Postoperative Chemoradiation for High-Risk Head and Neck Cancer
Bernier VS Cooper
Comparing EORTC #22931 and RTOG #9501
Background
- Locally advanced HNSCC: poor outcomes with surgery and postoperative RT alone
- Hypothesis: Adding concurrent chemotherapy may improve outcomes
- Two large randomized trials: EORTC #22931 and RTOG #9501
- Both published in the same issue of NEJM
Europe Vs the USA
Study Designs
| EORTC #22931 | RTOG #9501 | |
|---|---|---|
| Patients | 334 | 459 |
| Primary endpoint | Progression-free survival | Locoregional control |
| Radiotherapy | 66 Gy in 33 fractions | 60-66 Gy in 30-33 fractions |
| Chemotherapy | Cisplatin 100 mg/m2 on days 1, 22, and 43 | Cisplatin 100 mg/m2 on days 1, 22, and 43 |
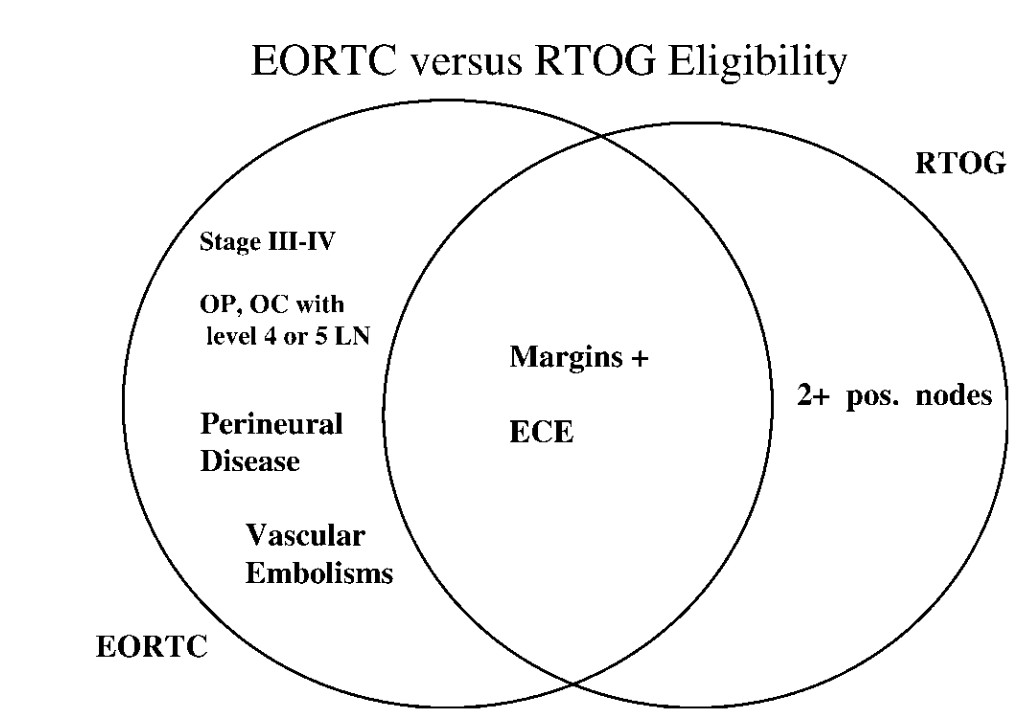
Patient Eligibility
| EORTC #22931 | RTOG #9501 |
|---|---|
|
|
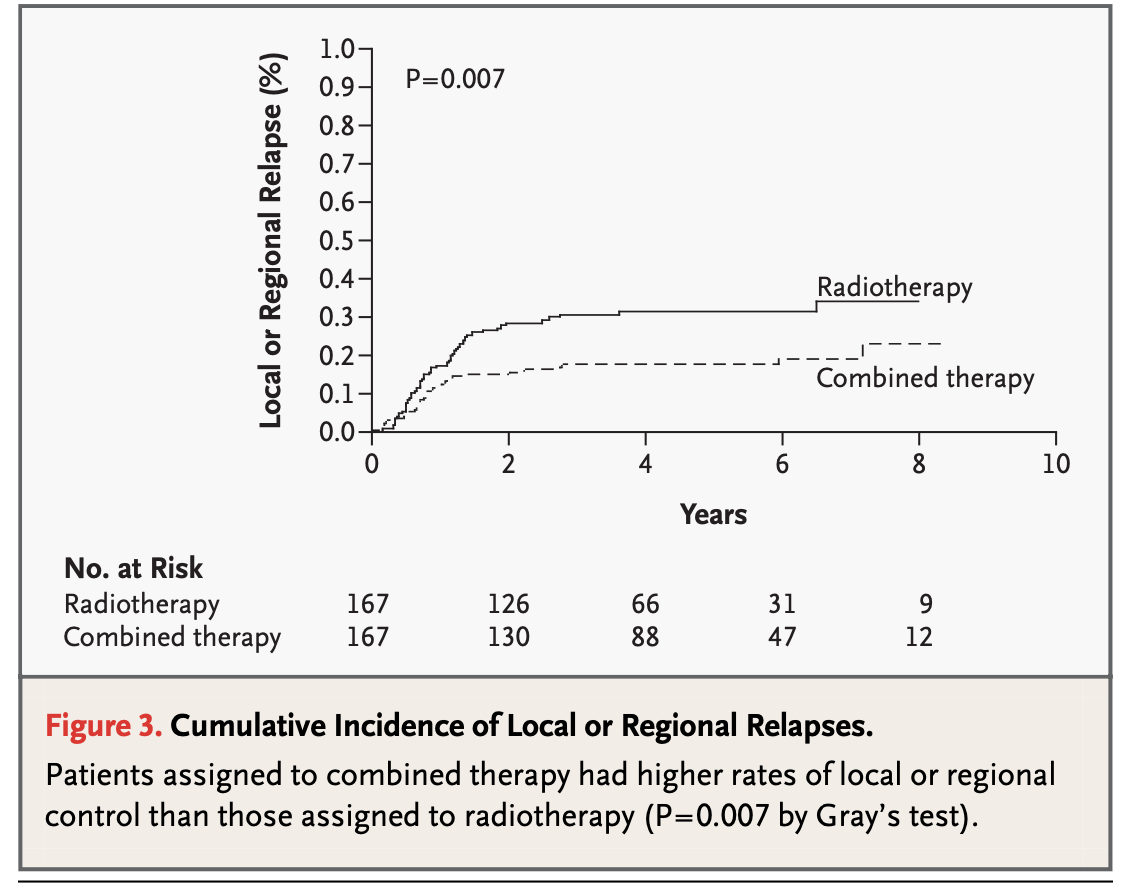
Results - Locoregional Control
-
EORTC #22931:
5-year estimate: 82% (CRT) vs 69% (RT), p=0.007
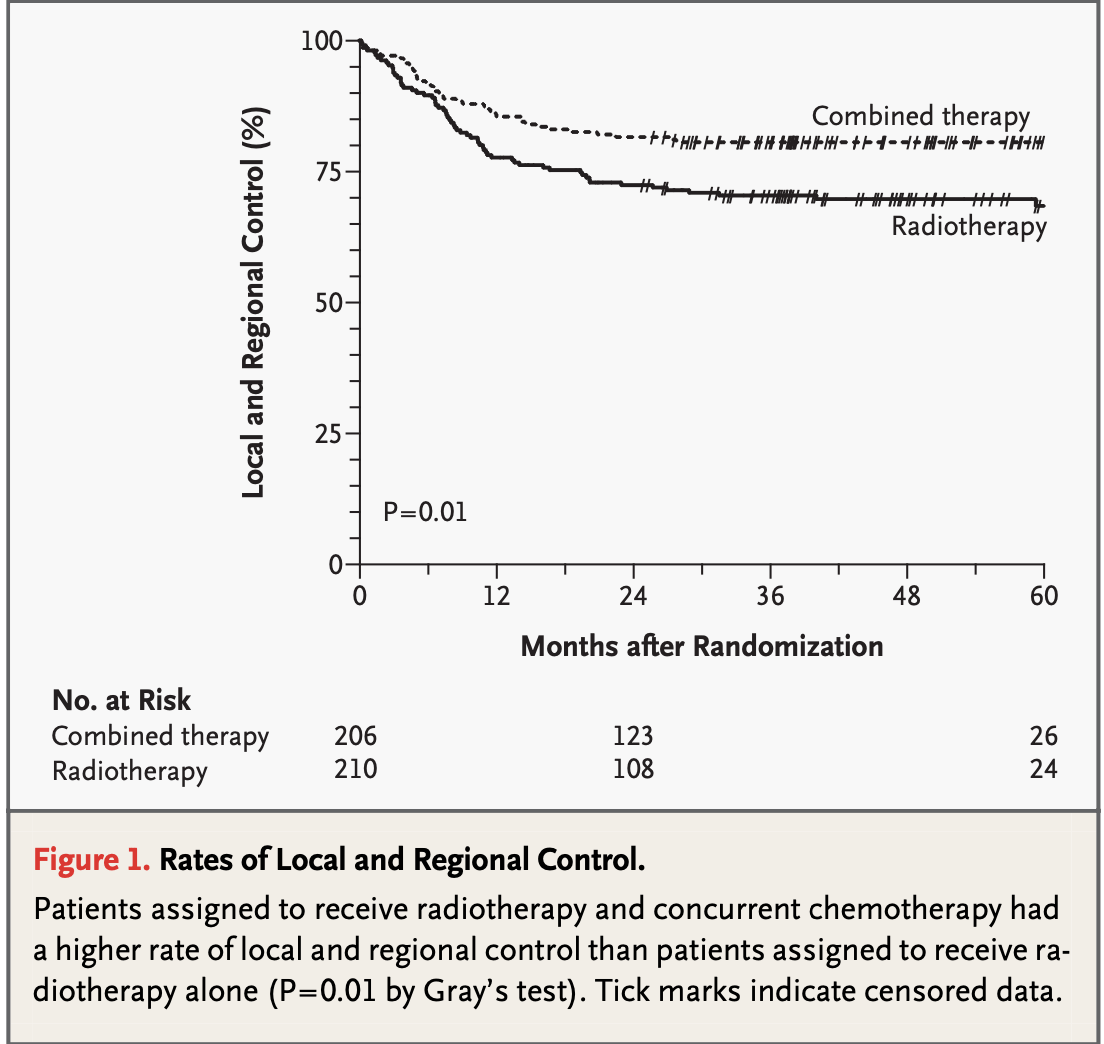
-
RTOG #9501:
2-year estimate: 82% (CRT) vs 72% (RT), p=0.01
5-year estimate: 84% (CRT) vs 74% (RT)
10-year estimate: 88% (CRT) vs 71% (RT)
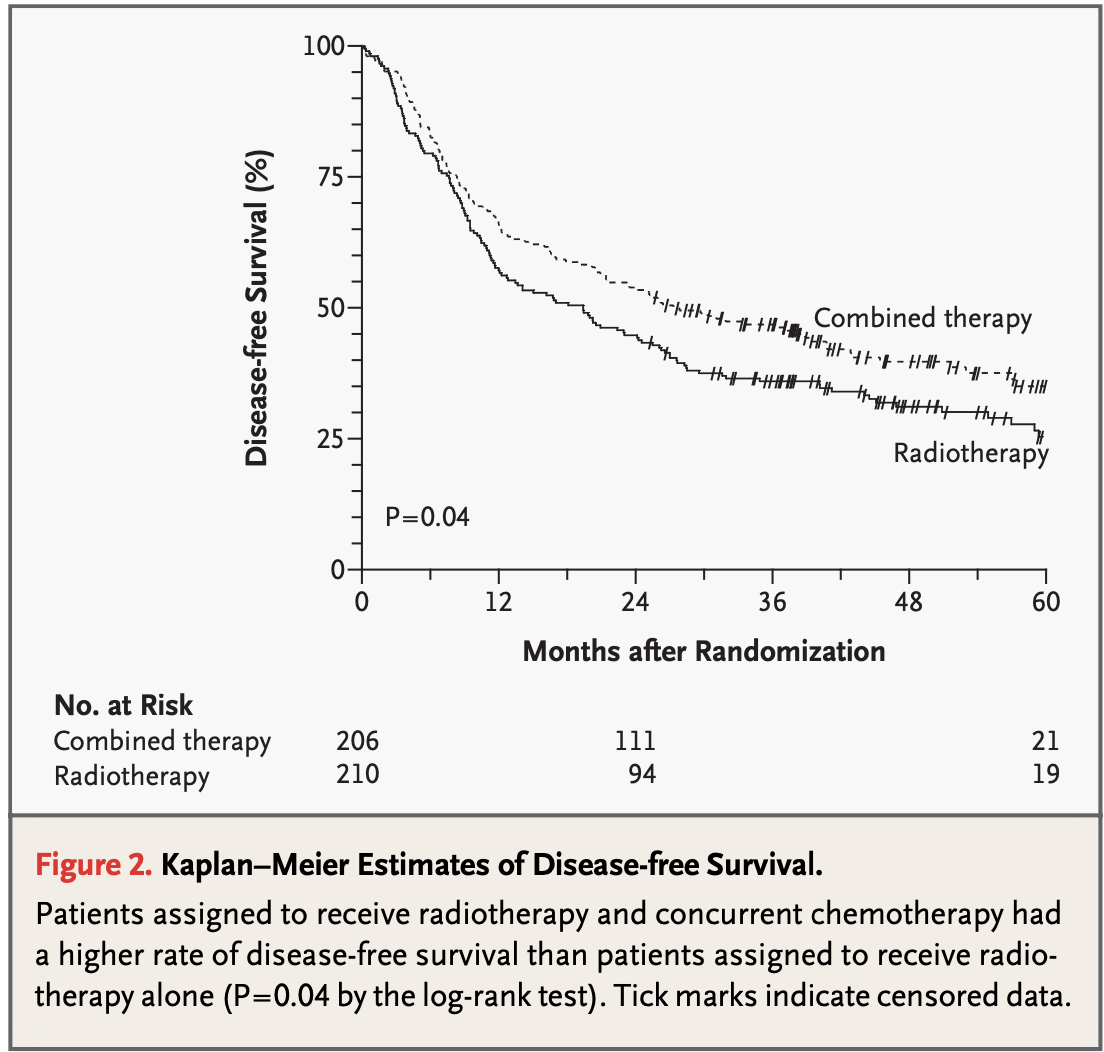
Results - PFS vs DFS
- EORTC #22931: 5-year:
