Prostate Cancer Trials: Salvage/Adjuvant and Oligometastatic Disease
Board Review Presentation
Radiation Oncology
Presentation Overview
- Part 1: Salvage/Adjuvant Trials
- Classical adjuvant vs observation trials
- Modern adjuvant vs early salvage trials
- Salvage + ADT trials
- Part 2: Oligometastatic Prostate Cancer
- Treatment of primary in M1 disease
- SBRT to oligometastases
- Systemic therapy intensification
Classical Adjuvant vs Observation RT Studies
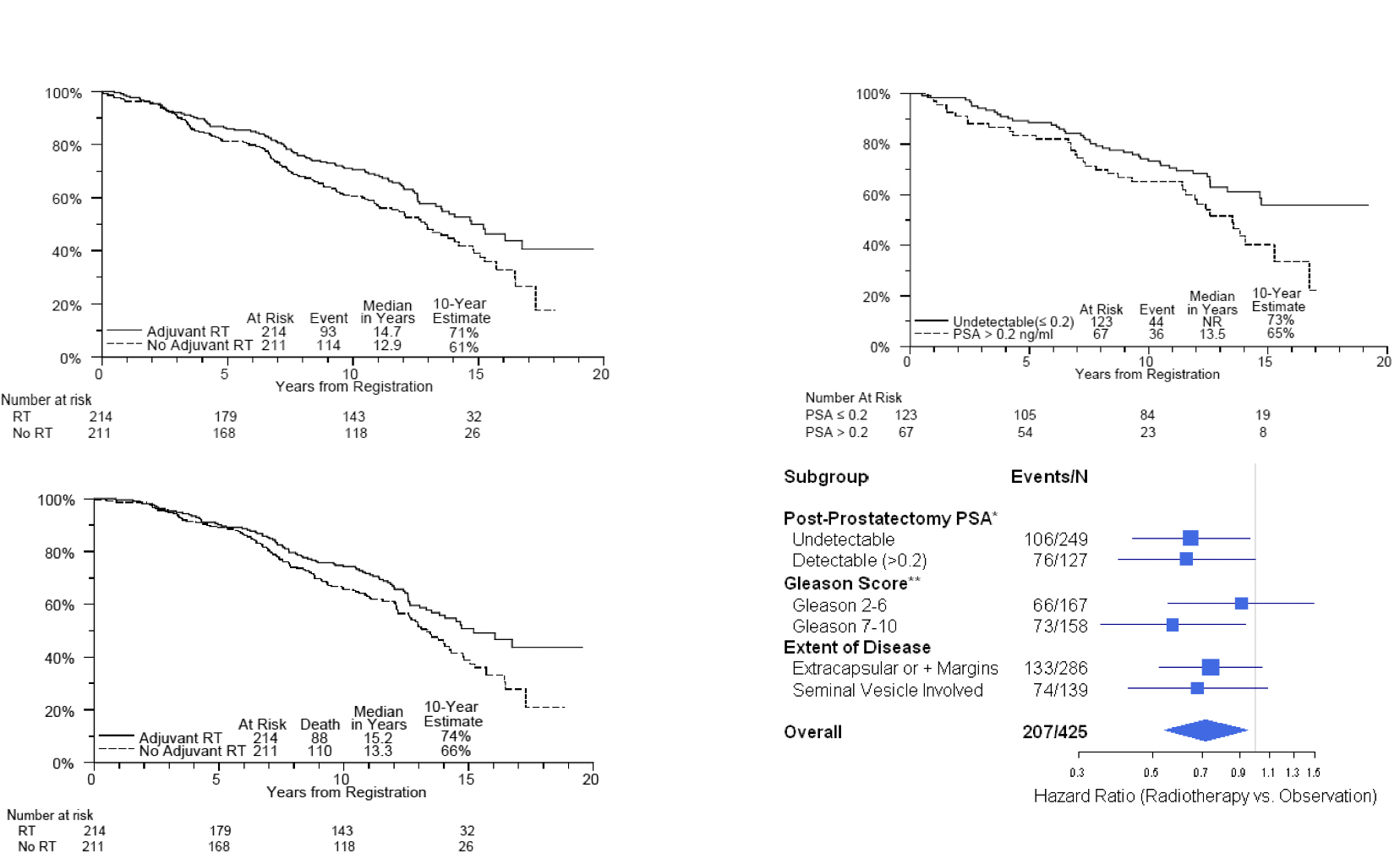
| SWOG/RTOG 9019 (~2006) | EORTC 22911 (~2005) | ARO 96-02 (~2009) | |
|---|---|---|---|
| Patients | • Post-RP • pT3 (SVI/ECE) or + margins • Any PSA level allowed ~1/3 had PSA >0.2, & 5-10% had PSA >1 |
• Post-RP • pT2-T3N0 with either +margin, ECE, or SVI • PSA ≤ 0.4 |
• Post-RP • pT3 (SVI/ECE) or + margin • Undetectable PSA (randomized prior to post-op PSA) |
| Arms | →adjuvant 60-64 Gy RT to prostate bed →wait and see [salvage RT in 33%, med PSA 1.0] No concurrent ADT |
→adjuvant 50 Gy to prostate bed +10 Gy boost →wait and see [delayed salvage RT 70Gy in 55%, at med PSA 1.7] |
→adjuvant 60 Gy to prostate bed [PSA <0.1 required] →wait and see RT strongly recommended at PSA rise |
| Results | 10-yr BPFS 58% vs. 28% OS and DMFS change seen at 15 years 10-yr OS 66% vs. 74% 15-yr OS 37% vs. 47% 15-yr DMFS 38% vs. 46% 10-yr toxicity: rectal complications 3% vs. 0% urethral strictures 18% vs. 9% urinary incontinence 6% vs. 3% |
10-yr BPFS 61% vs. 39% p<0.0001 10-yr LRR 7% vs. 16% p<0.0001 No change in DM, OS, or CSS at 10 years 10-yr toxicity: No grade 4 Grade 3 increased 5.3% vs. 2.5% Grade ≥2 GU increased 21% vs. 14% Grade ≥2 GI similar 2.5% vs. 1.9% |
