Self-assembly
and
beyond
Outline
- What is X?
- Colloidal X
- Nanoscale X
- Beyond X
X = Self-assembly
What is it?
Self-assembly is the formation of structures via the interaction of the constituents.
Most of the time*, self-assembly refers to weakly-interacting components that are able to diffuse and explore configurations until they (may) reach the state of thermodynamic equilibrium.
* Not always, you can have non-equilibrium self-assembly, or modular robots that act deterministically, etc.
Colloidal SA
What? Colloidal spheres (a few microns in size)
How? Depletion attraction. Microparticles surrounded by nanoparticles. When microparticles come close, nanoparticles don't fit in between and entropy then favours microparticles to stick together so that nanoparticles have more free volume to explore
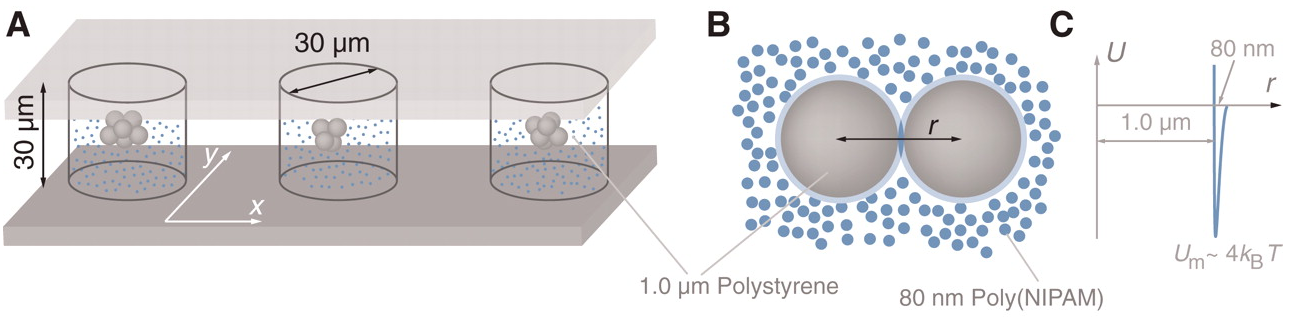
What do we see?
Clusters form that are free energy minima.
Free energy proportional to number of connections.
Therefore minima = maximum number of connections.
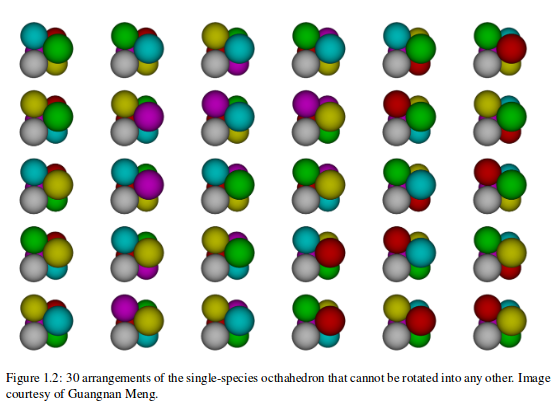
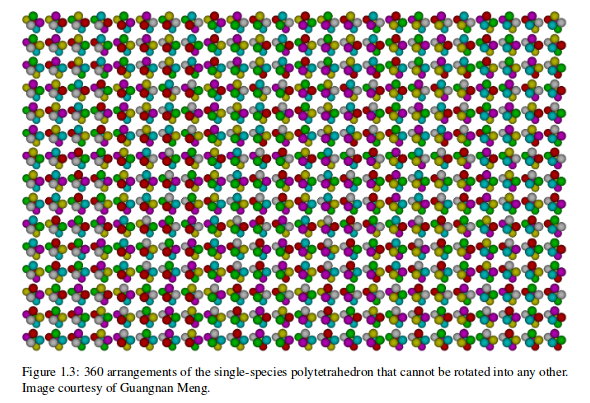
BUT, there are many structures with the same number of connections.
