Interpreting evolution of SARS-CoV-2 and other viruses
Jesse Bloom
Fred Hutch Cancer Center / HHMI
These slides at https://slides.com/jbloom/scripps-circuits-to-systems-2024

The Faroe Islands


"Measles had not prevailed on the Faroes since 1781, then it broke out early in April 1846."
"Of the 7782 inhabitants, about 6000 were taken with measles."
"Of the many aged people still living in the Faroes who had measles in 1781, not one was attacked the second time."
Panum was describing immune memory, which provides lifelong protection from measles.


What was reason for thinking coronavirus immunity would not be affected by viral mutations?
Coronaviruses have lower mutation rate than other RNA viruses
Coronaviruses are only RNA viruses with proofreading activity in their polymerase, and so have ~5- to 10-fold lower mutation rate than influenza virus
But rate is still high enough that evolution is not mutation-limited
The average single-nucleotide mutation to SARS-CoV-2 had occurred >10,000 independent times in human-transmitted SARS-CoV-2 by third year of pandemic.
Add some plot here
Evolution is not just mutation: also involves selection on phenotypic effects of mutations
We first asked: what is net effect of mutations to a coronavirus over decades of evolution in humans?
CoV-229E causes common colds and has been circulating in humans for a long time.
Typical person is infected every ~3 to 5 years.
We first asked: what is net effect of mutations to a coronavirus over decades of evolution in humans?
Evolution of CoV-229E spike

We experimentally generated CoV-229E spikes at ~8 year intervals so we could study them in the lab:
- 1984
- 1992
- 2001
- 2008
- 2016
Evolution of CoV-229E spike erodes neutralization by human serum antibodies