5-yr BPFS 72% RT vs. 54% P=0.0015 10-yr BPFS 56% vs. 35% No change in OS or DM Unknown how many received delayed salvage 10-yr toxicity: only one grade 3 late toxicity of GI. There were 3 grade 2 GU and 2 grade 2 GI. |
Modern Adjuvant vs Early Salvage RT Studies
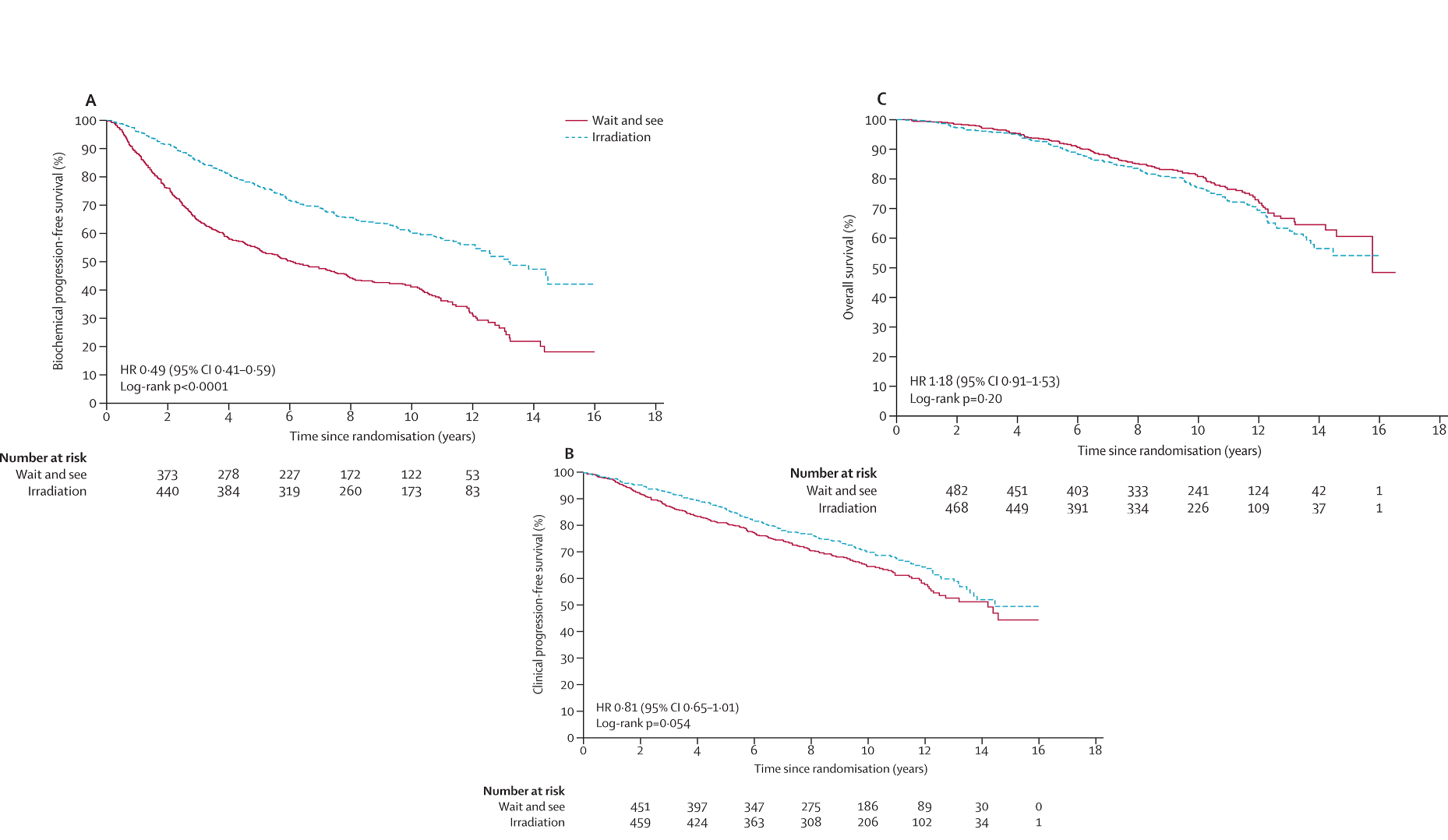
| TROG-RAVES (2020) | GETUG-AFU 17 (2020) | RADICALS-RT | |
|---|---|---|---|
| Patients | • pT3a/b (SVI/ECE), or positive margin • PSA ≤0.10 N = 333 |
• Positive margins required in all • pT3a/b, or pT4a • PSA ≤0.10 N = 424 |
• ≥1 of pT3a/b, T4, GS 7-10, pre-op PSA ≥10, or positive margins • PSA ≤0.2 N = 1396 |
| Arms | →adjuvant RT 64 Gy /32fx →salvage RT 64 Gy at PSA ≥0.2 >NO ADT or WPRT allowed |
Superiority →adjuvant RT 66 Gy /33fx →salvage RT 66 Gy at ≥0.2 and rising (WPRT of 46 Gy optional) > ADT required in all for 6 mos |
→adjuvant RT →salvage RT at PSA failure (PSA >0.1 & rising, or any PSA rising x3) RT: 66Gy/33fx (2/3rd) | 52.5Gy/20fx (1/3rd) (WPRT 56 Gy optional) >ADT none vs. 6 mos vs. 24 mos (not random) |
| Results | Salvage RT triggered in 50% 5-yr BPFS 86% vs. 87% (not noninferior) 8-yr BPFS 80% vs. 75% grade ≥2 GU toxicity 70% vs. 54% grade ≥2 GI toxicity 14% vs. 10% Trial terminated early due to low events |
Salvage RT triggered in 54% 5-yr EFS 90-92%, not different late grade ≥2 GU toxicity 27% vs. 7% late grade ≥2 GI toxicity 8% vs. 5% late ED grade ≥2 28% vs. 8% Trial terminated early due to low events |