- 47% (CRT)
- 36% (RT) p=0.04
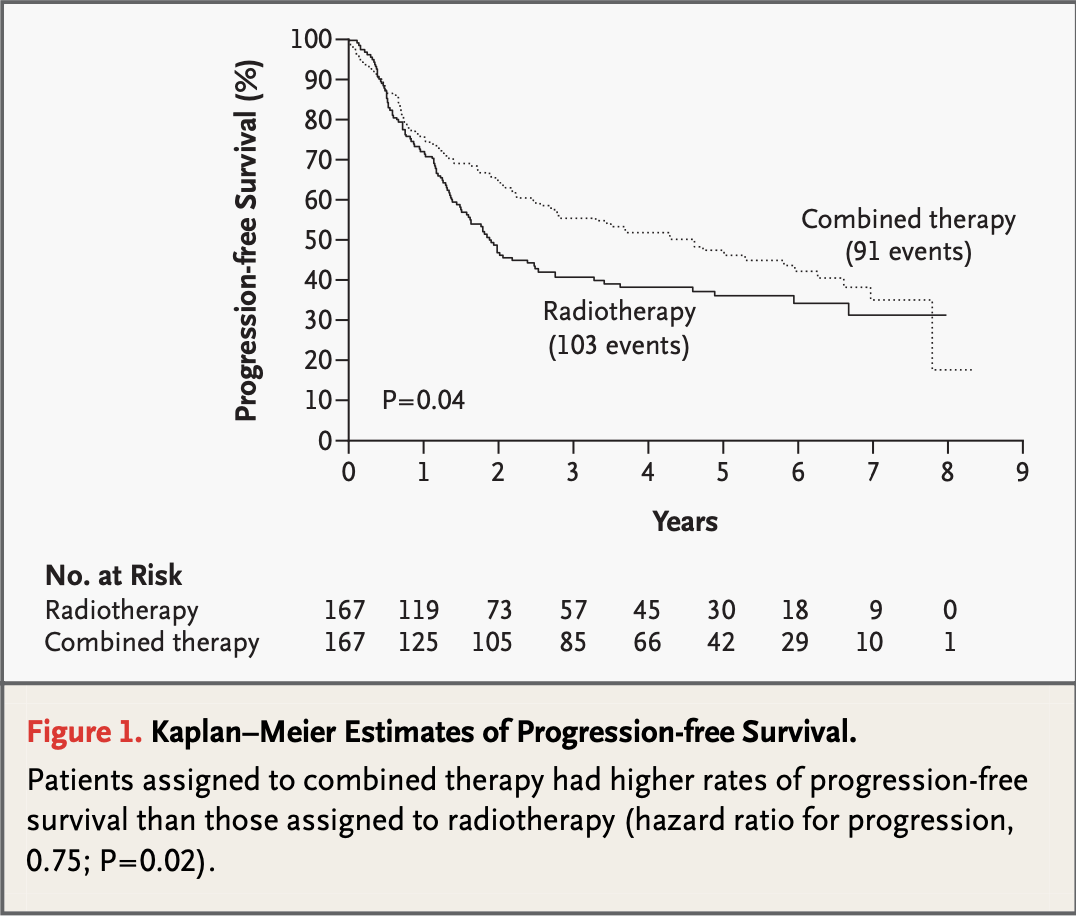
- RTOG #9501: 3-year:
- 47% (CRT)
- 38% (RT), p=0.04
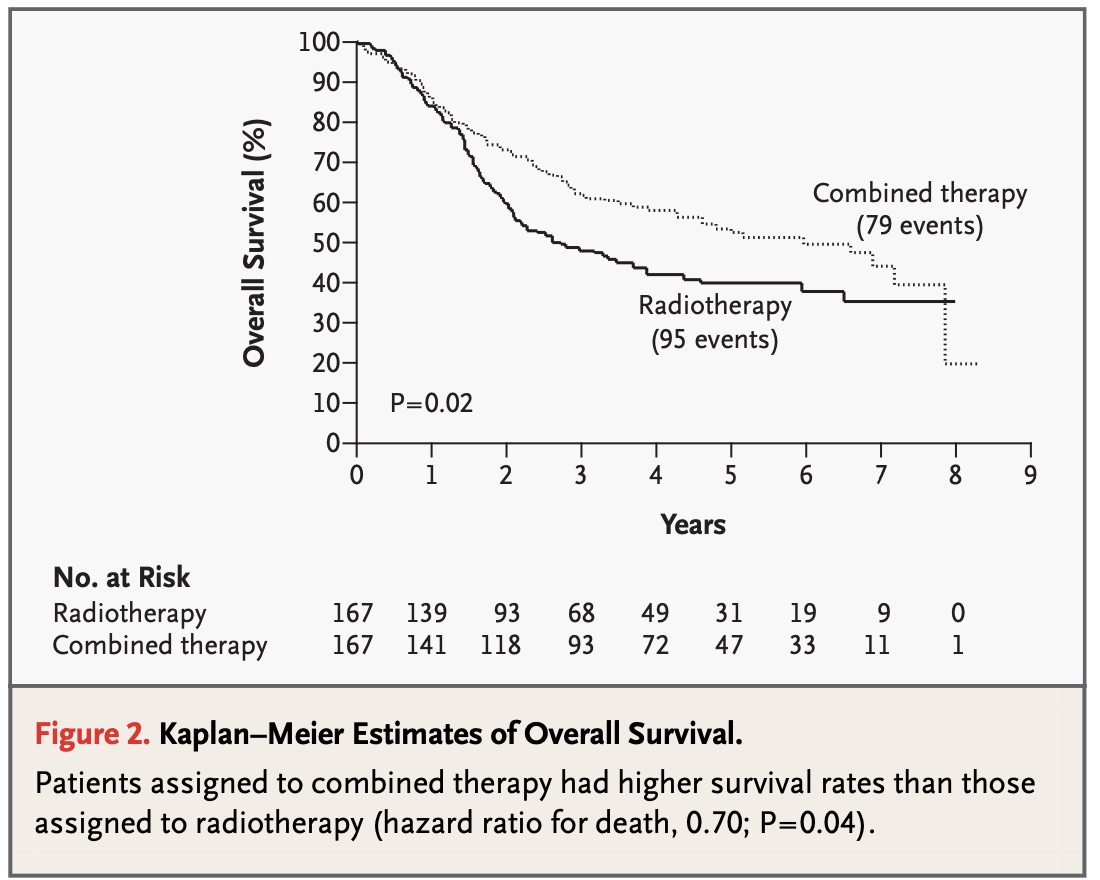
Results - Overall Survival
EORTC #22931
- 3-year estimate: 65% (CRT) vs 49% (RT)
5-year estimate: 53% (CRT) vs 40% (RT), p=0.02
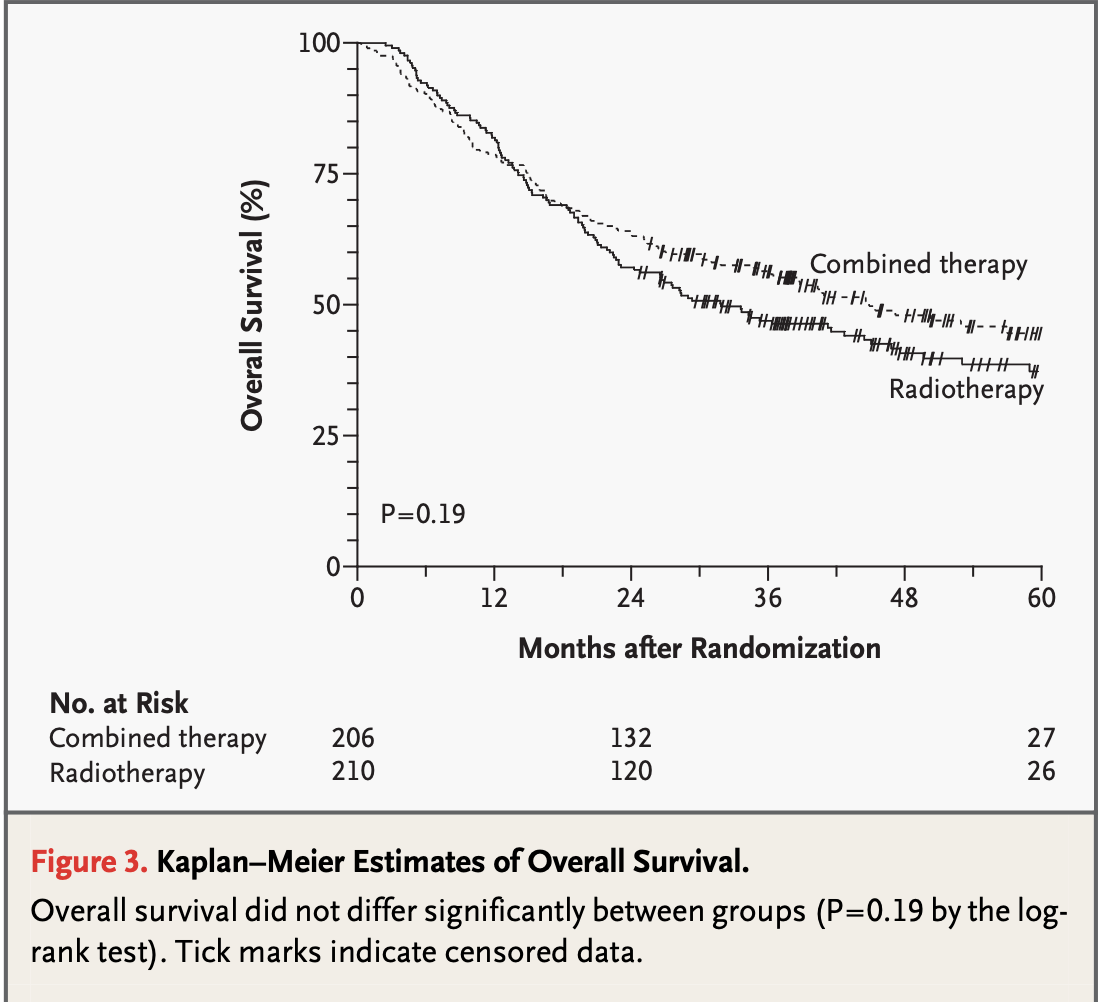
RTOG #9501:
- 2-year estimate: 63% (CRT) vs 57% (RT), p=0.19
5-year estimate: 29% (CRT) vs 27% (RT), p=0.31 - Subset with positive margins or ECE (RTOG #9501):
10-year estimate: 27% (CRT) vs 20% (RT), p=0.07
Results - Overall Survival
EORTC #22931
- 3-year estimate: 65% (CRT) vs 49% (RT)
