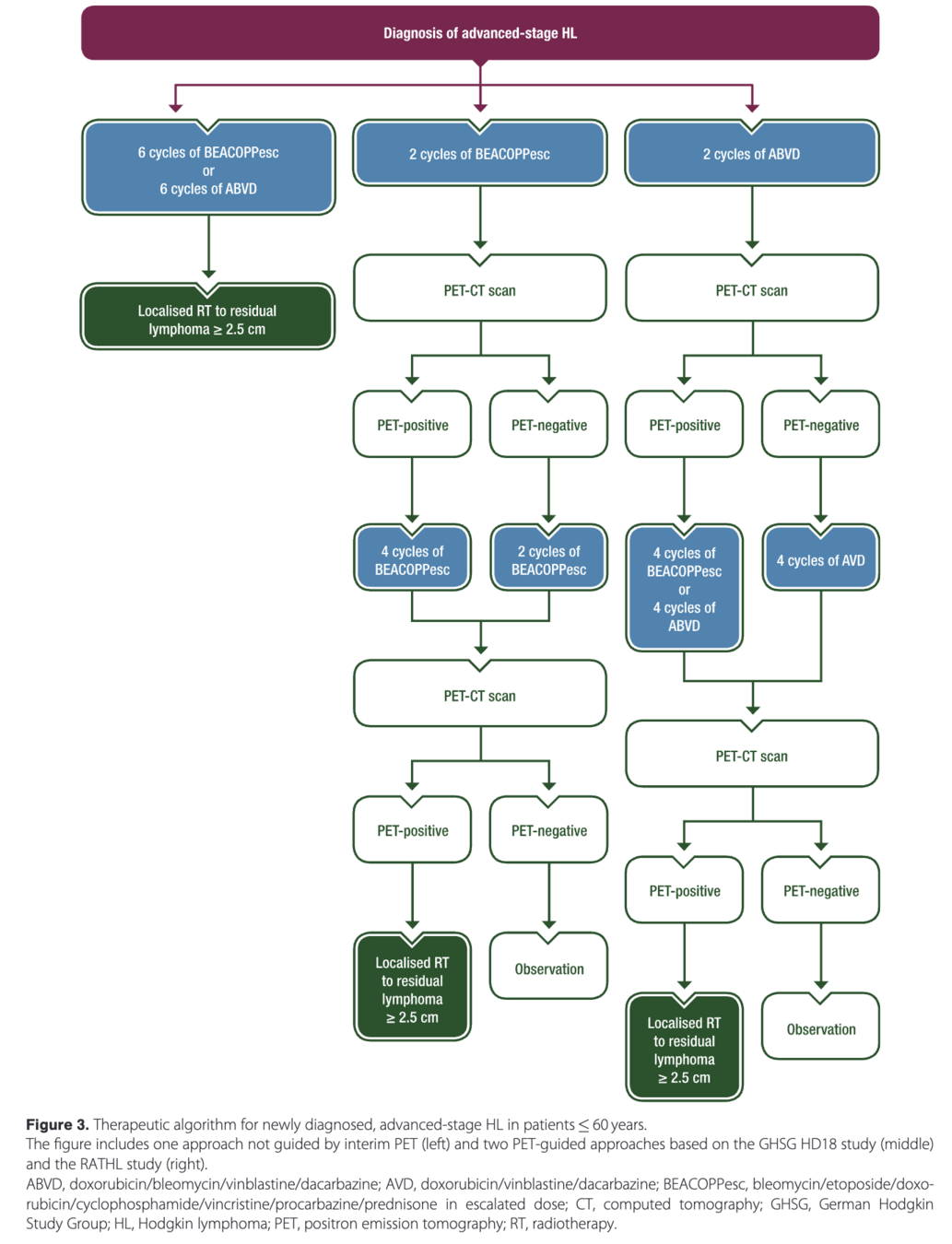
Trial schema
Radiation details
- No planned radiotherapy in either arm
- Radiotherapy was allowed at investigator discretion for:
- Residual PET 4 - positive disease after completion of chemotherapy
- bulky sites
-
32 patients received consolidation radiotherapy:
- 18 patients (4%) in the standard treatment group
- 14 patients (3%) in the PET-driven treatment group
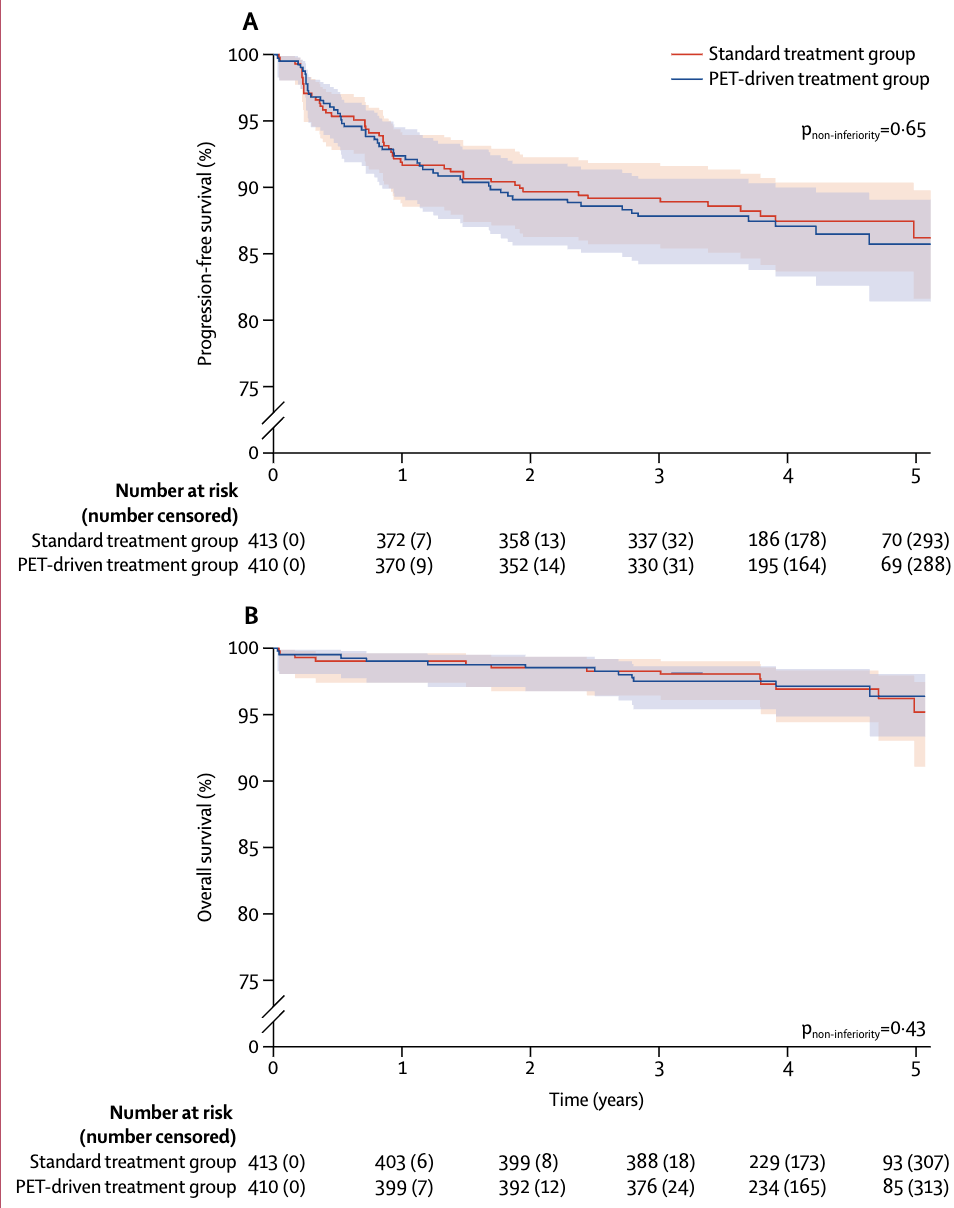
Primary outcome
