Toxicity Profiles and Survival Outcomes: IMPT vs IMRT for Nonmetastatic Nasopharyngeal Carcinoma
Li et al.
JAMA Network Open 2021
Journal Club Presentation
Introduction
- Nasopharyngeal carcinoma (NPC) is endemic to East and Southeast Asia
- Primary treatment: radiotherapy ± chemotherapy with curative intent
- IMRT has improved outcomes but 50-75% still experience grade 3-4 acute toxicities
- 10-20% of survivors experience serious late complications
- Proton therapy theoretical advantage: minimal exit dose beyond target
- Limited data on IMPT for NPC due to sporadic incidence in West and lack of proton centers in endemic regions
Study Design
- Retrospective cohort study at Memorial Sloan Kettering Cancer Center
- January 2016 - December 2019
- 77 patients with newly diagnosed nonmetastatic NPC
- Compared IMPT (n=28) vs IMRT (n=49)
- Propensity score matching for 48 EBV-positive patients (1:1)
- Primary outcomes: acute/chronic adverse events and oncologic outcomes (LRFS, PFS, OS)
- Median follow-up: 30.3 months overall
Eligibility Criteria
- Adults ≥18 years old
- Newly diagnosed nonmetastatic NPC
- Treated with curative intent RT ± chemotherapy
- Excluded: palliative RT or no follow-up after RT completion
- IMPT offered as alternative to IMRT off trial or when IMRT could not be safely delivered
- Barriers to IMPT: patient preference, insurance denial, logistics (proton center ~50 miles away)
Patient Characteristics
- Median age: 48.7 years (similar between groups)
- Male predominance: 67.5%
- 89.6% had EBV-positive disease
- 89.6% had WHO type 2b (nonkeratinizing undifferentiated carcinoma)
- Most had excellent performance status (KPS 90-100)
- IMPT group had more T4 disease (28.6% vs 12.2%, p=0.14)
- IMPT group received more high-dose cisplatin (57.1% vs 24.5%, p=0.004)
Radiation Protocol
- Dose to gross tumor volume: 69.96-70 GyE in 33-35 fractions
- High-risk anatomic sites: 56-63 GyE
- Low-risk anatomic sites: 54.12-56 GyE
- Concurrent chemotherapy:
- Weekly cisplatin 40 mg/m² (up to 7 cycles) OR
- Q3 week cisplatin 100 mg/m² (up to 3 cycles)