5-year estimate: 53% (CRT) vs 40% (RT), p=0.02
RTOG #9501:
- 2-year estimate: 63% (CRT) vs 57% (RT), p=0.19
5-year estimate: 29% (CRT) vs 27% (RT), p=0.31 - Subset with positive margins or ECE (RTOG #9501):
10-year estimate: 27% (CRT) vs 20% (RT), p=0.07
Toxicity
| Toxicity | EORTC #22931 | RTOG #9501 |
|---|---|---|
| Acute Grade 3-4 | 41% (CRT) vs 21% (RT) | 77% (CRT) vs 34% (RT) |
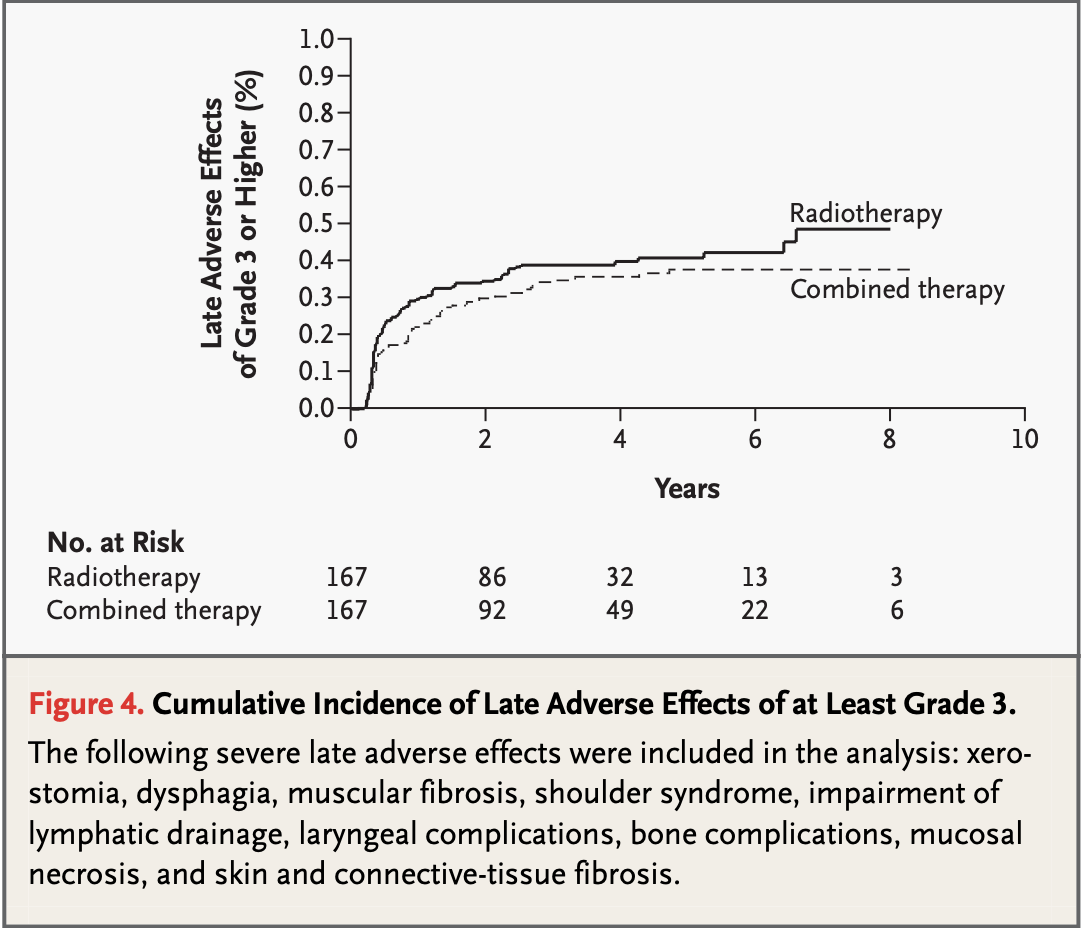
| Late Grade 3-4 | 21% (CRT) vs 17% (RT) | No significant difference |
- Increased acute toxicity with chemoradiation
- Similar late toxicity profiles
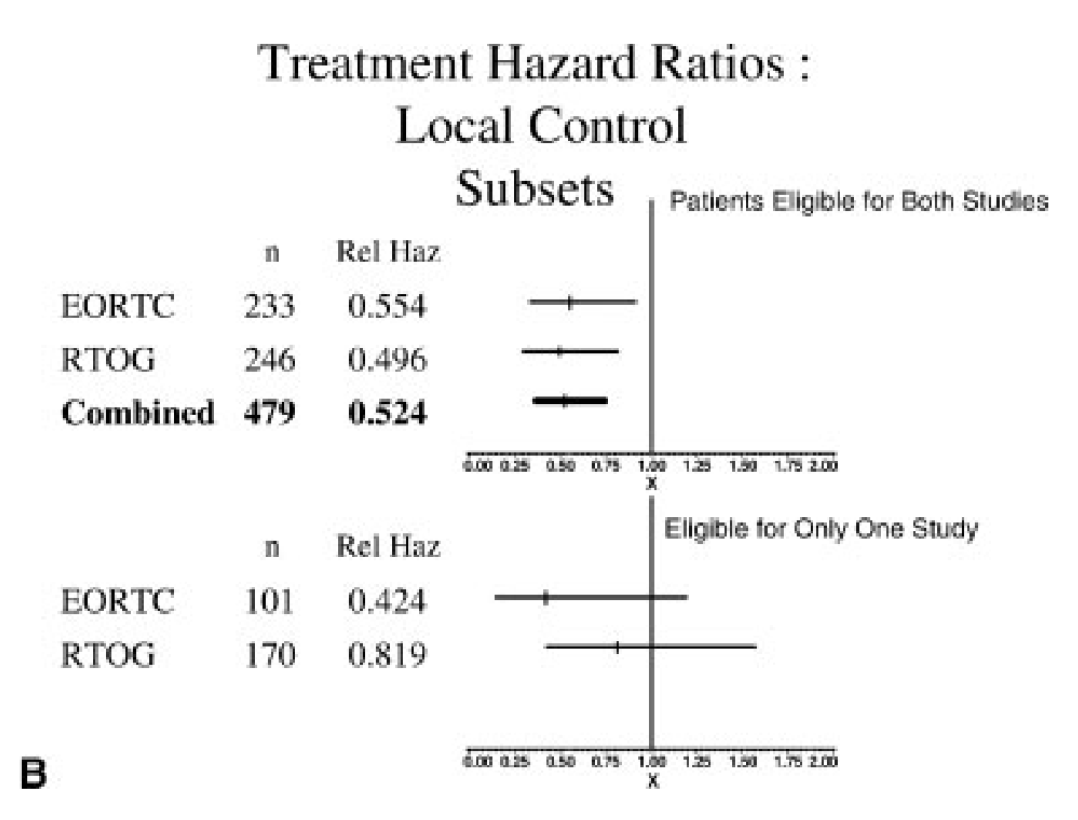
