97039: Global Health, Antimicrobial Drugs and Vaccines
Module 4: The potential for vaccines to reduce AMR
Russell Lewis, Associate Professor
Infectious Diseases, IRCSS S. Orsola-Malpighi Hospital
Department of Medical and Surgical Sciences
russeledward.lewis@unibo.it

Alma Mater Studiorum
Università di Bologna
Global Research and Development Priorities for AMR
| Priority | Pathogens included |
|---|---|
| Critical |
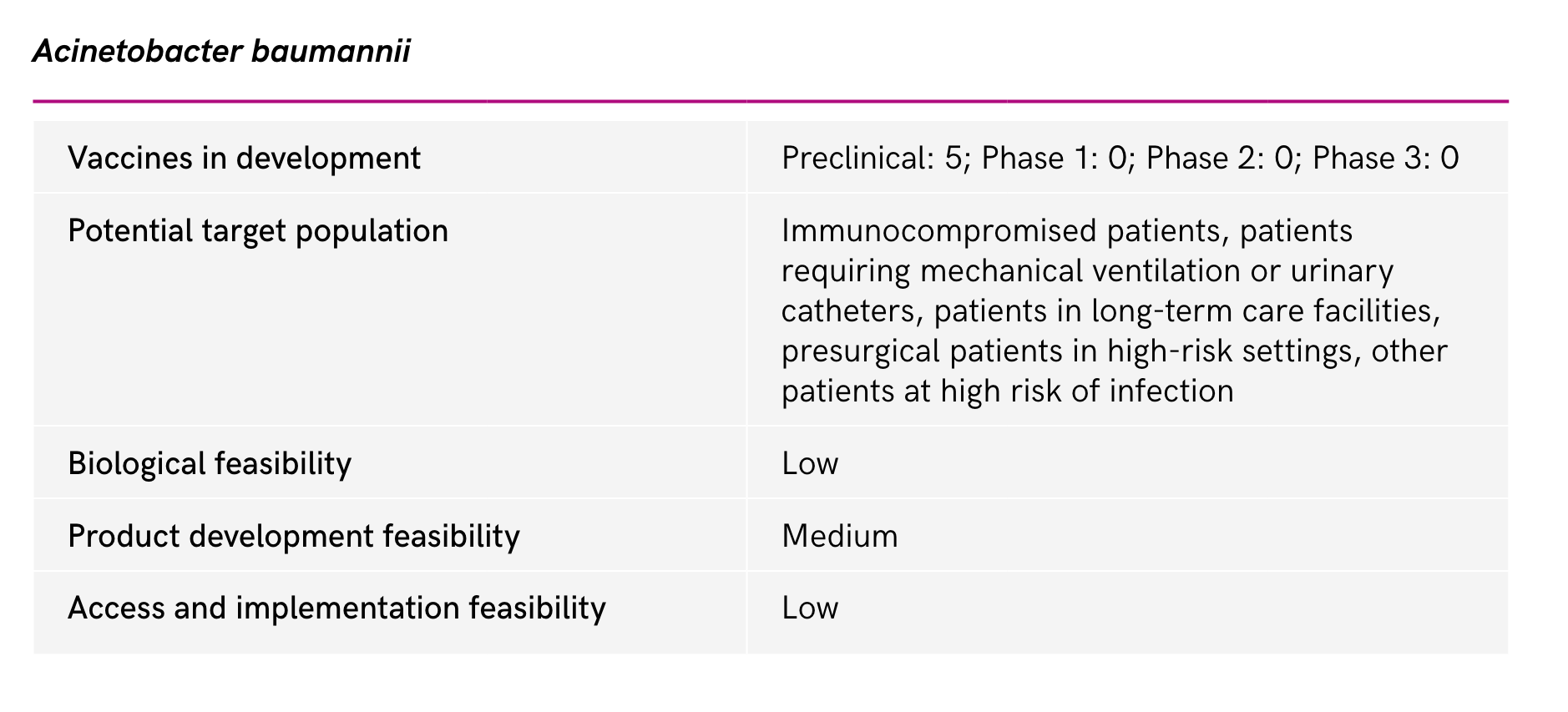
Acinetobacter baumannii (Carbapenem-resistant) Pseudomonas aeruginosa (Carbapenem-resistant) Enterobacterales (3rd generation cephalosporin, carbapenem-resistant) |
| High |
Enterococcus faecium, vancomycin-resistant Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant Helicobacter pylori, clarithromycin-resistant Campylobacter, fluoroquinolone-resistant Salmonella spp., fluoroquinolone-resistant Neisseria gonorrhoeae, 3rd gen. cephalosporin-resistant, fluoroquinolone-resistant |
| Medium |
Streptococcus pneumoniae, penicillin-non-susceptible Haemophilus influenzae, ampicillin-resistant Shigella spp., fluoroquinolone-resistant |
This table does not include Mycobacterium tuberculosis, which was already recognized as a global health priority pathogen
Source: World Health Organization
WHO Priority pathogens for future pandemics.
Vaccines can reduce AMR through multiple mechanisms

Streptococcus pneumoniae as a model

World Health Organization (WHO). Leveraging Vaccines to Reduce Antibiotic Use and Prevent Antimicrobial Resistance. 2020.
Impact of pneumococcal vaccination in the U.S.
Global burden of antimicrobial resistance

Murray CJ et al. The Lancet. 2022 Feb 12;399(10325):629–55.
Murray CJ et al. The Lancet. 2022 Feb 12;399(10325):629–55.



World Health Organization (WHO). Leveraging Vaccines to Reduce Antibiotic Use and Prevent Antimicrobial Resistance. 2020.
Estimated impact of typhoid vaccine


Source: Vaccines to tackle drug-resistant infections. Wellcome Trust and Boston Consulting 2018.