- Stage I: RT alone; Stage II-IVA: concurrent chemoRT ± adjuvant chemotherapy
Dosimetric Comparison
- IMPT achieved significantly lower doses to organs at risk:
- Mean oral cavity dose: 15.4 vs 32.8 GyE (p<0.001)
- Mean larynx dose: 16.0 vs 29.6 GyE (p<0.001)
- Mean parotid gland dose: 22.5 vs 25.2 GyE (p=0.01)
- These dosimetric advantages translated to clinical benefits in acute toxicity
Acute Toxicity Results
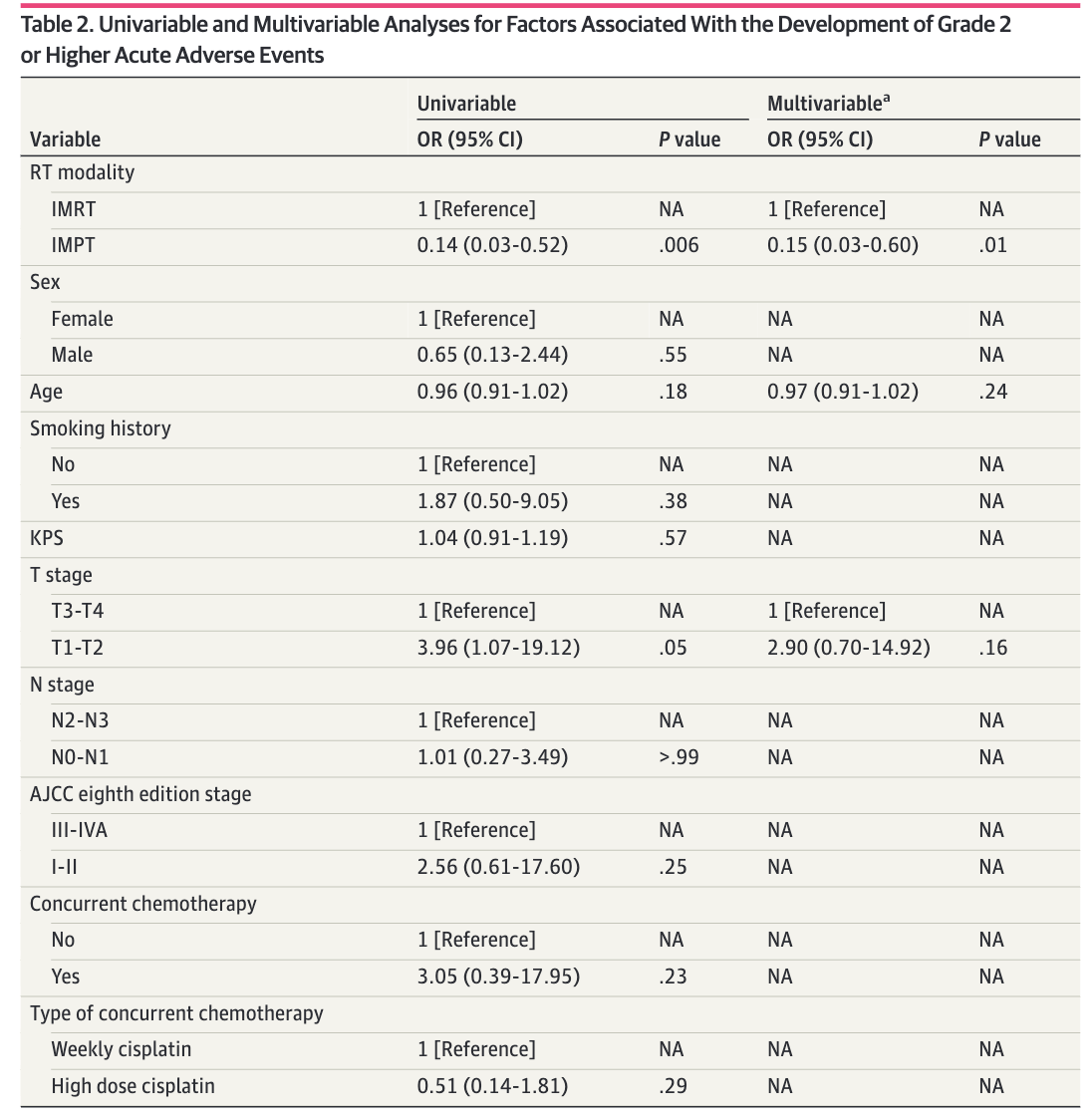
Late Toxicity Results
Figure: Kaplan-Meier Survival Curves
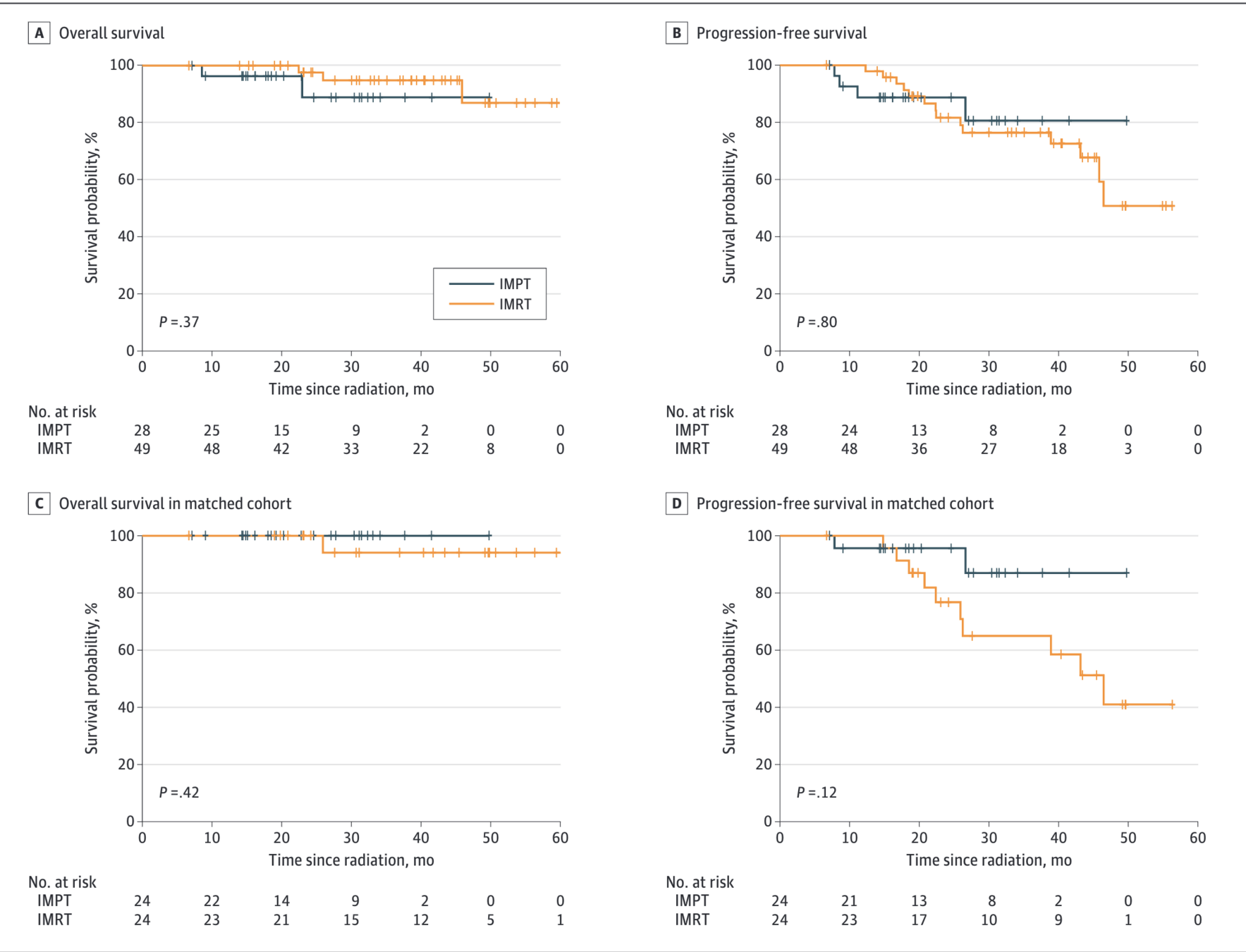
Figure: Locoregional Control
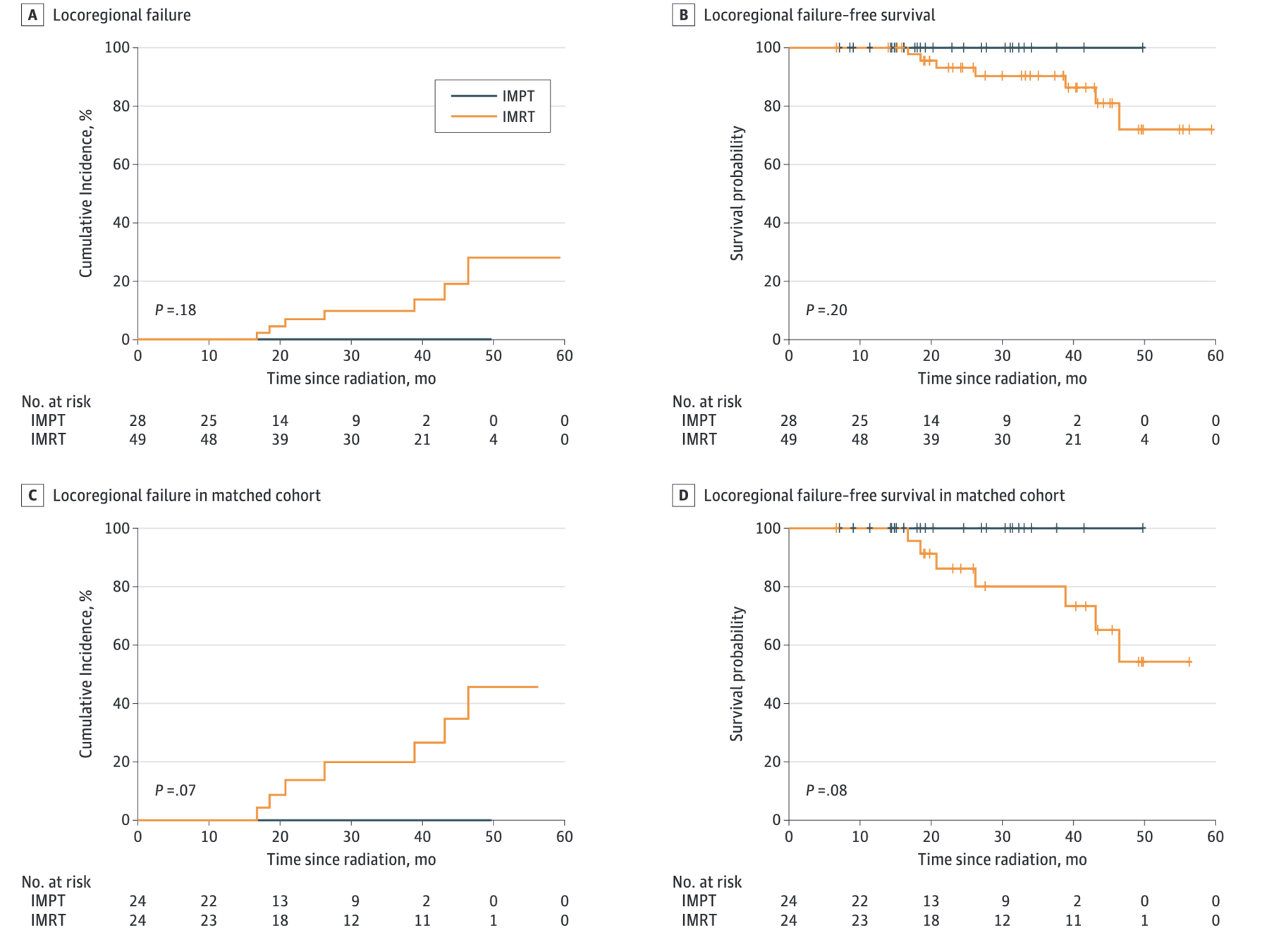
Oncologic Outcomes - Full Cohort
- Locoregional failure: 0 (IMPT) vs 7 (IMRT)
- Cumulative incidence of LRF at 30 months: 0% vs 9.6% (p=0.18)
- No significant differences in survival outcomes:
- LRFS: HR 0.00 (p<0.001) - no events in IMPT
- PFS: HR 0.86 (95% CI 0.28-2.68, p=0.80)
- OS: HR 2.29 (95% CI 0.36-14.67, p=0.37)
- Smoking history associated with poor LRFS and PFS
- EBV-positive status associated with better OS

Propensity Score-Matched Analysis