8-yr BPFS not different between adjuvant and salvage RT GU incontinence 5.3% vs. 2.7% (p=0.008) urethral stricture 8% vs. 5% (p=0.03) |
See ARTISTIC
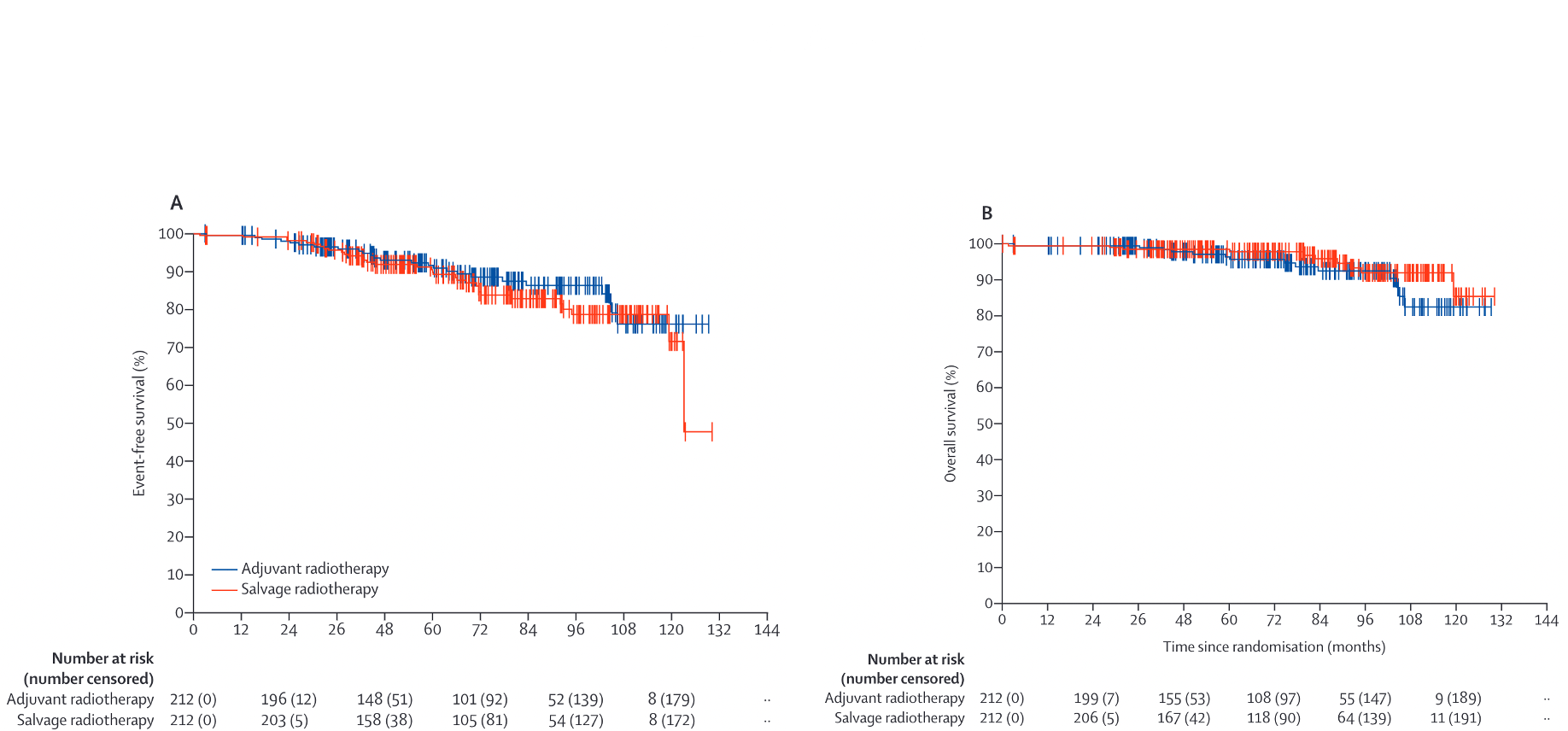
ARTISTIC Meta-analysis
- Design: Meta-analysis of RAVES, RADICALS-RT, and GETUG-AFU 17
- Patients: Combined data from 2,153 patients across three trials
- Results:
- 5-year event-free survival: 89% vs 88% (HR 0.95, 95% CI: 0.75-1.21)
- No difference in biochemical progression-free survival
- Lower rate of urinary toxicity with early salvage approach
- Approximately 50% of patients in salvage arms avoided radiation entirely
- Conclusion: Early salvage RT was non-inferior to adjuvant RT with lower toxicity
- Key Message: Early salvage approach spares ~50% of patients from RT without compromising oncologic outcomes
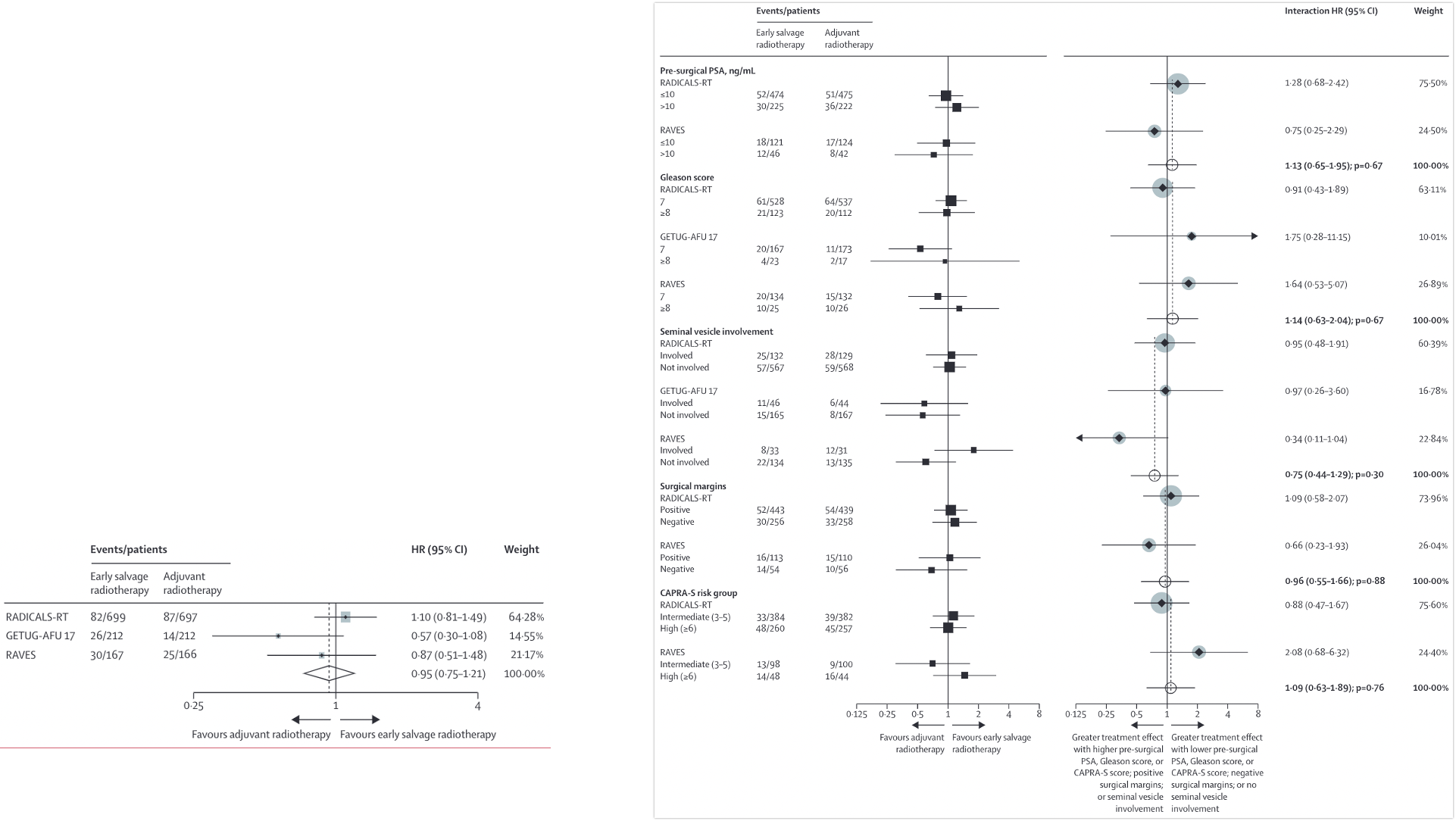

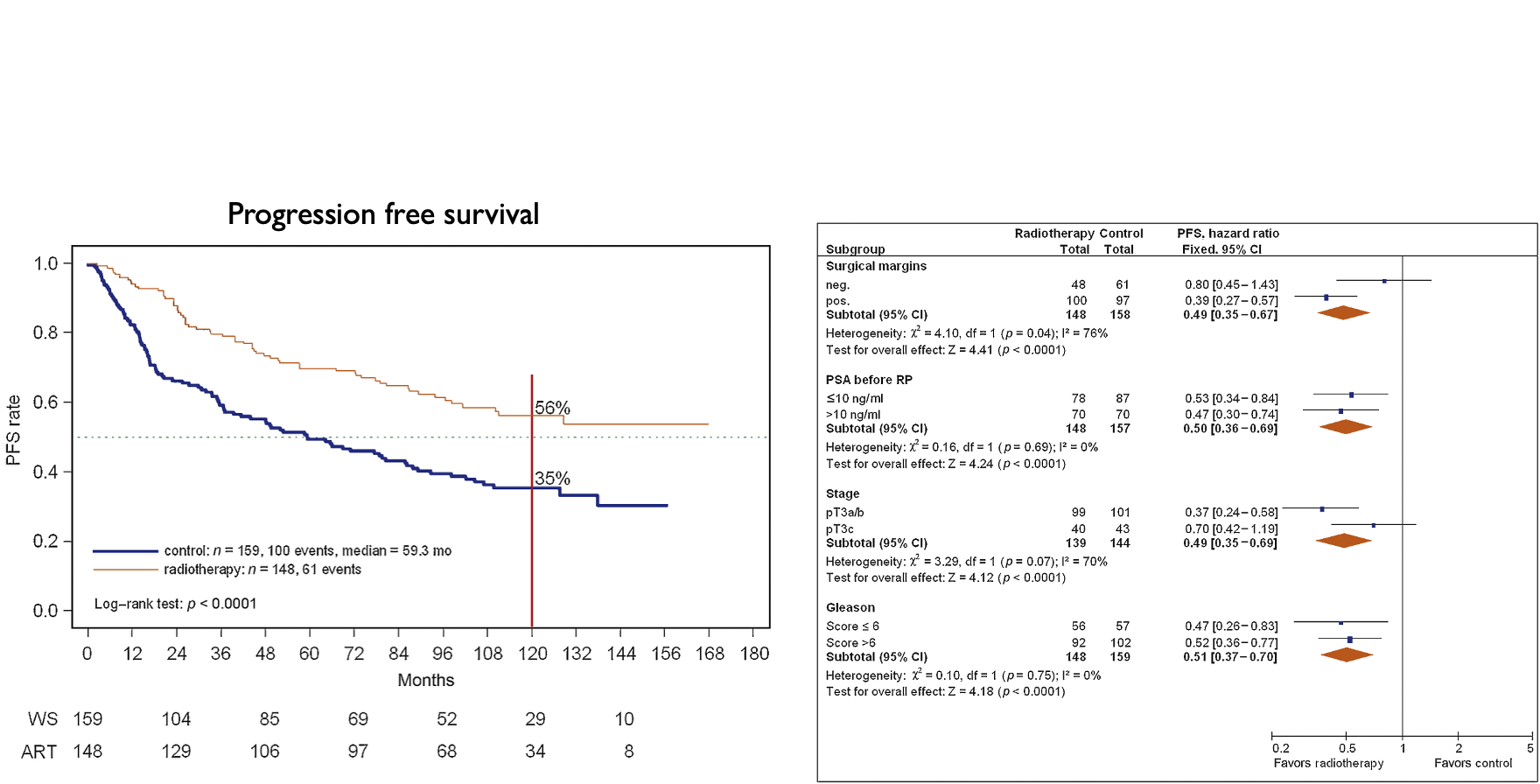
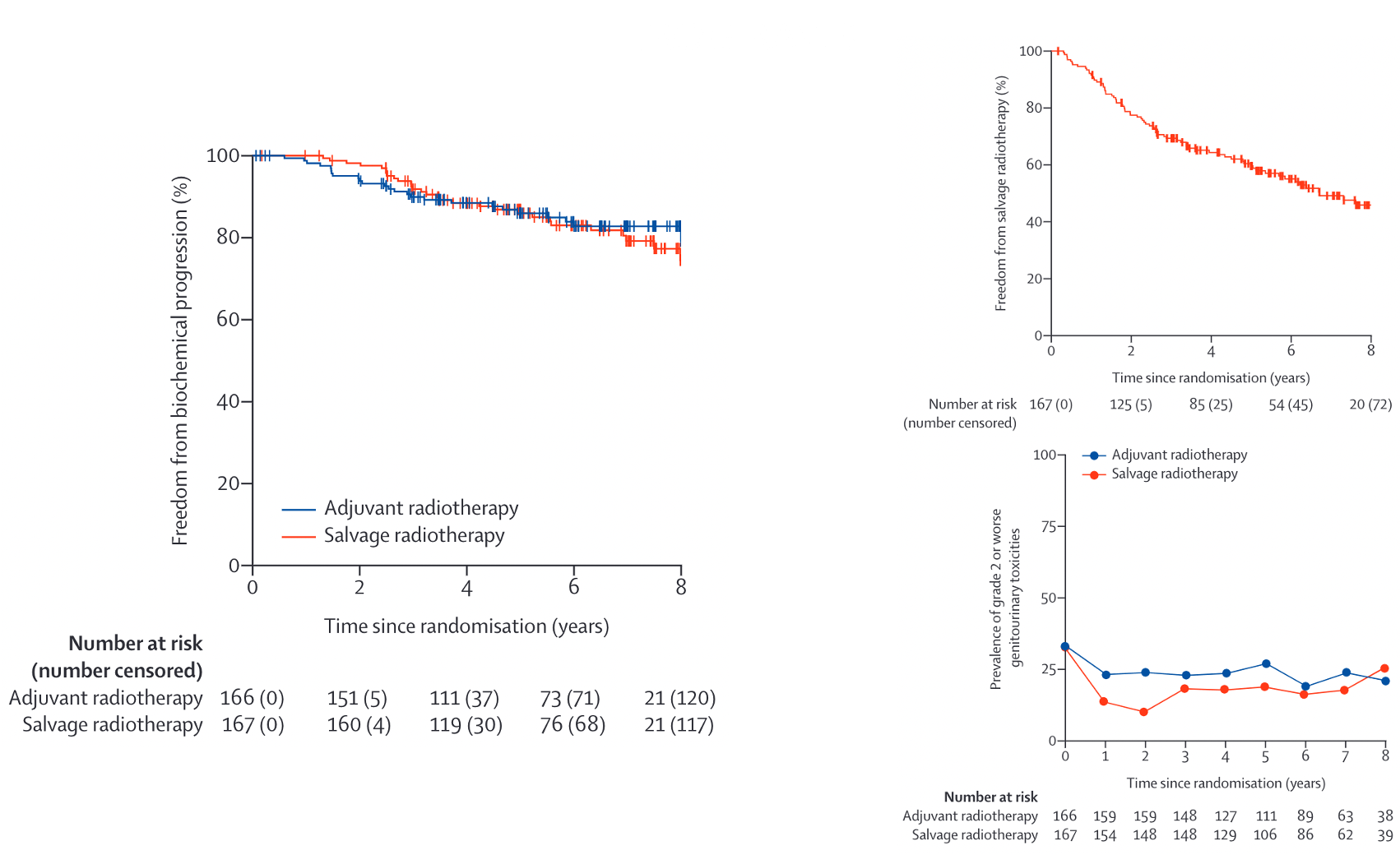
Salvage + ADT Studies
| RTOG 9601 | GETUG-AFU 16 | SPPORT | RADICALS-HD | |
|---|---|---|---|---|
| Patients | • pT3N0 | T2N0 with positive margins • PSA 0.2-4.0 after RP • N = 760 |
• pT2-T4a (bladder neck only) • PSA 0.2-2.0 • N = 743 |
• PSA ≥0.1 - 2.0 • T2-3N0/Nx, ±margin, GS ≤9 • Excluded pN1 • N = 1792 |
• ≥1 of: pT3a/b, T4, GS 7-10, PSA ≥10, or +margins • PSA ≤0.2 • 63% +margin, 70% ECE, 19% SVI • N = 1480 (0v6m), 1523 (6v24m) |
| Arms | →RT 64.8 Gy →RT + 24 mo bicalutamide |
→RT 66 Gy alone →RT + 6 mo ADT 46 Gy WPRT if Partin ≥10% |
→PB RT 64.8-70.2 Gy →PB RT + 4-6 mo ADT →WPRT + PB RT + 4-6 mo ADT |
Two 2-way comparisons: →RT vs RT + 6m ADT →RT + 6m vs RT + 24m ADT RT: 66 Gy/33 fx or 52.5 Gy/20 fx |