Source: Vaccines to tackle drug-resistant infections. Wellcome Trust and Boston Consulting 2018.
- H. influenzae, S. pneumoniae and S. Typhi:
- Although uptake of H. influenzae vaccine is relatively high globally at ~70%, continued efforts can be made to maintain and further expand coverage, particularly in certain geographies.
- Increasing uptake of the S. pneumoniae vaccine presents a significant opportunity; this vaccine is effective for 13 serotypes and used in high, middle and low-income countries, but currently only has ~40% coverage.
- A new, conjugated S. typhi vaccine has recently been pre-qualified by the WHO and is supported by Gavi for introduction in 2019, following effectiveness trials. Upon completion, efforts should focus on successfully introducing a vaccination programme.
Source: Vaccines to tackle drug-resistant infections. Wellcome Trust and Boston Consulting 2018.
Pathogens on the WHO list with effective vaccines include:

-
Pathogens on the WHO list in this category include E. coli (enteric), non-typhoidal Salmonella and Shigella:
- The high antigenic diversity of E. coli (enteric) is a challenge for vaccine development, but inclusion of LT toxoid and fimbrial antigens in a potential vaccine may help cover 70-80% of strains.
- A non-typhoidal Salmonella vaccine appears technically promising and potentially impactful, given high disease burden in Africa.
- A vaccine against Shigella would represent a major opportunity in this segment due to high incidence and significant associated mortality, particularly in low- and middle-income countries.
Pathogens on the WHO list where immunity is understood:
Source: Vaccines to tackle drug-resistant infections. Wellcome Trust and Boston Consulting 2018.
Pathogens where additional R&D could improve feasibility
-
Pathogens on the WHO list in this category include M. tuberculosis (due to sub-optimal effectiveness of BCG vaccine), N. gonorrhoeae, P. aeruginosa, S. aureus and E. coli (urinary):
- There is a strong case for vaccine development for M. tuberculosis given its health impact and AMR threat. However, current difficulties in understanding pathogen biology and translatability of pre-clinical research must be overcome.

- The first vaccine against M. tuberculosis, the Bacillus Calmette–Guérin (BCG) vaccine, was developed 100 years ago
- It is the most widely administered vaccine in the world. BCG provides moderate to good protection against severe forms of TB in infants and young children (averting thousands of paediatric deaths annually).
- However, the efficacy of BCG is variable in preventing adult forms of disease and wanes over time. Moreover, BCG does not reduce transmission of M. tuberculosis.
Pathogens where additional R&D could improve feasibility


Challenges:
-
Biological: Immunity poorly understood, multiple pathogeneic strains
- 4CMenB vaccine, licensed against group B meningococcal infections, also provides protection from gonorrhoea.
- Social: Culture acceptance of vaccine for STD
- Combine N. gonorrhoeae and N. meningitidis

- Four vaccine candidates against ETEC have reached clinical trials and been discontinued or become inactive over the last 10 years
- There are multiple different markets for an ETEC vaccine, including infants in LMICs, travellers and the military.
- Future vaccines may combine protection of ETEC and Shigella
- No active candidates are in clinical development against P. aeruginosa.
- Vaccine candidates have failed to demonstrate reductions in all-cause mortality

- No active candidates are in clinical development against P. aeruginosa.
- Vaccine candidates have failed to demonstrate reductions in all-cause mortality
Even if a promising vaccines for MDR gram-negative are scientifically feasible...
- Very large efficacy trials would be required for low-prevalence disease
- Target population of critically-ill patients with multiple comorbidities and compromised immune responses in ICUs- efficacy endpoints?
- When would the vaccine need to be administered?
- Some high risk groups could be identified-i.e. cystic fibrosis
Clostridium difficile


- Designing clinical trials is complicated as the end point, diarrhoea, is hard to assess, especially in elderly patients, and those who are ill and might be high and due to multiple causes
- The role of the microbiome in modulating the immune response to vaccines is not well understood. In addition, faecal microbiome transplants and biotherapeutic agents have shown recent success against C. difficile and may reduce the need for a vaccine.
- An antibody to C. difficile toxin has been successfully developed, suggesting that if a vaccine could deliver local antibodies in the gut, it may be successful.
Staphylococcus aureus


The scientific challenges of developing a
S. aureus vaccine
- Staphylococcus aureus is part of the normal flora (e.g.,different then S. pneumoniae, N, meningitis)
- S. aureus causes multiple diseases-which should be targeted?
- Multiple virulence factors-which antigens?
- Antibody levels and bactericidal tests do not predict protection from S. aureus infections
Staphylococcus aureus vaccine:
Potential harms

Fowler VG et al. JAMA. 2013 Apr 3;309(13):1368–78.

Fowler VG et al. JAMA. 2013 Apr 3;309(13):1368–78.

Fowler VG et al. JAMA. 2013 Apr 3;309(13):1368–78.
undetectable Il-2 and IL17α on day of immunization strongly correlated with mortality (hyper-immune response?)
- Antigen expression of mRNA vaccines occurs in vivo, which removes the need for some costly and time-consuming manufacturing requirements associated with other vaccine platforms requiring in vitro expression.
- Research is needed to improve the thermostability of mRNA vaccines, which currently require ultra-cold chain for long-term storage.
- Further research is also needed to incorporate multiple antigens into mRNA vaccines and assess safety in infants and children.
- Currently licensed mRNA vaccines are against viral pathogens, further research is needed to understand if viable mRNA bacterial vaccines can be effective
Are mRNA vaccines the future?