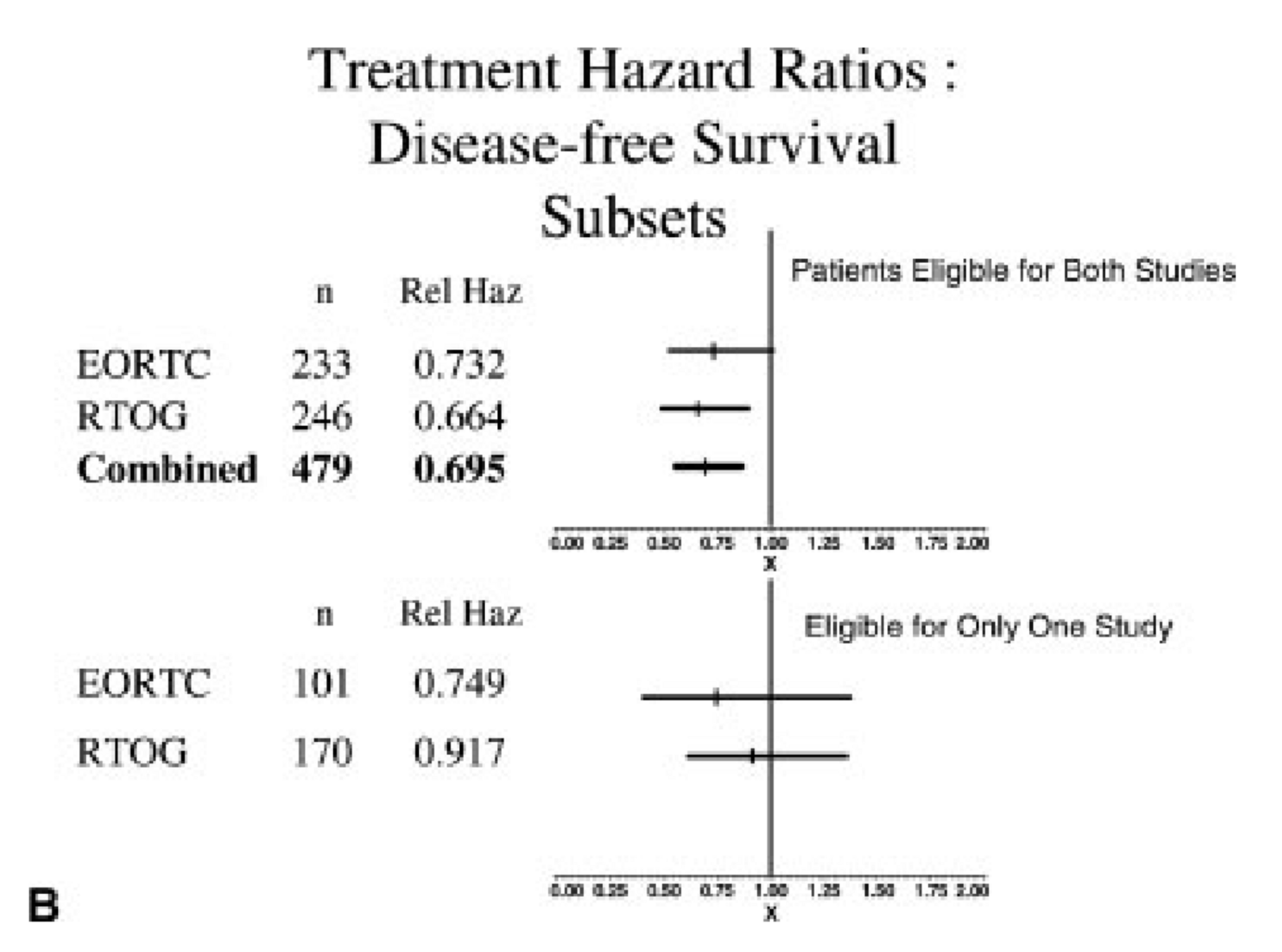
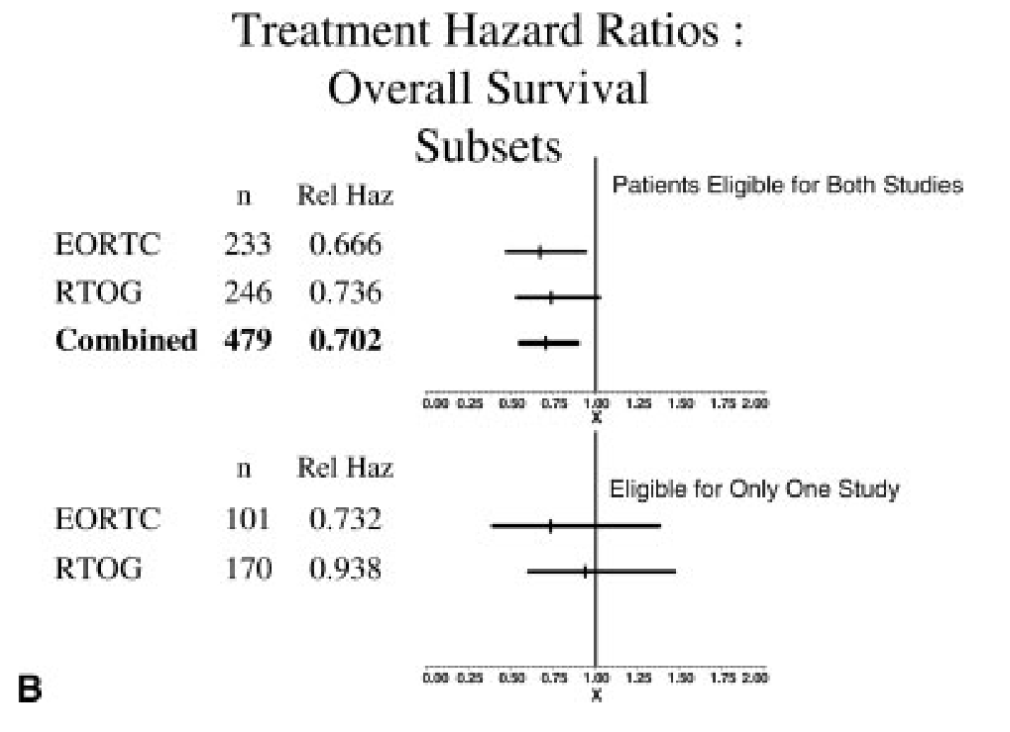
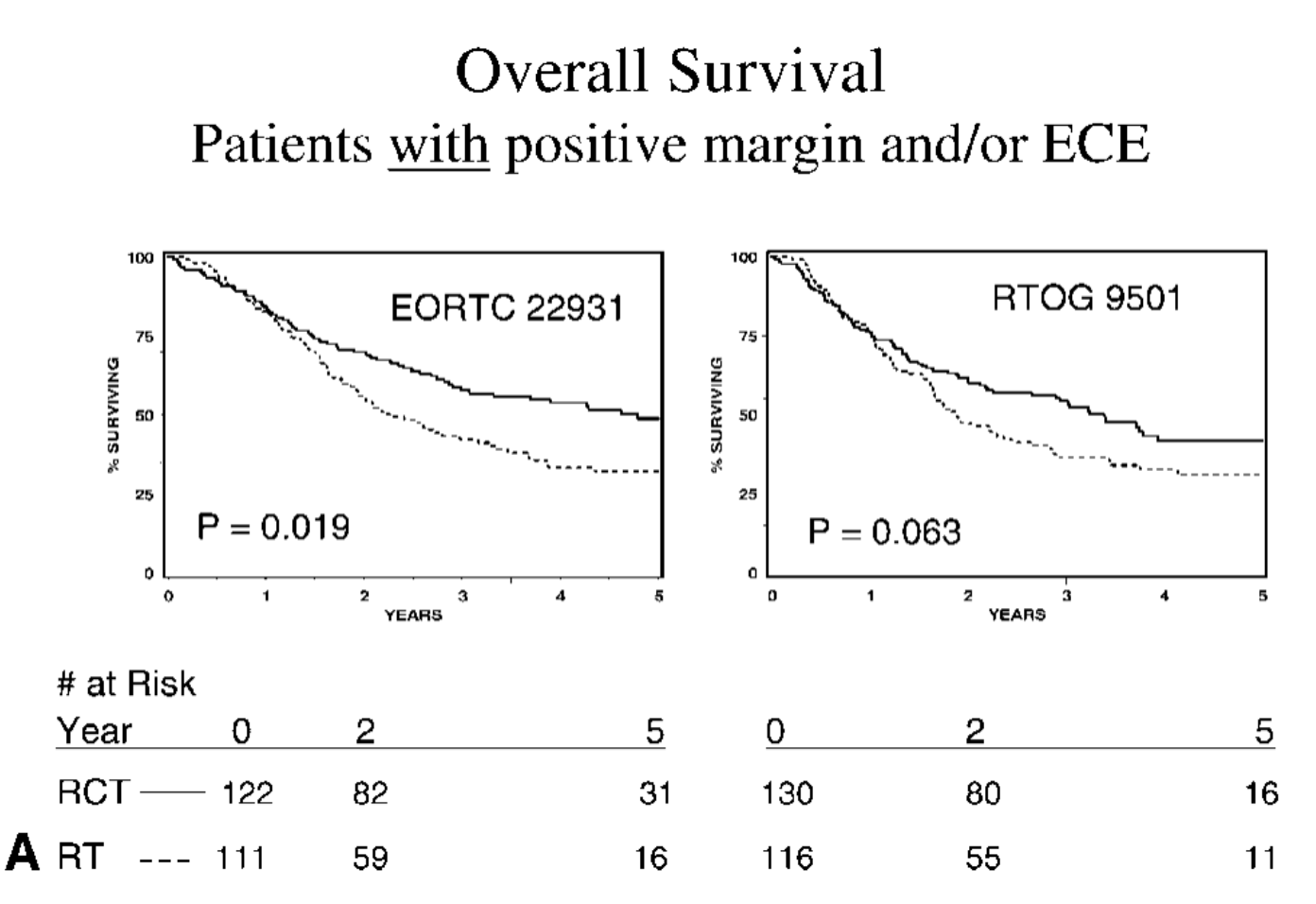
Comparative Analysis - Risk Factors
-
ECE and/or microscopically involved surgical margins were the most significant prognostic factors
-
Patients with these risk factors benefited most from chemoradiation in both trials
-
Patients with ≥2 positive lymph nodes without ECE did not seem to benefit significantly from chemoradiation
-
No interaction between number of lymph nodes and benefit with chemo (DM p=0.8, OS p=0.161, DFS p=0.45)
Conclusions
-
Postoperative chemoradiation improves outcomes in high-risk HNSCC