| Overall Survival |
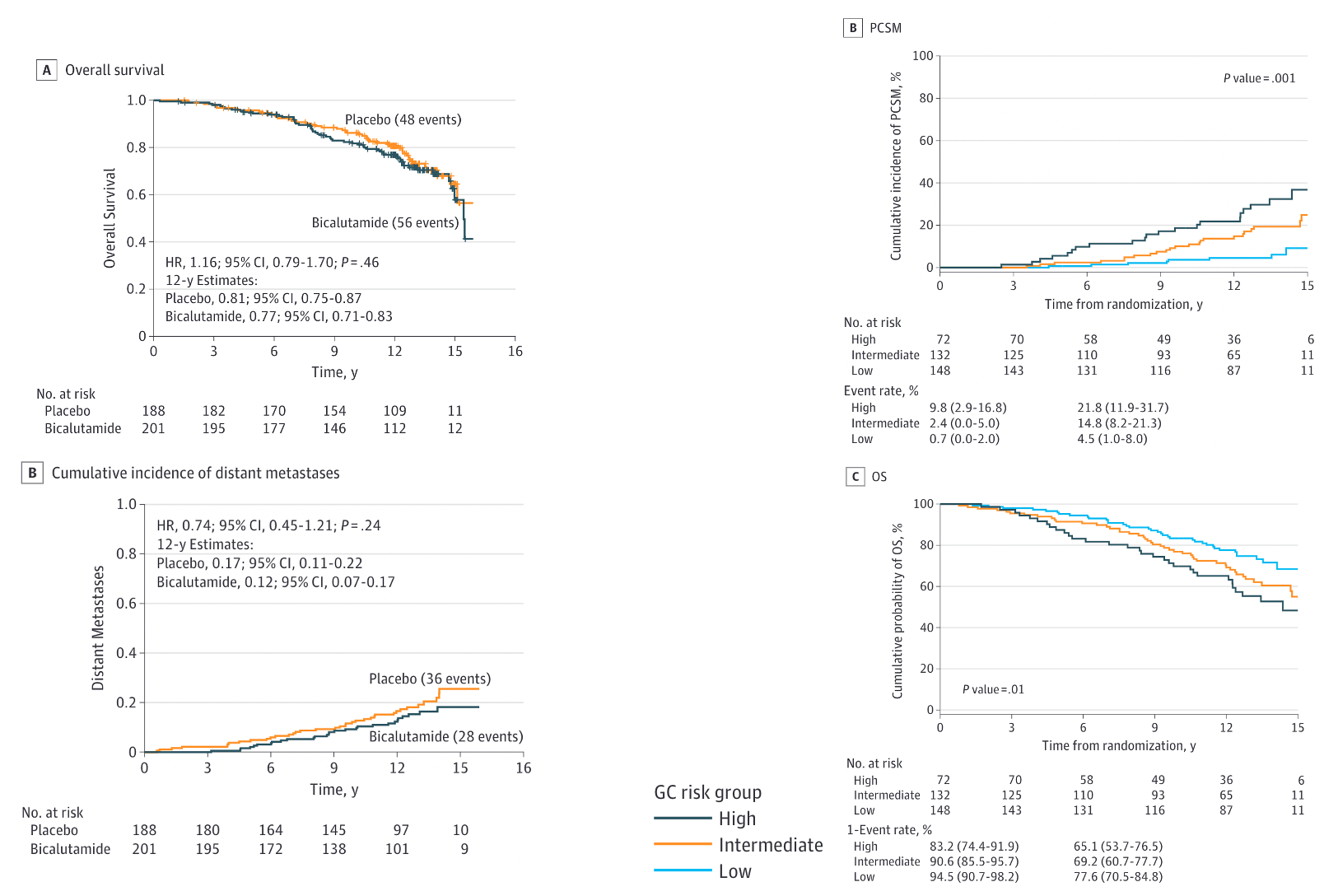
IMPROVED (p=0.04): 12-yr: 76% vs 71% HR 0.77 (0.59-0.99) Benefit if PSA >0.7 No benefit if PSA ≤0.6 |
IMPROVED (p=0.036): 10-yr: 76% vs 86% HR 0.69 (0.47-1.01) |
NOT DIFFERENT: 5-yr: 94-96% (immature data) |
NO BENEFIT: No OS improvement with any ADT 0v6m: HR 0.88 (0.65-1.19) 6v24m: HR 0.88 (0.66-1.17) |
| Metastasis-Free Survival |
IMPROVED: 12-yr: 76.3% vs 71.3% HR 0.73 (0.61-0.87) |
IMPROVED (p=0.034): 10-yr: 69% vs 75% HR 0.73 (0.54-0.98) |
Data not reported |
0v6m: NO BENEFIT HR 0.89 (0.69-1.14) 6v24m: IMPROVED (p=0.029) 10-yr: 72% vs 78% HR 0.77 (0.61-0.97) |
| Progression-Free Survival |
IMPROVED: 10-yr FFP: 46% vs 30% 12-yr DM: 14.5% vs 23.0% 12-yr PC death: 6% vs 13% |
IMPROVED (p<0.0001): 10-yr: 49% vs 64% HR 0.54 (0.43-0.68) 10-yr DM: 31% vs 25% |
IMPROVED (p<0.0001): 5-yr FFP: 71% vs 81% vs 87% 5-yr DM: 9% vs 6% vs 5% 5-yr castrate resistance: 3% vs 2% vs 1% |
10-yr BPFS ~75% (no difference) 10-yr DM: 7% vs 10% (adj vs salv, NS) No difference by PSA level, GS, margins, or RT schedule |
| Key Points |
• Decipher can help if PSA 0.5-0.7 • Grade 3-4 GI similar: 7% • Grade 3-4 GU: 2.7% vs 1.6% |
• Grade 3 GU: 8% vs 7% • No toxicity difference |
• WPRT improved outcomes • 5-yr regional failure: 5% vs 2% vs 1% • Late G2+ toxicity similar |
• Largest salvage ADT trial • Early salvage preferred (adj vs salv) • Stricture: 13% vs 6% (adj vs salv) |
KEY MESSAGE: 24m ADT improves outcomes for PSA >0.7 (RTOG 9601). RADICALS-HD shows 24m > 6m ADT for MFS, but 6m = none. No subgroup differences found.