Strongest evolutionary selection in RBD
Sites of evolutionary change in the spike of CoV-229E over the last four decades
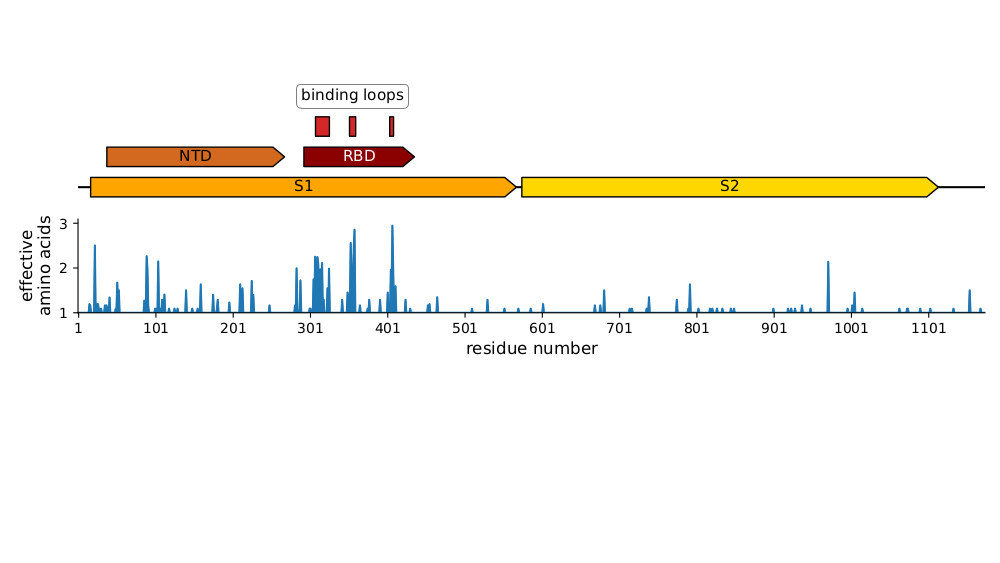
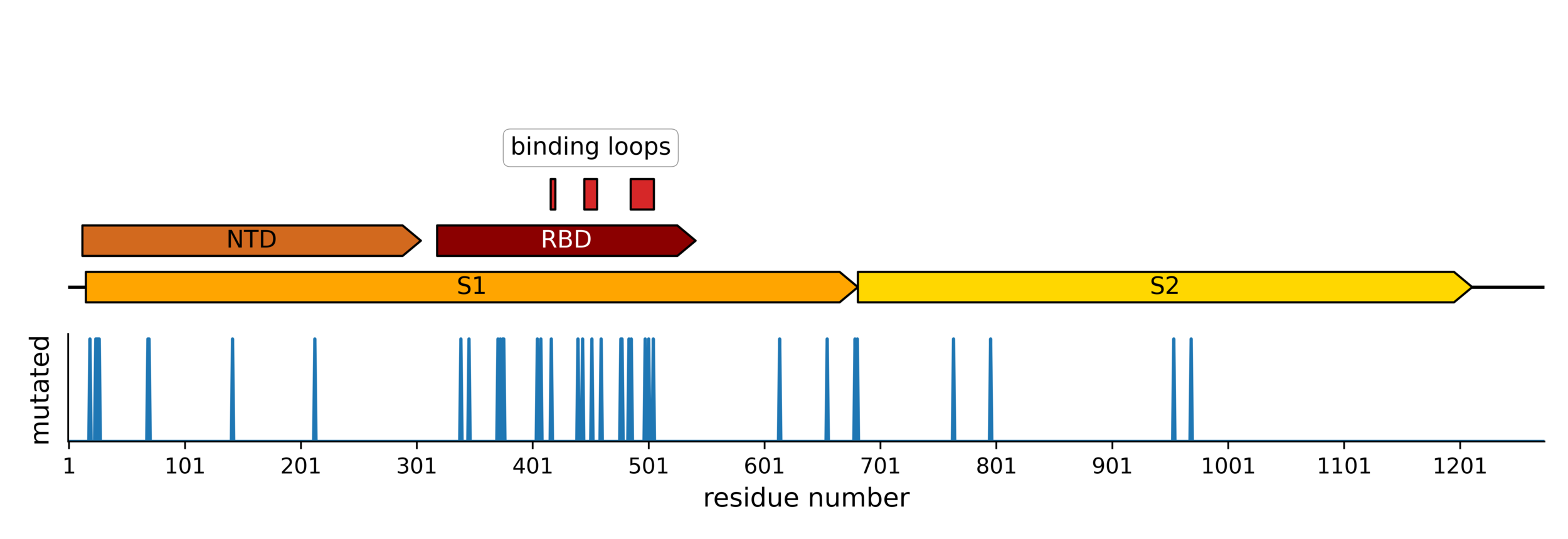
Sites of mutations in SARS-CoV-2 Omicron BQ.1.1 spike relative to Wuhan-Hu-1

Ideally vaccines would elicit evolution-resistant neutralizing antibodies (like those made by person at right) rather than evolution-sensitive antibodies (like those made by person at left)
Evolution erodes neutralization by sera from different people at different rates