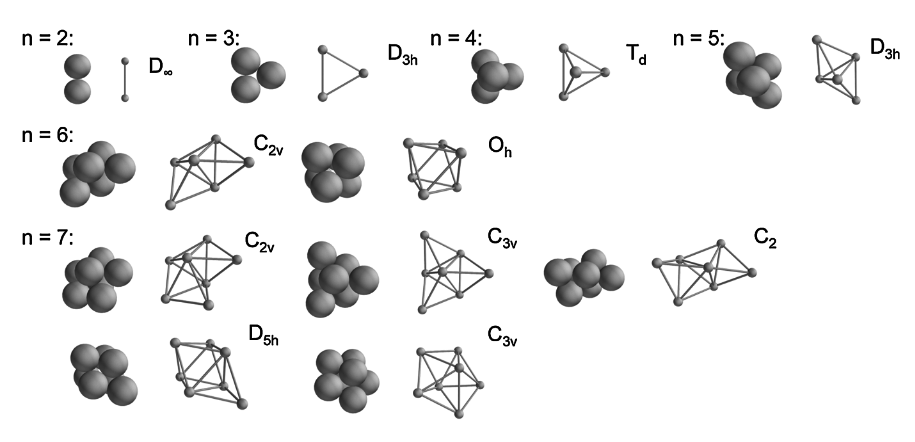
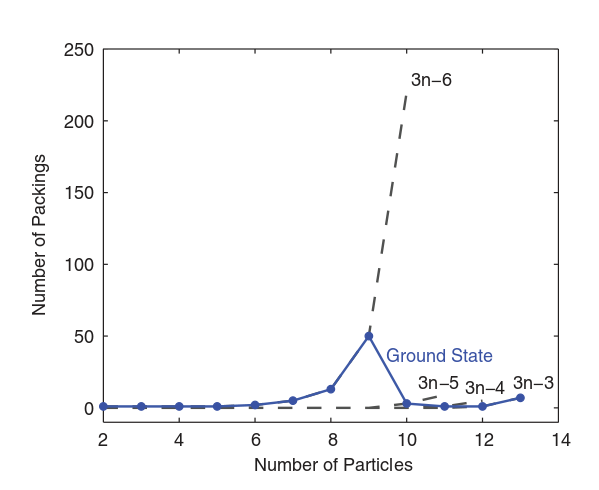
Enumerating them turns out to be surprisingly hard. Need computers' help.
And they are not all the same!
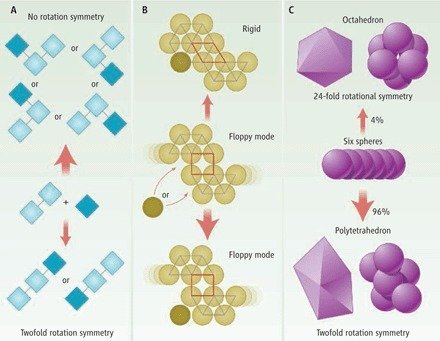
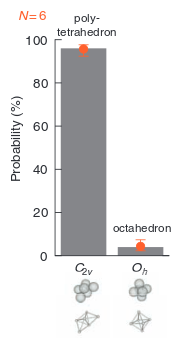
Rotational and vibrational entropy, as well as kinetic effects disfavours symmetry!
Ard Louis has some ideas on a different mechanism for why often symmetry is favoured in Nature. See tomorrow at 2pm
Can we program this?
Yes.
Use DNA coating.
But there are limits
Nanoscale SA
i.e. shrink by 1000
What? DNA
How? Watson-Crick base pairing
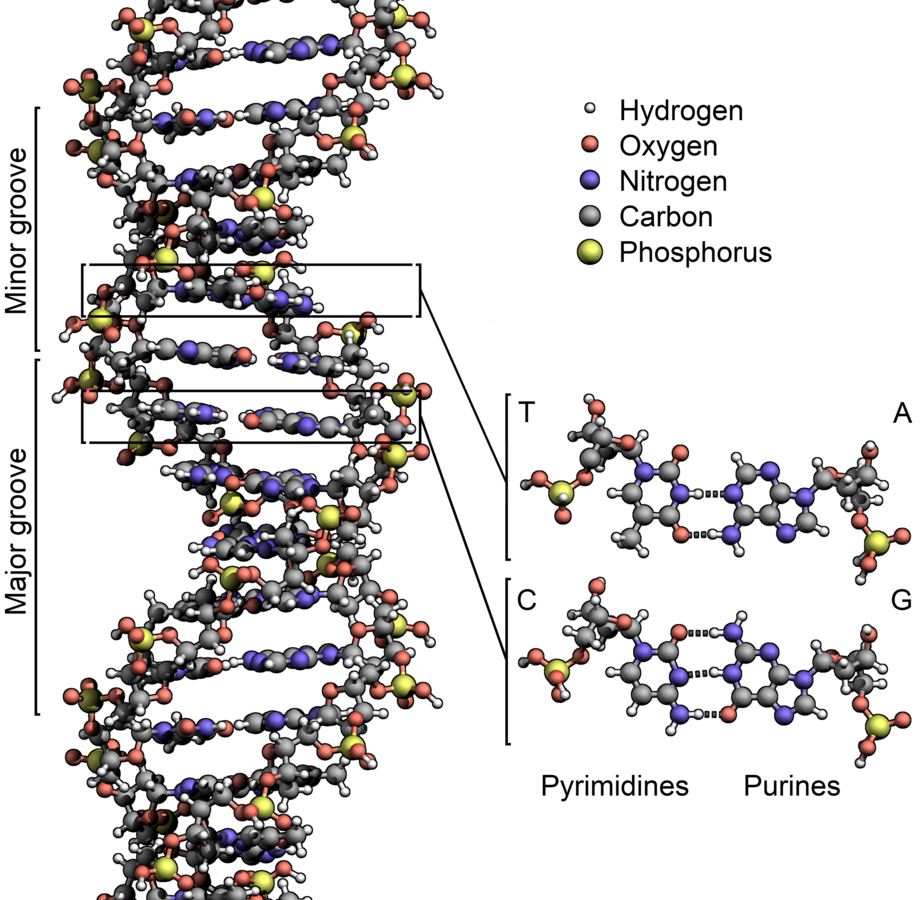
DNA Origami

DNA Origami
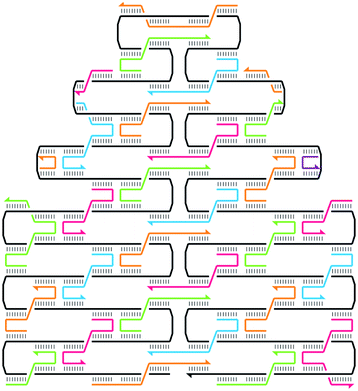
Also variant called DNA bricks, doesn't need base single strand