-
Greatest benefit in patients with ECE and/or positive margins
-
Consider chemoradiation for patients with stage III-IV disease, perineural invasion, vascular embolism, or level IV-V nodes (oral cavity/oropharynx)
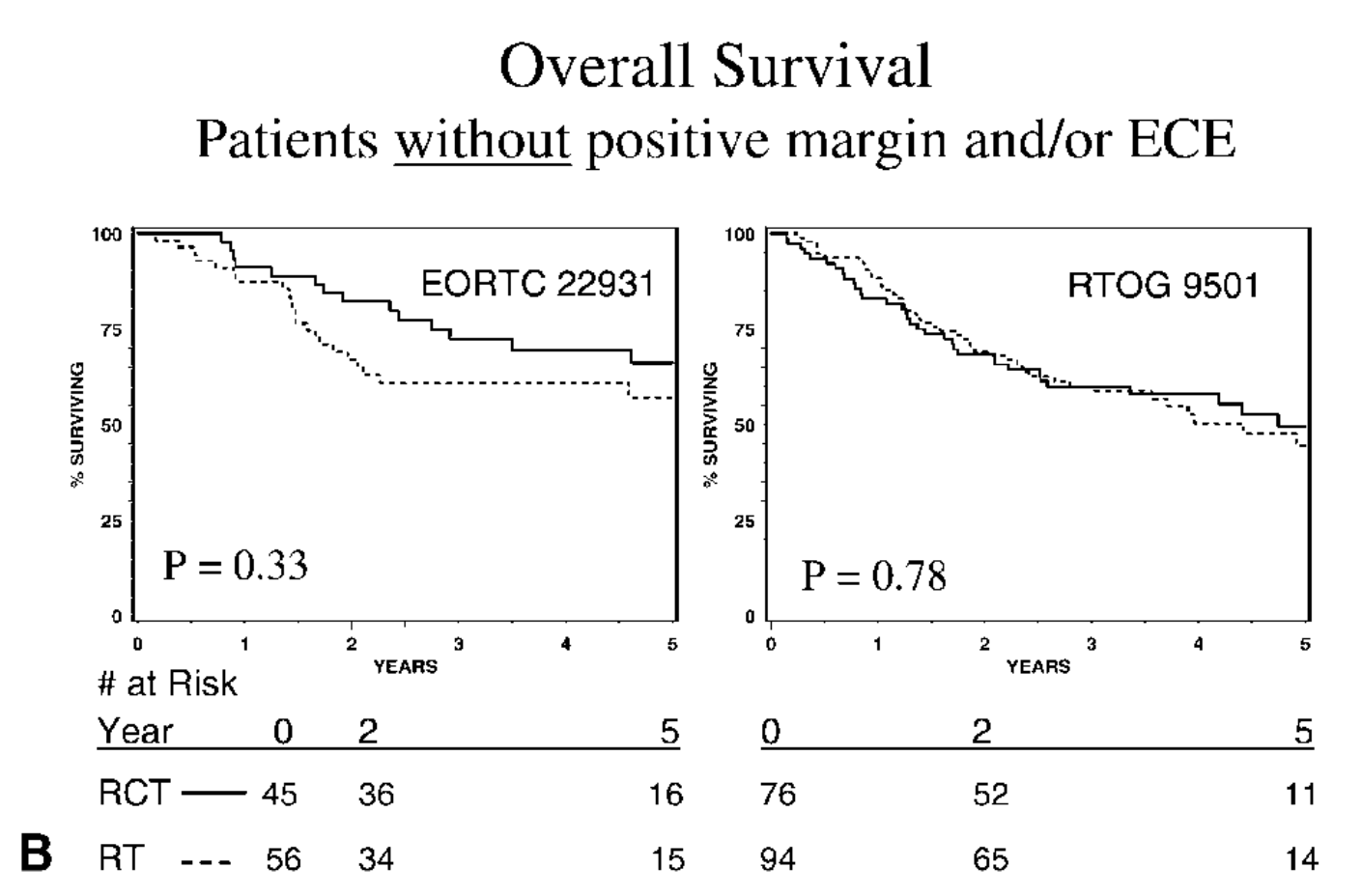
-
No OS benefit if only intermediate risk factors present (p=0.33 in EORTC and p=0.78 in RTOG)

Implications for Practice
- Chemoradiation should be standard for patients with ECE and/or positive margins
- Careful patient selection based on risk factors is crucial
- Balance potential benefits with increased toxicity
- Multidisciplinary approach to treatment decision-making
- Consider updated analysis (Lu 2022) showing no benefit with chemo for rising nodal count in OS or DM
Limitations and Future Directions
- Retrospective subgroup analysis
- Differences in eligibility criteria between trials
- Need for prospective validation of risk stratification
- Ongoing research on de-escalation strategies for lower-risk patients
- Investigation of novel systemic therapies (e.g., immunotherapy) in the adjuvant setting
- Need for updated analysis of intermediate risk factors with modern statistical approaches
Wait but what about lymph nodes..... ??