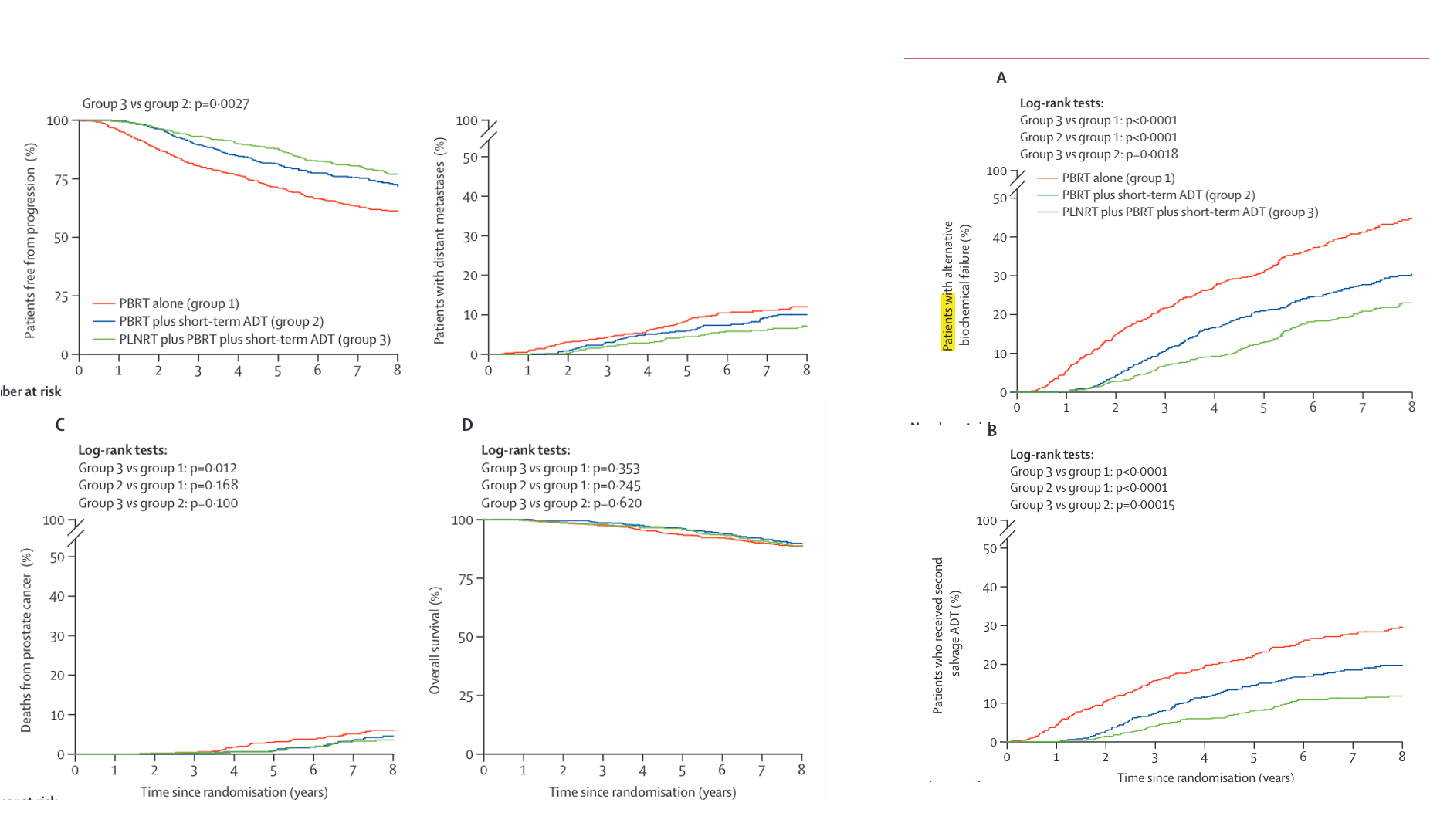
Radicals HD
Salvage + ADT Studies
| RTOG 9601 | GETUG-AFU 16 | SPPORT | |
|---|---|---|---|
| Patients | • pT3N0 | T2N0 with positive margins • elevated PSA 0.2-4.0 after RP • N = 760 |
• pT2-T4a (bladder neck only) • rising PSA of 0.2-2.0 • N = 743 |
• PSA ≥0.1 - 2.0 • T2-3N0/Nx, with or without positive margin, Gleason ≤9, excluded pN1 • N = 1792 |
| Arms | →RT 64.8 Gy to prostate fossa →RT with 24 months bicalutamide concurrently and adjuvant |
→66 Gy RT alone →RT with 6 mos ADT 46 Gy WPRT given if Partin score ≥10% before RP and no LND |
→prostate bed (PB) RT 64.8-70.2 Gy →PB RT + 4-6 mos ADT →WPRT 45 Gy + PB RT + 4-6 mos ADT |
| Results | 10-yr FFP: improved 46% vs. 30% 12-yr OS improved 76% vs. 71% 12-yr DM 14.5% vs. 23.0% 12-yr PC death 6% vs. 13% No OS or DM benefit in PSA ≤0.6 OS benefit in PSA <0.7 with high Decipher score Late grade 3-4 GI toxicity similar at 7% Late grade 3-4 GU toxicity similar, 2.7 vs 1.6% |
ADT reduced DM, but did not improve OS 5, 10-yr PFS 62%/49% vs. 80%/64% ADT 10-yr DM 31% vs. 25% ADT No change in OS Grade 3 GU 8% vs. 7% (no toxicity difference apparently) |
5-yr FFP 71% vs. 81% vs. 87% 5-yr DM 9% vs. 6% vs. 5% 5-yr OS 94-96%, not different 5-yr BF (Phoenix) 20% vs. 14% vs. 8% 5-yr BF (≥0.4) 31% vs. 21% vs. 13% 5-yr castrate resistance 3% vs. 2% vs. 1% 5-yr regional failure 5% vs. 2% vs. 1% Late grade 2+ toxicity (>3 mos) not different, except for worse 1-yr toxicity |
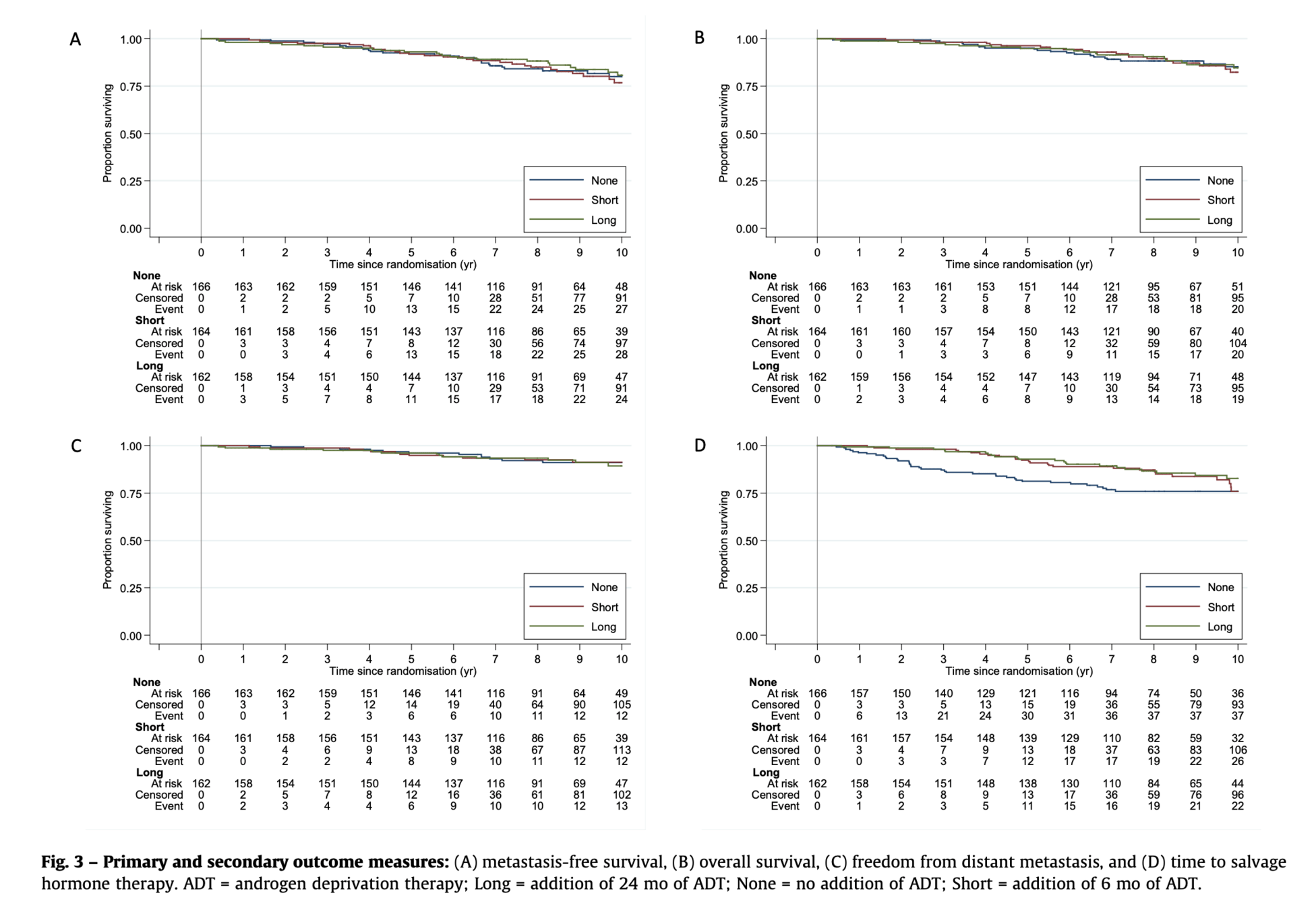
DADSPORT Meta-analysis
Duration of Androgen Suppression with Post-operative Radiotherapy
Meta-analysis of RTOG 9601, GETUG-AFU 16, NRG/RTOG 0534 (SPPORT), and RADICALS-HD
Included Trials: 4 trials • 4,452 men • 701 deaths • Median follow-up: 8 years • 100% of randomized patients
| Treatment Comparison | No. of Trials | Events/Men | HR (95% CI), p-value | Significance |
|---|---|---|---|---|
| Overall Survival | ||||
| HT (any duration) vs no HT | 4 | 701/4452 | 0.89 (0.77-1.03), p=0.13 | NS |
| 6m HT vs no HT | 3 | 419/3364 | 0.90 (0.74-1.09), p=0.28 | NS |
| 24m HT vs no HT | 2 | 282/1088 | 0.89 (0.72-1.10), p=0.29 | NS |
| Sensitivity analysis (excl. PSA >1.5) | 4 | 650/4334 | 0.93 (0.80-1.08), p=0.35 | NS |
| Metastasis-Free Survival | ||||
| 6m HT vs no HT | 3 | 653/3364 | 0.82 (0.70-0.96), p=0.013 | IMPROVED |
Overall Survival
No clear improvement with HT
Similar effects for 6m and 24m duration
Results consistent in sensitivity analysis
Metastasis-Free Survival
6m HT significantly improves MFS
HR 0.82 (18% relative reduction)
24m HT data pending from 2 trials
KEY MESSAGE: 6 months of ADT improves MFS but not OS in salvage RT setting (aggregate data meta-analysis)