- 48 patients (24 IMPT vs 24 IMRT) with EBV-positive disease
- Matched on: T4 disease, nonsmoking status, high-dose cisplatin
- 2-year LRFS: 100% (IMPT) vs 86.2% (IMRT), p=0.08
- 2-year PFS: 95.7% (IMPT) vs 76.7% (IMRT), HR 0.31, p=0.14
- 3-year OS: 100% (IMPT) vs 94.1% (IMRT), p=0.42
- No locoregional recurrence or death in IMPT group
- Smoking remained significant predictor of poor outcomes
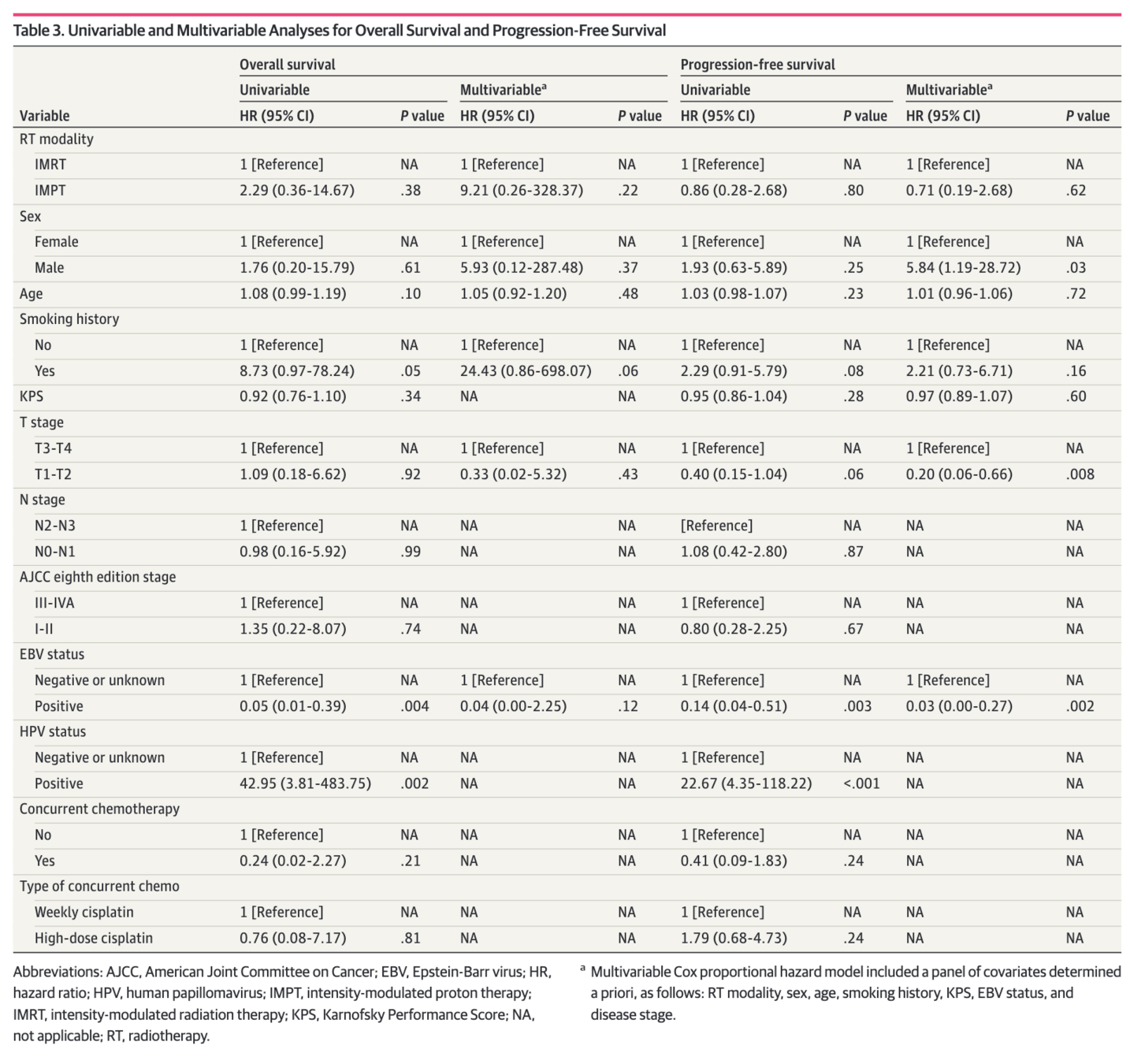
Conclusions
- IMPT was associated with significantly reduced acute toxicity burden vs IMRT
- Rare late complications with IMPT (median follow-up ~2 years)
- Excellent oncologic outcomes with 100% locoregional control at 2 years in IMPT group
- Results suggest IMPT should be discussed as potential primary RT modality when available
- Prospective trials warranted to optimize patient selection
- Particularly relevant as proton centers expand in endemic regions
Strengths
- Largest comparative analysis of IMPT vs IMRT for primary NPC treatment
- Contemporary cohort with modern techniques
- Comprehensive toxicity assessment with CTCAE grading
- Propensity score matching to address selection bias
- Detailed dosimetric comparisons
- Consistent treatment protocols at single institution
Limitations
- Retrospective design with inherent selection bias
- Small sample size, especially in IMPT group (n=28)
- Imbalanced follow-up time: 23.0 months (IMPT) vs 37.0 months (IMRT)
- Socioeconomic factors not captured (insurance status)
- Limited patient-reported outcome data
- Single institution experience
- May miss late recurrences and toxicities occurring after 2 years
Discussion Points
- How should we select patients for IMPT vs IMRT for NPC? What factors should guide this decision?
- Is the follow-up adequate to capture meaningful late toxicity differences? What late effects are we most concerned about?
- How do we balance the dosimetric advantages with practical barriers (access, cost, insurance)?
- Should IMPT be standard of care for NPC when available, or do we need randomized data first?
- What is the potential impact as proton centers expand in Asia where NPC is endemic?