- 5-year progression-free survival:
- Standard arm: 86.2% (95% CI 81.6-89.8)
- PET-driven arm: 85.7% (95% CI 81.4-89.1)
- HR 1.084 (95% CI 0.737-1.596), p=0.65
- Met pre-specified non-inferiority margin of 10%
Secondary outcomes
- 5-year overall survival:
- Standard arm: 95.2%
- PET-driven arm: 96.4% (HR 0.936, p=0.43)
- Complete response rate: Similar between arms
- Toxicity:
- Grade 3-4 adverse events lower in PET-driven arm
- Fewer secondary malignancies in PET-driven arm
- More pregnancies reported in PET-driven arm
Results by arm
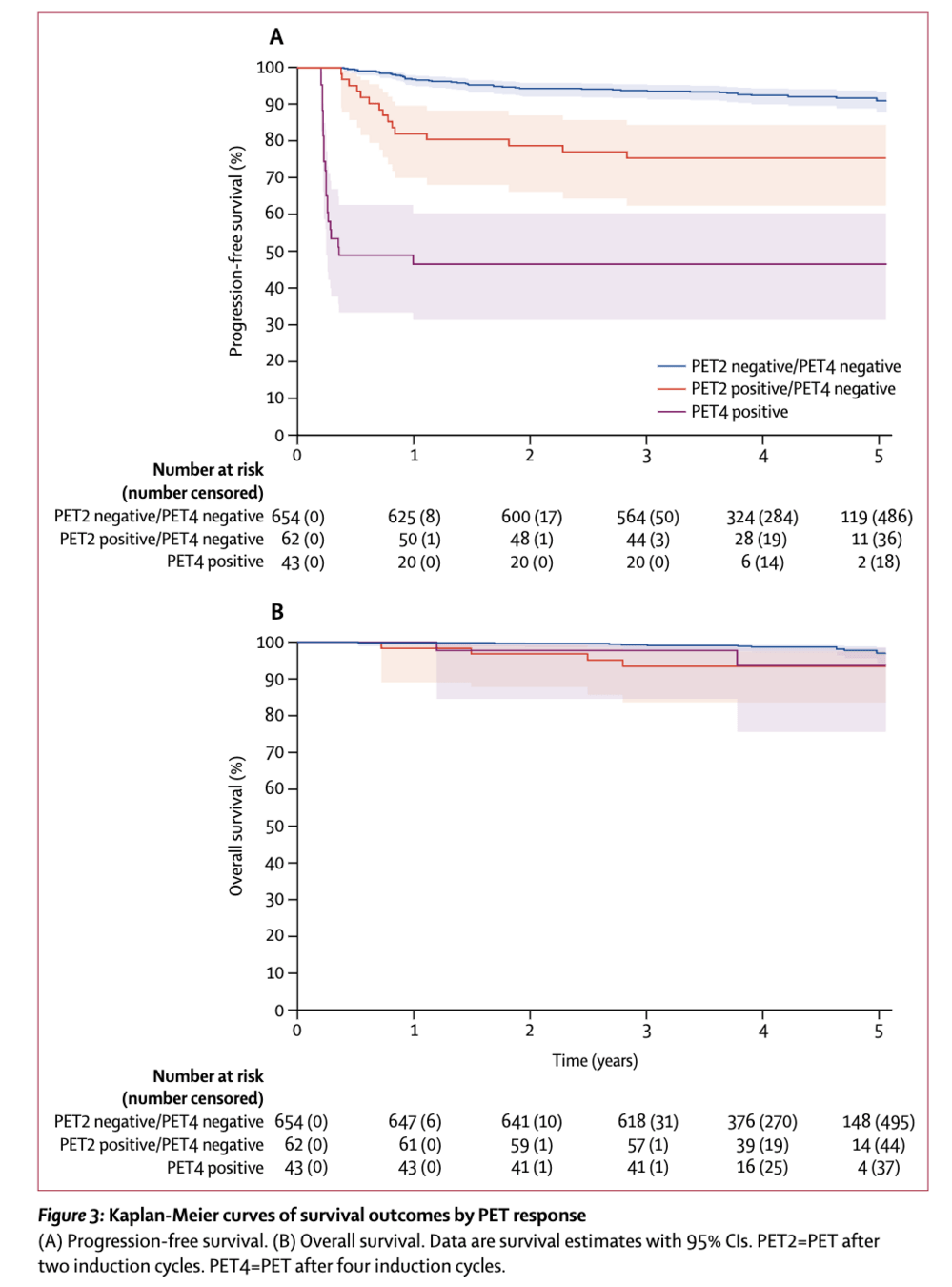
Results by PET response
Results by PET response
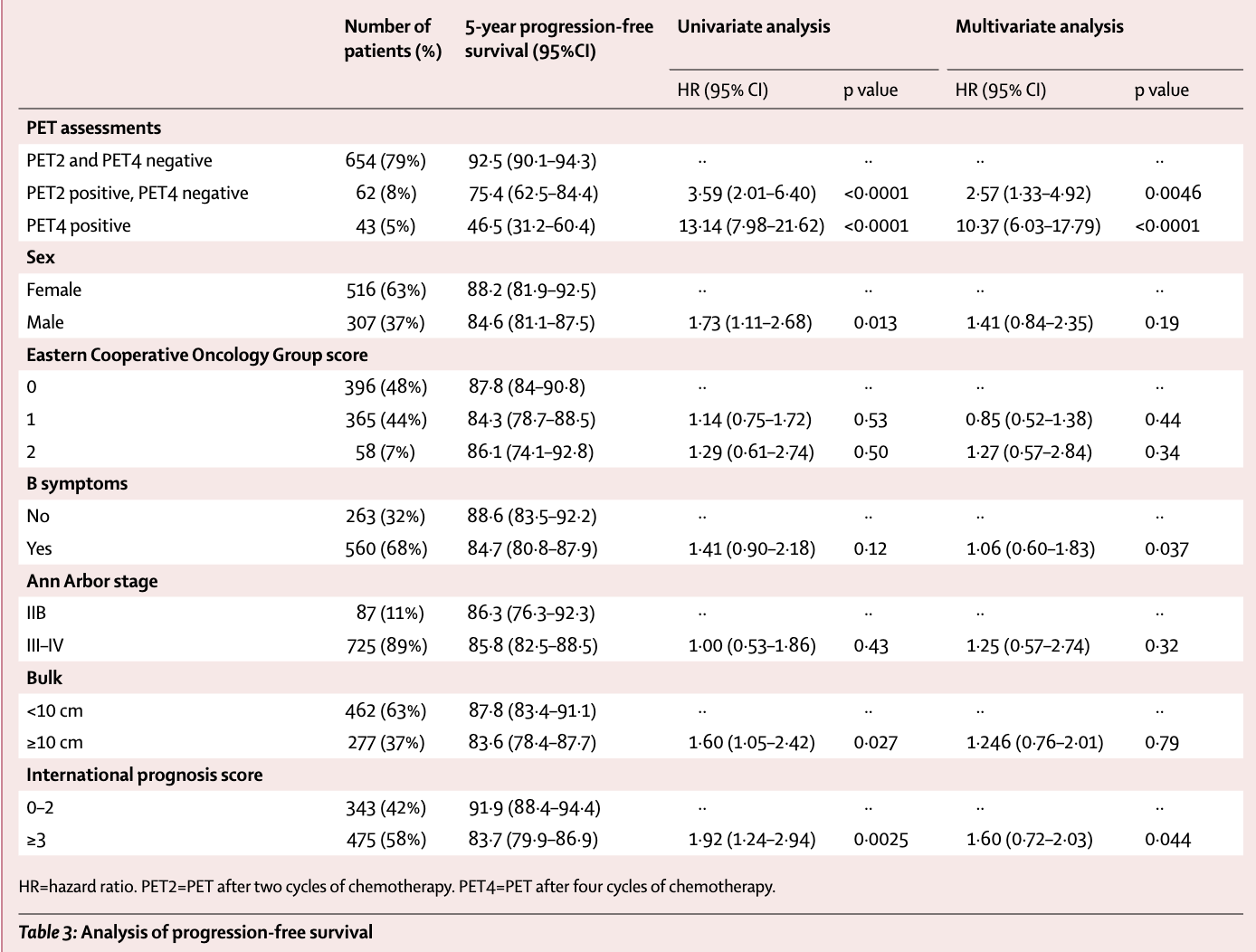
Results by PET response

Conclusions
- PET-adapted treatment strategy was non-inferior to standard escalated BEACOPP
- 84% of patients were able to de-escalate to ABVD after 2 cycles of escalated BEACOPP
- PET-adapted approach reduced toxicity without compromising efficacy
- Combining PET2 and PET4 results provided strong prognostic information
- PET-adapted strategy could be considered for routine management of advanced Hodgkin lymphoma
Strengths and limitations
- Strengths:
- Large, randomized phase 3 trial
- Long-term follow-up (median 50.4 months)
- Centralized PET review
- Limitations:
- Wide non-inferiority margin (10%)
- No details on radiotherapy use
- Limited data on long-term toxicity