Included Trials: 4 trials • 4,452 men • 701 deaths • Median follow-up: 8 years • 100% of randomized patients
Important: Most trials excluded pN1 patients (SPPORT excluded pN1; RTOG 9601 was pN0/pNx only)
| Treatment Comparison | No. of Trials | Events/Men | HR (95% CI), p-value | Significance |
|---|---|---|---|---|
| Overall Survival | ||||
| HT (any duration) vs no HT | 4 | 701/4452 | 0.89 (0.77-1.03), p=0.13 | NS |
| 6m HT vs no HT | 3 | 419/3364 | 0.90 (0.74-1.09), p=0.28 | NS |
| 24m HT vs no HT | 2 | 282/1088 | 0.89 (0.72-1.10), p=0.29 | NS |
| Sensitivity analysis (excl. PSA >1.5) | 4 | 650/4334 | 0.93 (0.80-1.08), p=0.35 | NS |
| Metastasis-Free Survival | ||||
| 6m HT vs no HT | 3 | 653/3364 | 0.82 (0.70-0.96), p=0.013 | IMPROVED |
Overall Survival
No clear improvement with HT overall
Similar effects for 6m and 24m duration
Results consistent in sensitivity analysis
Metastasis-Free Survival
6m HT significantly improves MFS
HR 0.82 (18% relative reduction)
24m HT data pending from 2 trials
Who Benefits from 24 Months ADT? (Individual Trial Data)
RTOG 9601 showed OS benefit with 24m ADT if:
- Pre-salvage PSA >0.7 ng/mL
- High Decipher genomic score (even at PSA 0.5-0.7)
- No benefit if PSA ≤0.6 ng/mL
Consider risk stratification:
- High-risk features may warrant 24m ADT
- Meta-analysis masks subgroup effects
- Individual patient data meta-analysis pending
⚠️ Critical Limitation: Node-Positive Disease
These results do NOT apply to pN1 patients! Most trials excluded node-positive disease. For pN1 patients, longer ADT duration (18-36 months) remains standard based on other evidence (e.g., RTOG 8531, EORTC 22961).
KEY MESSAGE: For pN0 disease - consider 24m ADT if PSA >0.7. For pN1 disease - longer ADT (18-36m) remains standard (not addressed in DADSPORT)
Key Takeaways: Salvage/Adjuvant Trials
-
Early salvage is now preferred over adjuvant RT
- Similar oncologic outcomes
- ~50% of patients avoid RT entirely
- Lower toxicity (especially GU)
-
ADT benefit depends on pre-salvage PSA
- RTOG 9601: Greatest benefit with PSA >0.7 ng/mL
- Consider genomic classifiers (Decipher) for PSA 0.5-0.7
- Limited benefit with PSA <0.5
-
Pelvic nodal RT considerations
- SPPORT: Adding WPRT + ADT improved FFP
- Consider in high-risk features (multiple risk factors)
-
Optimal salvage RT timing
- Initiate at PSA 0.2-0.5 ng/mL
- Modern trials used very low PSA thresholds
Part 2: Oligometastatic Prostate Cancer
- Key Questions in Oligometastatic Disease:
- Should we treat the primary tumor in M1 disease?
- What is the role of metastasis-directed therapy?
- How do we optimize systemic therapy?
- What defines "oligometastatic" disease?