We now know SARS-CoV-2 evolves to erode neutralizing antibodies, just like CoV-229E
neutralization from original COVID-19 vaccine
Original vaccine induced hight neutralizing antibody titers against early viral strains
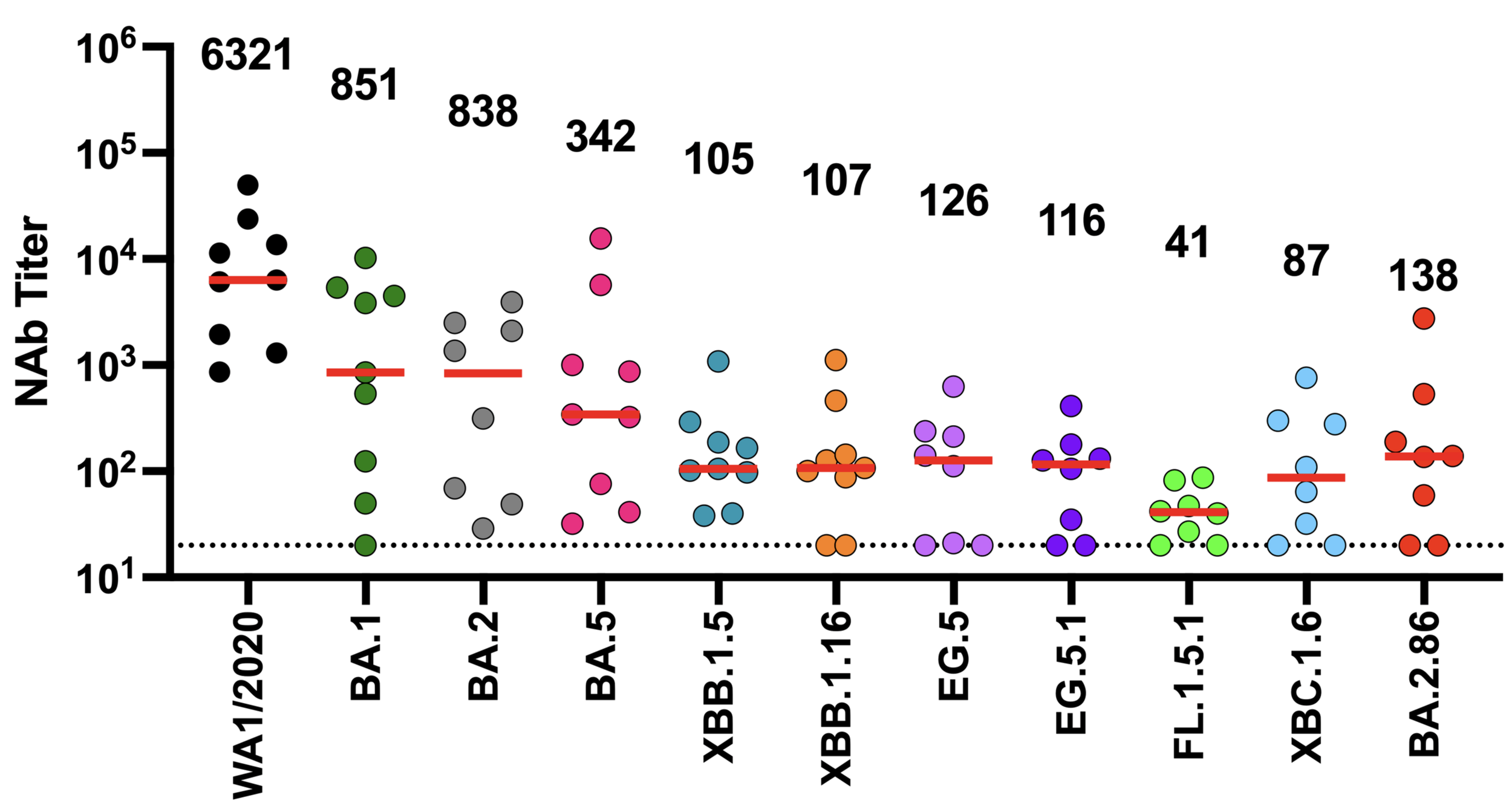
We now know SARS-CoV-2 evolves to erode neutralizing antibodies, just like CoV-229E
newer viral variants
neutralization from original COVID-19 vaccine

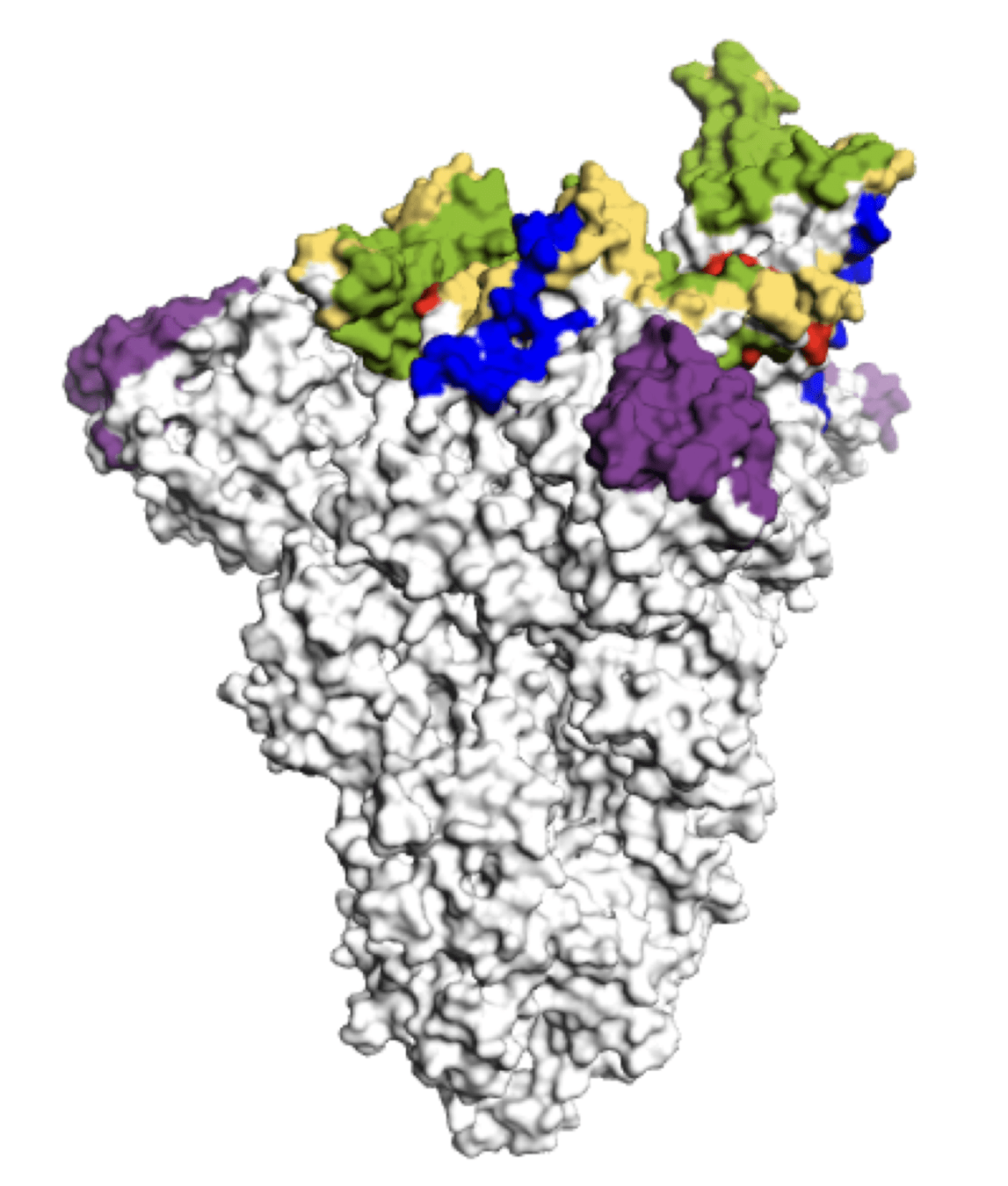
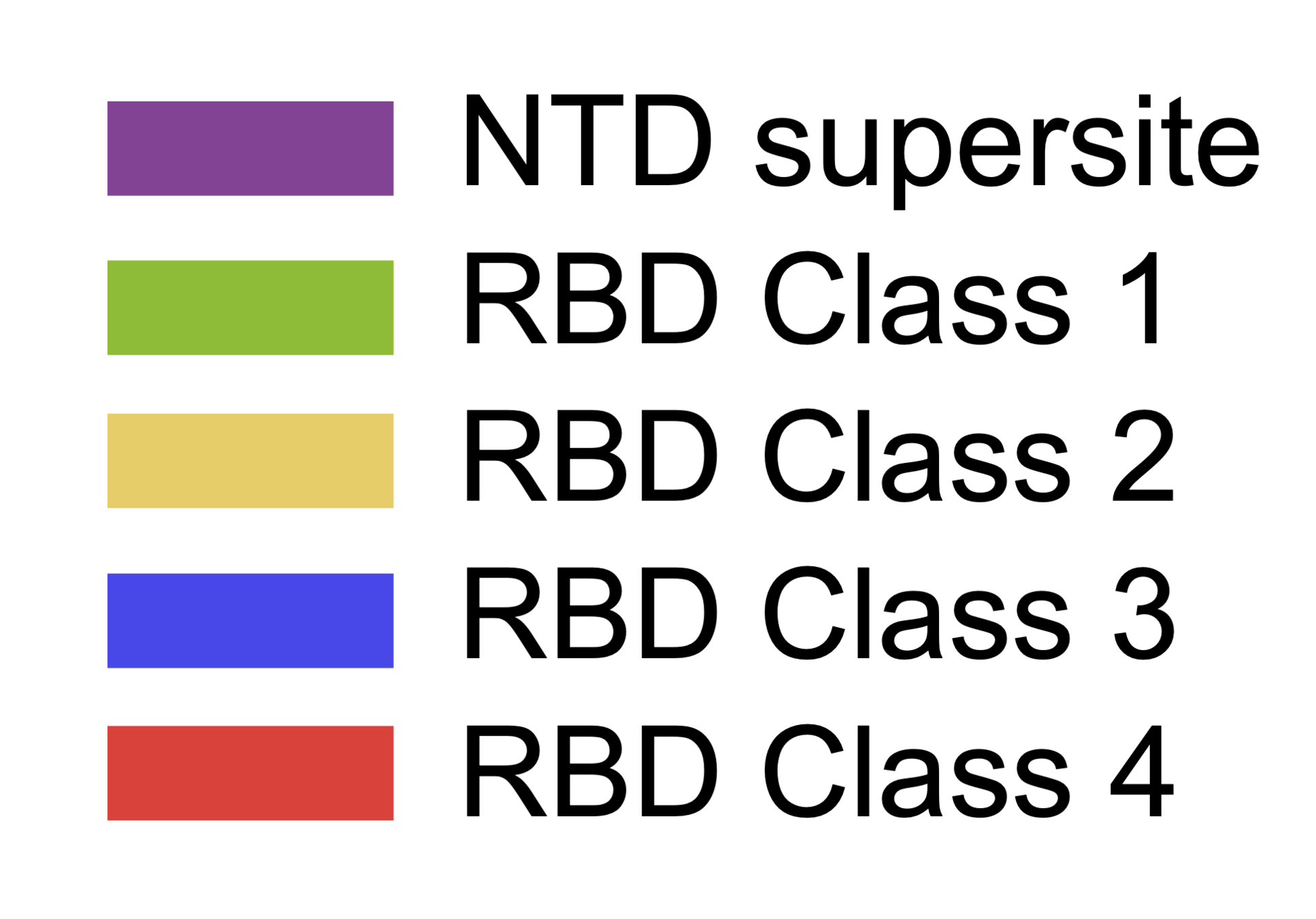
Main regions where neutralizing antibodies bind
Molecular basis of this antigenic evolution?

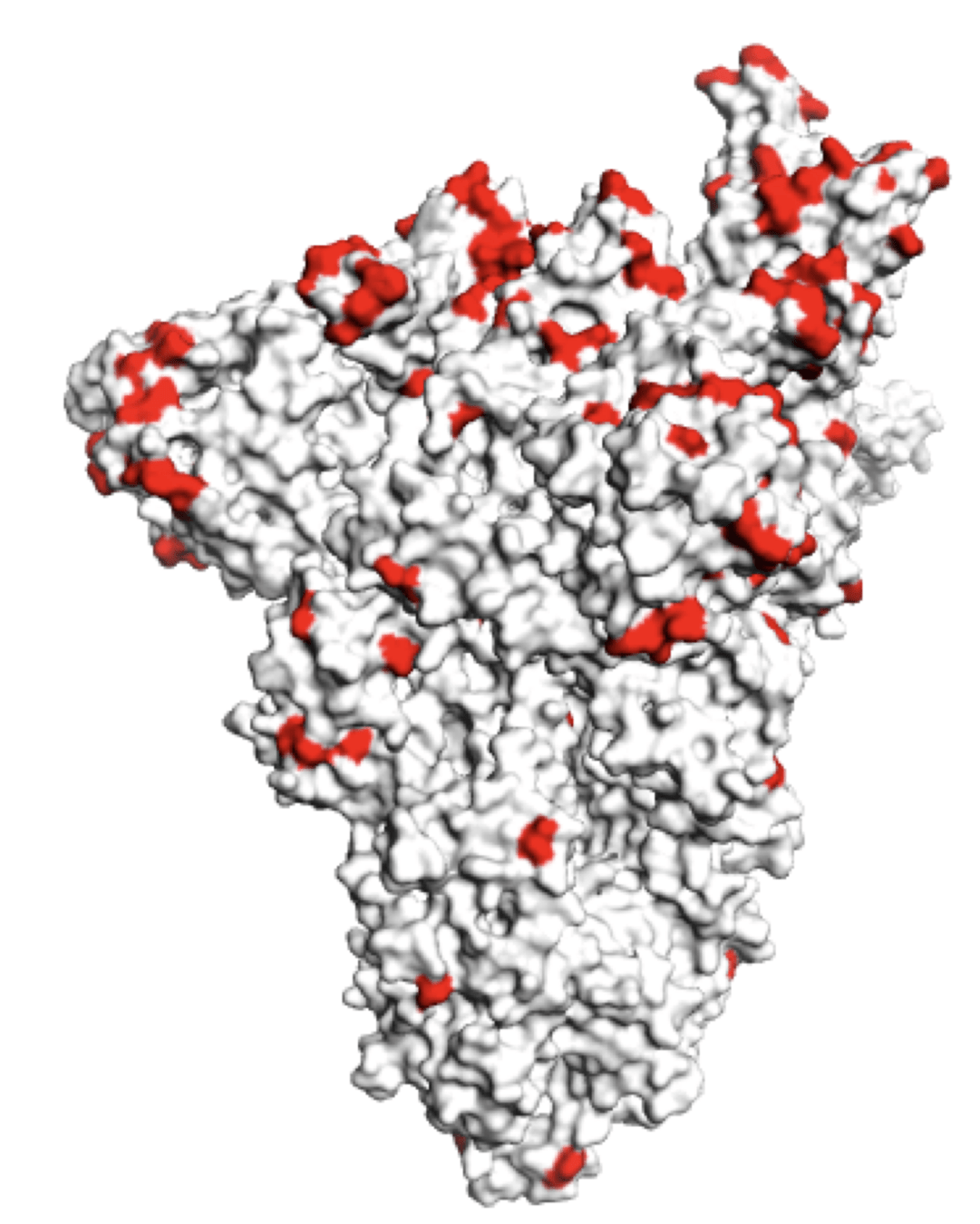

Molecular basis of this antigenic evolution?Mutations where antibodies bind
Main regions where neutralizing antibodies bind
Sites of mutations in recent (BA.2.86) SARS-CoV-2 strain relative to early 2020 strain
So overall, some human RNA viruses evolve to escape immunity, others don't
Why only some RNA viruses evolve to escape immunity is a deep question that has not been fully answered (see here for some theories).
But all these viruses have high mutation rates, so explanation has more to do with phenotypic effects of mutations than rate at which they arise.