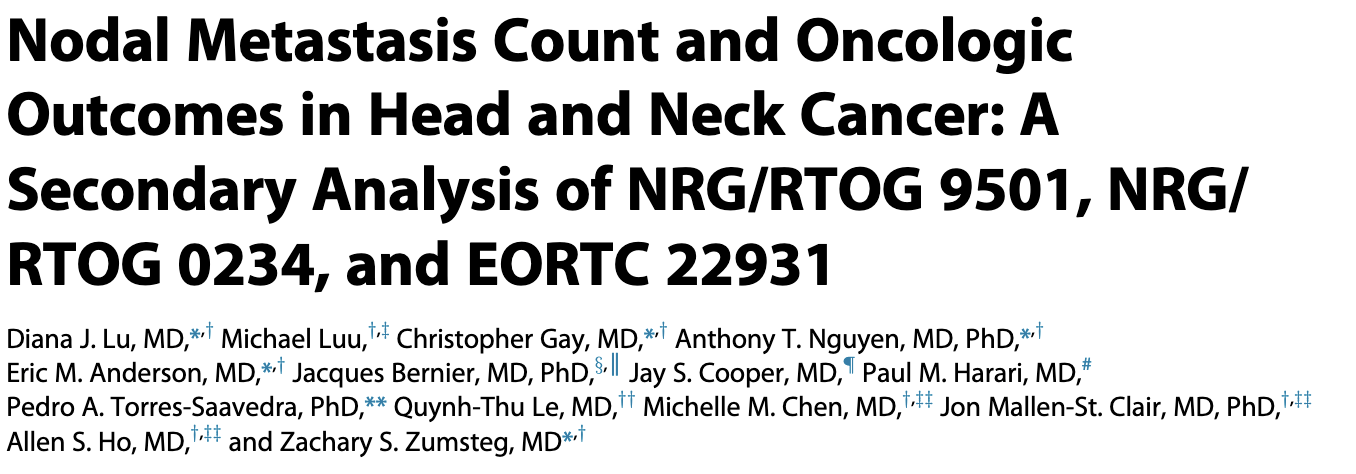
Impact of Nodal Metastasis Count on Outcomes
- Secondary analysis of RTOG 9501, RTOG 0234, and EORTC 22931 (947 patients)
- Increasing number of positive lymph nodes associated with worse outcomes
- Strongest association up to 5 positive nodes
- The association of +LN on outcomes was strongest up to 5 +LNs, with each metastatic LN being associated with an independent additional 19% increased risk of death
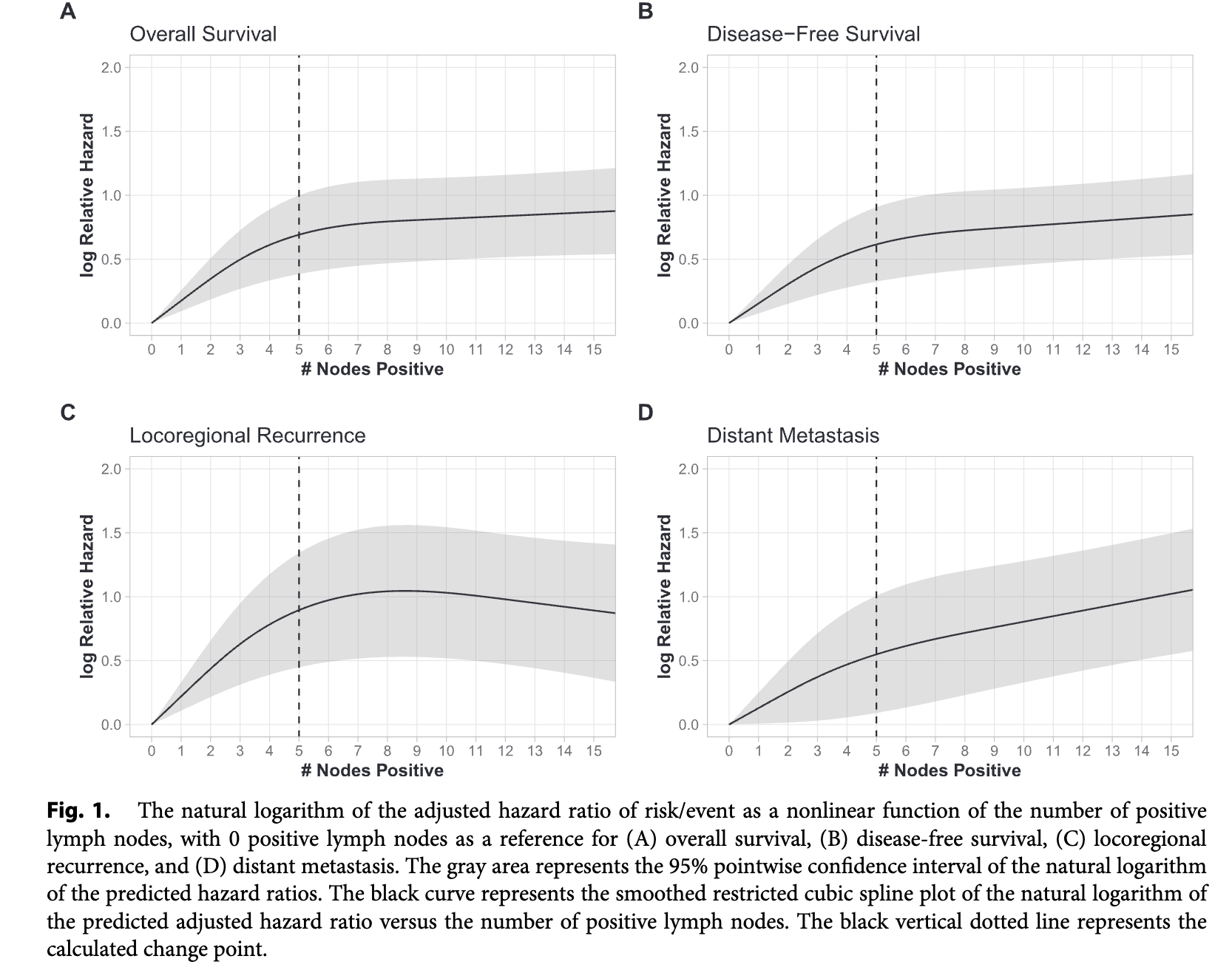
Impact of Nodal Metastasis Count
Should I give chemo for >5+ lymph nodes?
No
This secondary analysis it was not designed to test the benefit of chemotherapy based on nodal count alone.
No significant interaction:
The study found no statistically significant interaction between nodal count and the effect of systemic therapy on outcomes
(OS p=0.161, DFS p=0.45, DM p=0.802, LRR p=0.07).