- Trial Categories:
- Treatment of primary in de novo M1
- SBRT to oligometastases
- Systemic therapy intensification
STAMPEDE Trial: Trial Arms
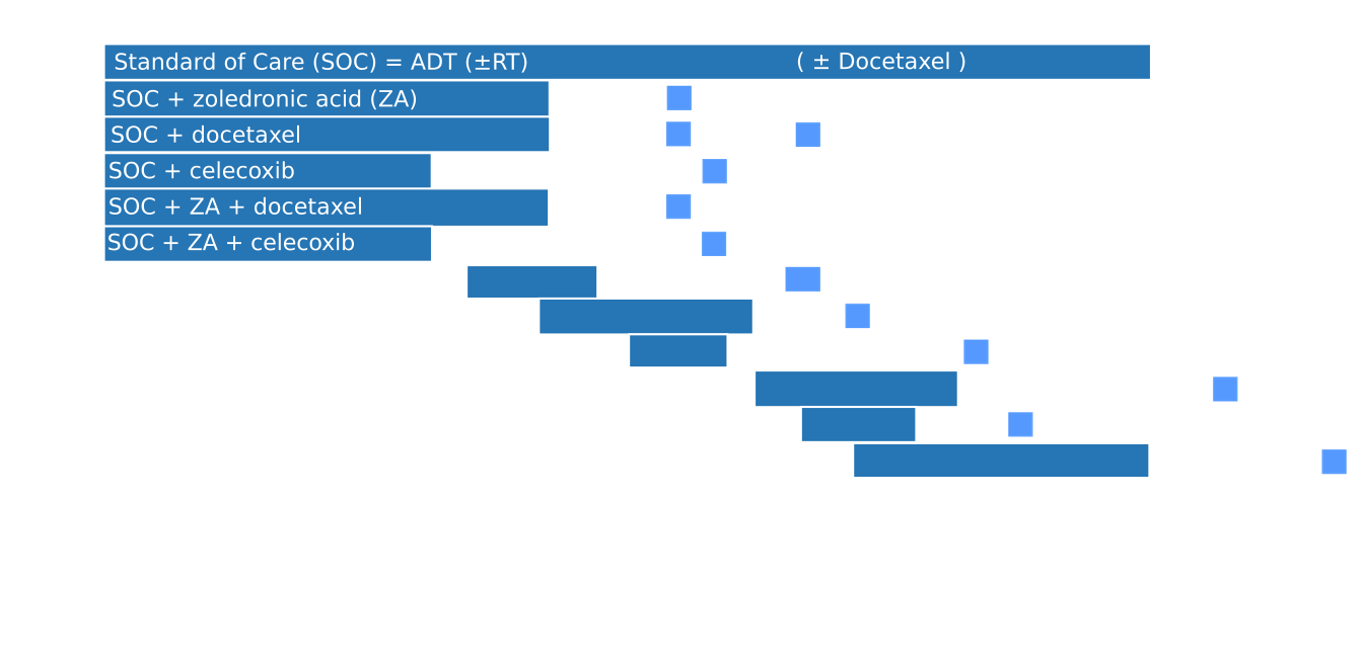
RT to Primary in M1 Disease
Eligibility: 2/3 of: T3/T4, PSA >40, GS 8-10 OR newly diagnosed N+/M1 | Arms: ADT + docetaxel ± RT (55 Gy/20 fx or 36 Gy/6 fx weekly)
Metastatic Burden Definitions
Low burden: <4 bone mets with none outside vertebra/pelvis AND no visceral mets
High burden: ≥4 bone mets with ≥1 outside vertebra/pelvis OR any visceral mets
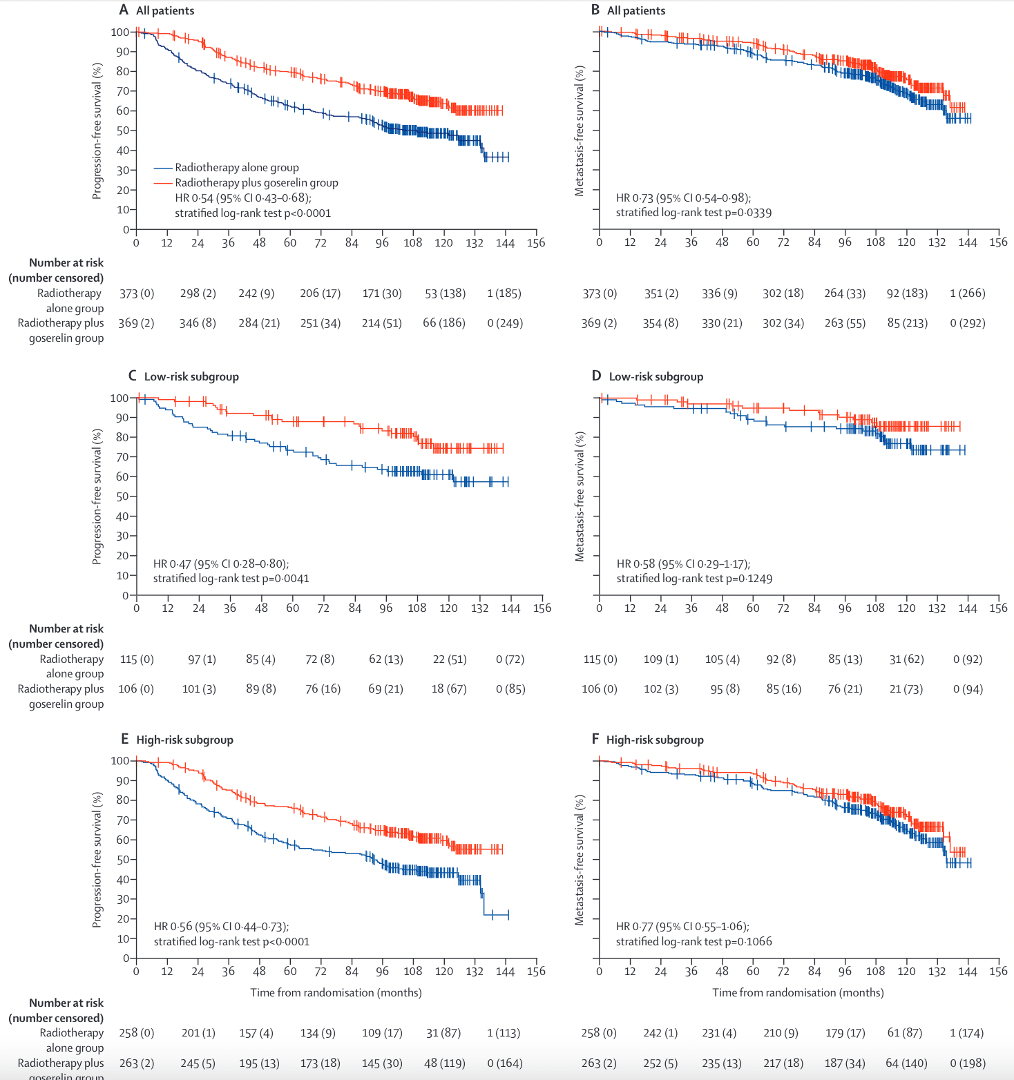
Low Burden (Prespecified Analysis)
Overall Survival: IMPROVED (p=0.007)
- 3-yr OS: 81% vs 73%
- 5-yr OS: 65% vs 53%
- Median OS: 85.5 vs 63.6 months
- HR 0.68 (95% CI: 0.52-0.90)
Failure-Free Survival: IMPROVED (p<0.0001)
- 3-yr FFS: 40% vs 23%
Exploratory: ≤3 Bone Mets
OS IMPROVED: 3-yr 85% vs 75%
FFS IMPROVED: 3-yr 53% vs 33%
High Burden: NO BENEFIT
- 3-yr OS: 52-53% both arms (NS)
- 5-yr OS: No difference (NS)
- 3-yr FFS: 15-16% both arms (NS)
Additional Findings
RT Fractionation:
55 Gy/20 fx better FFS
vs
36 Gy weekly (no OS difference)
Toxicity: Grade 3-4 during RT: 5%, after RT: 4%
KEY MESSAGE: RT to prostate significantly improves OS in low-burden M1 disease (NNT = 8 for OS benefit at 3 years)
Systemic Therapy Intensification in mHSPC
| CHAARTED | LATITUDE | TITAN | PEACE-1 | |
|---|---|---|---|---|
| Patients | • mHSPC • N = 790 • Any volume |
• High-risk mHSPC • N = 1199 • ≥2 of: GS≥8, ≥3 bone lesions, visceral mets |
• mHSPC • N = 1052 • Any volume • Prior docetaxel allowed |
• mHSPC • N = 1172 • 2x2 factorial • ADT + docetaxel as SOC |
| Arms | → ADT alone → ADT + docetaxel x6 |
→ ADT + placebo → ADT + abiraterone + prednisone |
→ ADT + placebo → ADT + apalutamide |
→ ADT + docetaxel → ADT + docetaxel + abiraterone ± RT to primary |
| Overall Survival |
IMPROVED (p<0.001): • Median: 44.0 vs 57.6 mo • HR 0.61 (0.47-0.80) High volume: 32.2 vs 51.2 mo Low volume: NR vs 63.5 mo (NS) |
IMPROVED (p<0.0001): • Median: 36.5 vs 51.8 mo • HR 0.66 (0.56-0.78) • 3-yr OS: 49% vs 66% |
IMPROVED (p=0.005): • 24-mo OS: 74.8% vs 82.4% • HR 0.67 (0.51-0.89) • Benefit in all subgroups |
IMPROVED in high volume (p=0.017): • High: 3.5 vs 5.1 yrs • HR 0.72 (0.55-0.95) • Low volume: NR (immature) |
| Progression-Free Survival |
IMPROVED (p<0.001): • Median clinical PFS: 19.8 vs 33.0 mo • HR 0.60 (0.52-0.70) |
IMPROVED (p<0.0001): • Median rPFS: 16.8 vs 33.0 mo • HR 0.47 (0.40-0.55) |
IMPROVED (p<0.001): • Median rPFS: NR vs NR • HR 0.48 (0.39-0.60) • 24-mo rPFS: 68.2% vs 83.3% |
IMPROVED (p<0.0001): • Median rPFS: 2.0 vs 4.5 yrs • HR 0.50 (0.40-0.62) • High: 1.6 vs 4.1 yrs • Low: 2.7 vs NR |
| Key Toxicity | • Grade 3-4 neutropenia: 12% vs 15% • Febrile neutropenia: 6% vs 7% |
• Grade 3+ HTN: 10% vs 22% • Grade 3+ hypokalemia: 3% vs 12% |
• Rash: 6% vs 28% • Hypothyroidism: 1% vs 8% |
• Grade 3+ toxicity: 52% vs 63% • HTN: 13% vs 22% |
KEY MESSAGE: All trials show consistent OS benefit with systemic intensification.