3D DNA origami

Functional DNA structures (machines)
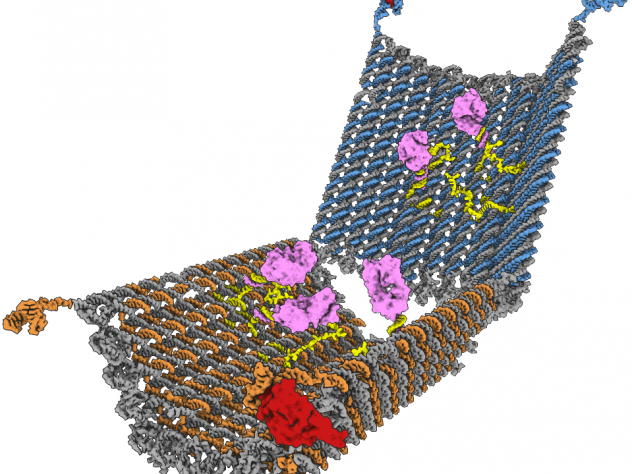
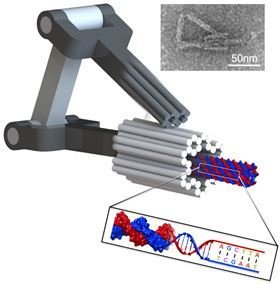
Problems with self-assembly
- Slow (hours, days, weeks, depending)
- Inefficient (low yield, specially for large or complex structures).
Beyond self-assembly
Beyond Equilibrium -> Non-equilibrium self-assembly. Active matter, pattern formation
Hierarchical self-assembly -> Build small building blocks, then put them together, and so on
Direct assembly:
- Guided/patterned assembly
- Build a better tool to help you assemble (life does it with ribosomes)
A Nano 3D printer?
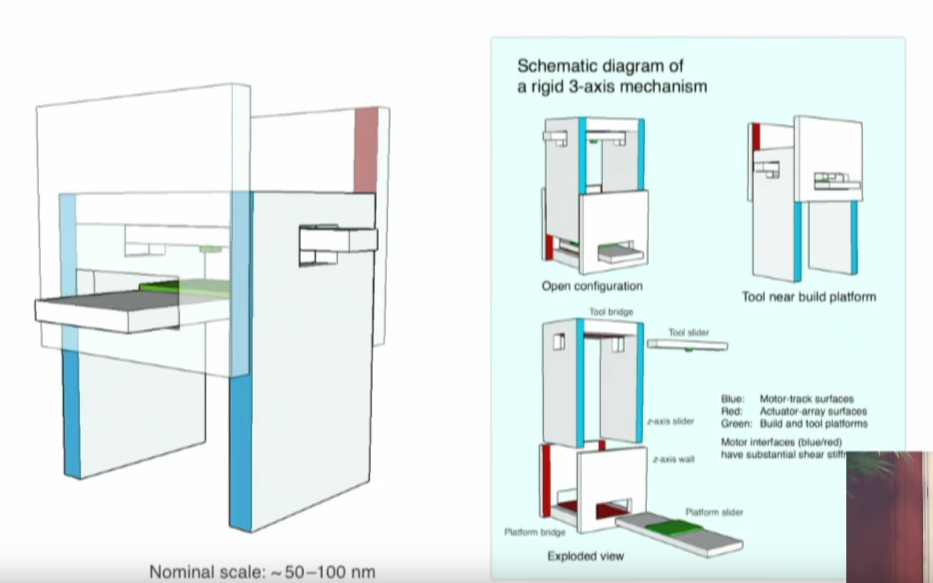
Watch Eric Drexler's presentation on the Oxford Martin School website for more.