Doublet therapy ADT + novel hormonal standard for all metastatic prostate cancer,
Triplet therapy (ADT + docetaxel + novel hormonal) for high-volume disease
PEACE-1:
2x2 Factorial Design
N = 1172
ADT + docetaxel
abiraterone
RT (74 Gy/37 fx)
abiraterone + RT
Key Results:
-
Adding abiraterone to ADT + docetaxel:
- Median PFS: 2.0 vs 4.5 years
- High burden median OS: 3.5 vs 5.1 years
-
Low burden + RT analysis:
- 3-yr PFS: 3.0 yrs (SOC) vs 4.4 (abi) vs 2.6 (RT) vs 7.5 (RT+abi)
- RT + abi significantly better than abi alone (p=0.019)
- Median OS: 6.9 yrs (no RT) vs 7.5 yrs (RT), p=0.81
ORIOLE
Recurrent hormone-sensitive PCa • 1-3 metastases • s/p primary treatment
No ADT x 6 months • Testosterone >50 ng/dL
N = 54
n = 36
• Progression: 19% (p=0.003)
SECONDARY ENDPOINTS:
• Median PFS: Not reached vs 5.8 mo (HR 0.30, p=0.002)
• New PSMA lesions at 6 mo: 16% vs 63% (p=0.006)
• Total consolidation failure: 31%
• T cell clonal expansion: Significant (p=0.03)
PATTERN OF FAILURE:
• 7/11 failures: untreated PSMA+ lesions
• Only 1 in-field failure
n = 18
• Progression: 61%
SECONDARY ENDPOINTS:
• Median PFS: 5.8 months
• New PSMA lesions at 6 mo: 63%
• Total consolidation failure: 91%
NOTE:
• Higher baseline Gleason scores in observation arm (imbalance)
KEY FINDINGS: SBRT significantly reduced progression (primary endpoint met), prolonged PFS, and induced systemic immune response
STOMP
Recurrent PCa • ≤3 mets on choline PET • Testosterone >50 ng/dL
PSA doubling time <12 months
N = 62
n = 31
• ADT-free survival: 21 mo (p=0.11)
• 3-yr ADT-free survival: improved
SECONDARY ENDPOINTS:
• No symptomatic progression
• No local progression of treated lesions
• 11 patients had repeat SBRT
• QOL: Similar to observation
KEY FINDING:
• 8-month delay in ADT initiation
• Fewer metastases in treatment arm (imbalance)
n = 31
• ADT-free survival: 13 months
ADT TRIGGERS:
• Progression to >3 metastases
• Local progression of known mets
• Symptomatic progression
NOTE:
• More metastases at baseline vs treatment arm
KEY FINDING: MDT delayed ADT initiation by 8 months (phase II criteria: p<0.20 considered significant)
ORIOLE & STOMP Pooled Analysis
- Combined analysis of 116 patients from ORIOLE and STOMP
- Results:
- Median PFS: 11.9 vs 5.9 months favoring SBRT
- Median time to castrate resistance: 18.3 vs 17 months (NS)
- Median OS: Not reached in either arm
- Subgroup analysis:
- Benefit consistent across subgroups
- No difference by number of metastases (1 vs 2-3)
- No difference by location (nodal vs bone)
- Conclusion: SBRT to oligometastases doubles PFS compared to observation
Advanced Oligometastatic Trials
| EXTEND | ARTO | SABR-COMET | |
|---|---|---|---|
| Patients | • Oligorecurrent PCa • ≤5 metastases • Hormone-sensitive • N = 87 |
• Castrate-resistant PCa • ≤3 non-visceral mets • Starting abiraterone • N = 157 |
• 1-5 mets, any histology • Controlled primary • 18% prostate cancer • N = 99 |
| Arms | → Intermittent ADT alone → SBRT + intermittent ADT (2 mo ADT pre-SBRT) |
→ Abiraterone + prednisone → SBRT + abiraterone + prednisone |
→ Palliative SOC → SBRT + palliative SOC (30-60 Gy/3-8 fx) |
| Overall Survival | Not reported (ongoing) | Not mature |
IMPROVED (p=0.006): • Median: 28 vs 50 mo • 5-yr: 18% vs 42% • 8-yr: 14% vs 27% |
| Progression-Free Survival |
IMPROVED (HR 0.25, p<0.001): • Median: 15.8 mo vs NR • Improved T-cell activation |
IMPROVED (HR 0.35): • Median time to progression improved |
IMPROVED (p=0.001): • Median: 6.0 vs 12 mo • 8-yr: 0% vs 21% |
| Key Secondary Endpoints |
Eugonad PFS IMPROVED: • 6.1 mo vs NR • Allows intermittent ADT |
6-mo biochemical response: • ≥50% decline: 68% vs 92% • PSA ≤0.2: 23% vs 56% |
• Local control: 46% vs 63% • 11/25 long-term survivors had no progression • 5/25 had salvage SBRT |
| Toxicity | • Grade 3+: minimal • No grade 4-5 events |
• Grade 3+: 6% • Well tolerated |
• Grade 5: 4.5% (n=3) vs 0% • Grade 3+: increased • No deaths in other SBRT trials |
KEY MESSAGES: SBRT improves PFS across settings. SABR-COMET showed OS benefit (phase II). EXTEND allows intermittent ADT. ARTO benefits even in CRPC.
Key Takeaways: Oligometastatic Disease
-
Treatment of primary in M1 disease:
- Benefits low-volume disease (≤3 mets or CHAARTED low burden)
- No benefit for high-volume or visceral metastases
- Standard dose (55 Gy/20 fx) preferred over weekly hypofractionation
-
Metastasis-directed therapy:
- Doubles PFS in oligorecurrent disease
- Can delay ADT initiation by 8-12 months
- May allow for intermittent ADT (EXTEND trial)
- Benefits seen even in CRPC (ARTO trial)
-
Systemic therapy intensification:
- Doublet therapy: ADT + novel hormonal agent or docetaxel now standard for mHSPC
- Triplet therapy: (ADT + docetaxel + abiraterone) for high-volume disease
- Consider RT to primary: (+ RT to primary) for low-volume disease
-
Future directions:
- Optimal patient selection (PSMA PET, genomics)
- Sequencing of local and systemic therapies
- Role of immunotherapy combinations
- Role of lutetium in early stage
Summary
- Salvage/Adjuvant RT:
- Early salvage preferred over adjuvant (ARTISTIC meta-analysis)
- Add ADT for PSA >0.7 (RTOG 9601)
- Consider WPRT for high-risk features (SPPORT)
- Oligometastatic Disease:
- Treat primary for low-volume M1 (STAMPEDE)
- SBRT to oligomets improves PFS and delays ADT
- Systemic intensification improves OS in mHSPC
- Clinical Integration:
- PSMA PET changing detection of oligometastatic disease
- Genomic classifiers help personalize treatment
- Multidisciplinary approach essential