Rate of viral antigenic evolution
Measles
Mumps
Influenza
SARS-CoV-2
1. Define a virus's evolutionary potential (eg Starr et al 2020; Starr et al 2022)
2. Quantify how easily a virus can escape a specific antibody or vaccine (eg Greaney et al 2021; Carr et al 2024)
3. Forecast and interpret viral evolution (eg, Dadonaite et al 2024)
Measuring phenotypic effects of viral mutations can enable us to:
1. Define a virus's evolutionary potential (eg Starr et al 2020; Starr et al 2022)
2. Quantify how easily a virus can escape a specific antibody or vaccine (eg Greaney et al 2021; Carr et al 2024)
3. Forecast and interpret viral evolution (eg, Dadonaite et al 2024)
Measuring phenotypic effects of viral mutations can enable us to:
All viruses have entry proteins, which are critical determinants of host range and immunity
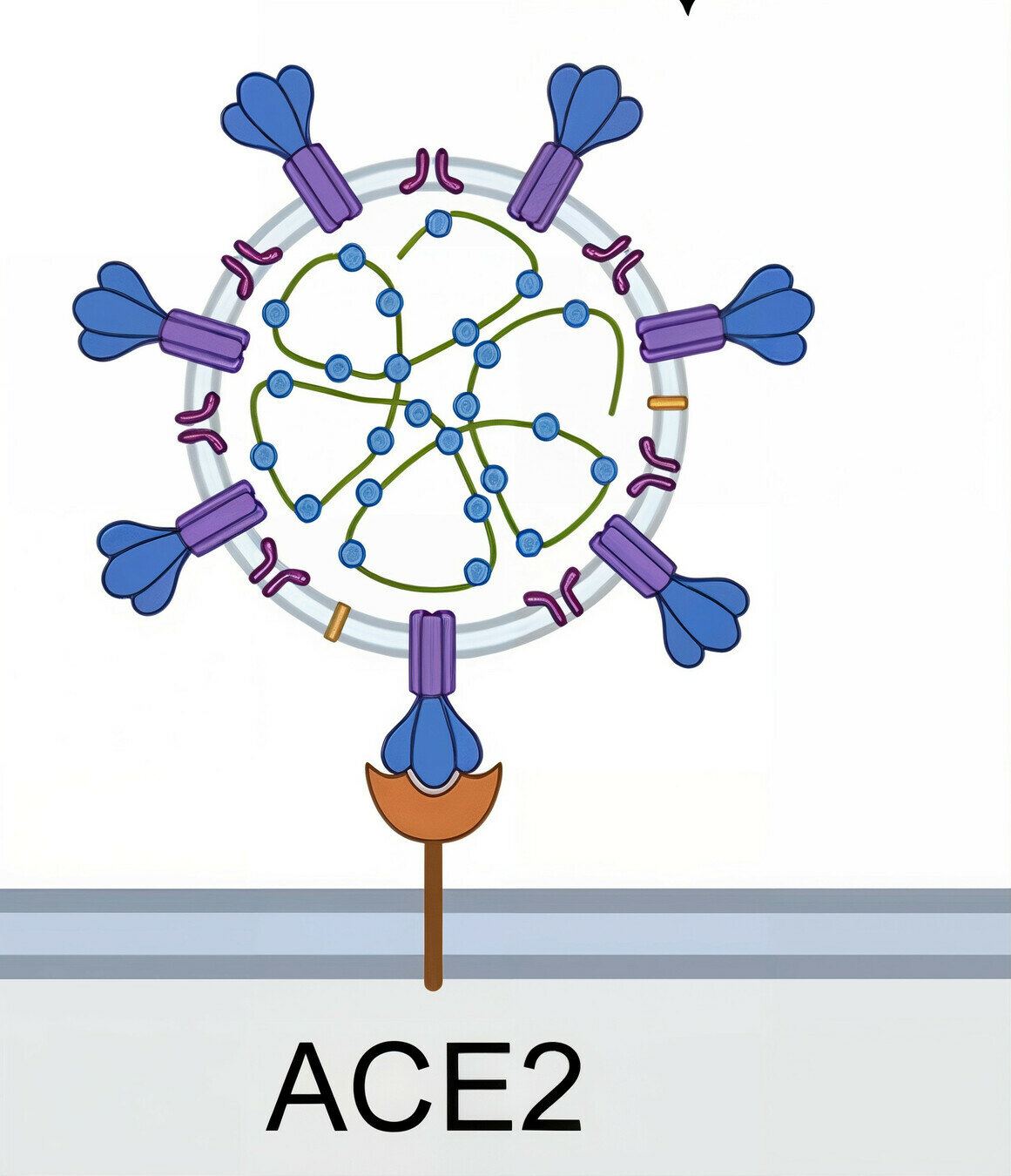
target cell membrane
SARS-CoV-2 virion
Example: SARS-CoV-2's entry protein spike first binds ACE2 on target cells
spike protein
Image adapted from here
ACE2
After binding receptor, spike then fuses the viral and cell membranes to enable viral entry
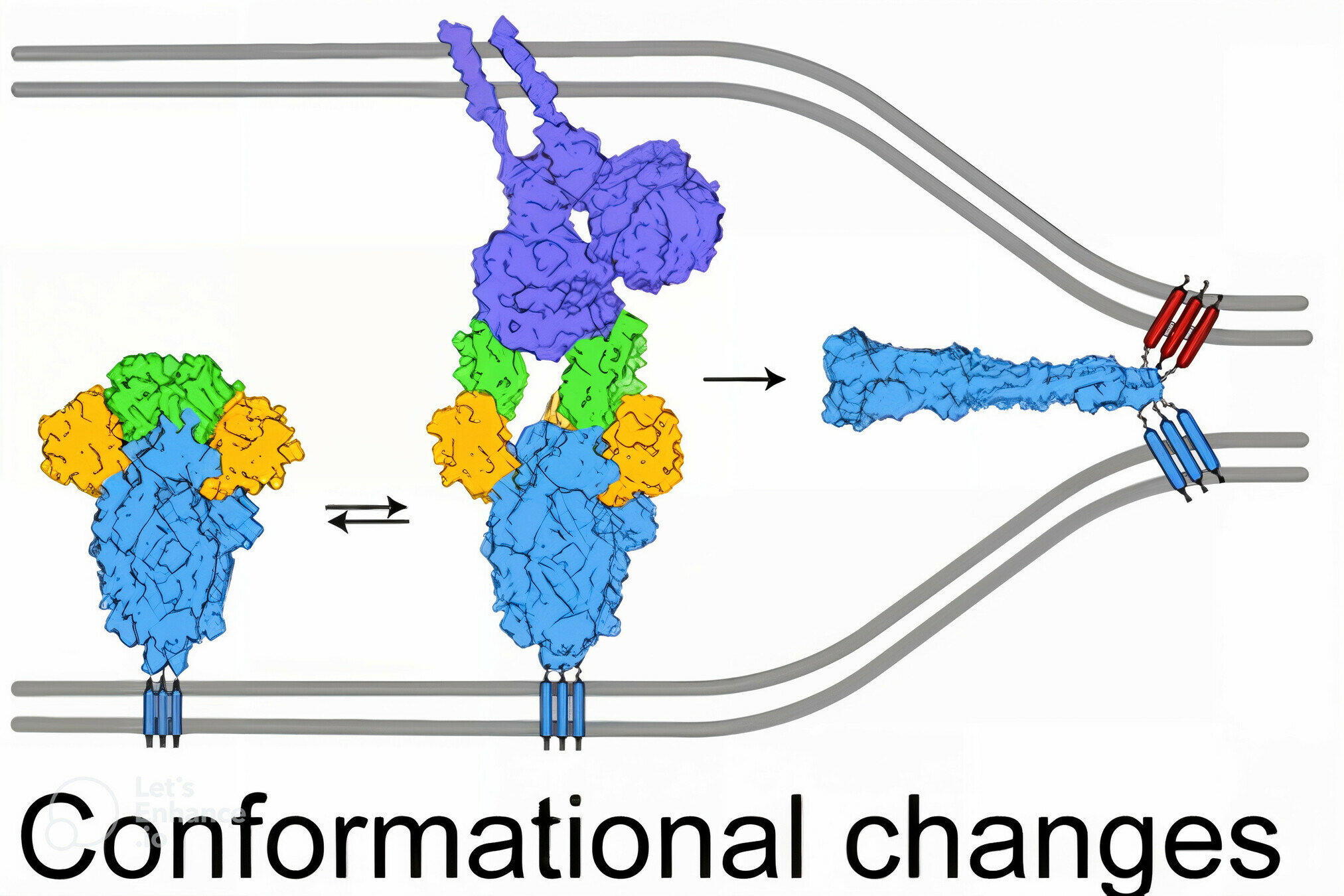
viral membrane
cell membrane
spike
spike conformational change
Image adapted from here
ACE2
Antibodies to spike can block viral entry, such as by preventing receptor binding or fusion

antibody
Image adapted from here
Deep mutational scanning to measure effects of mutations to viral entry proteins
Deep mutational scanning involves pooled selections on libraries of protein mutants, read out by deep sequencing


Initially we used yeast display of receptor binding domain (RBD) of SARS-CoV-2 spike
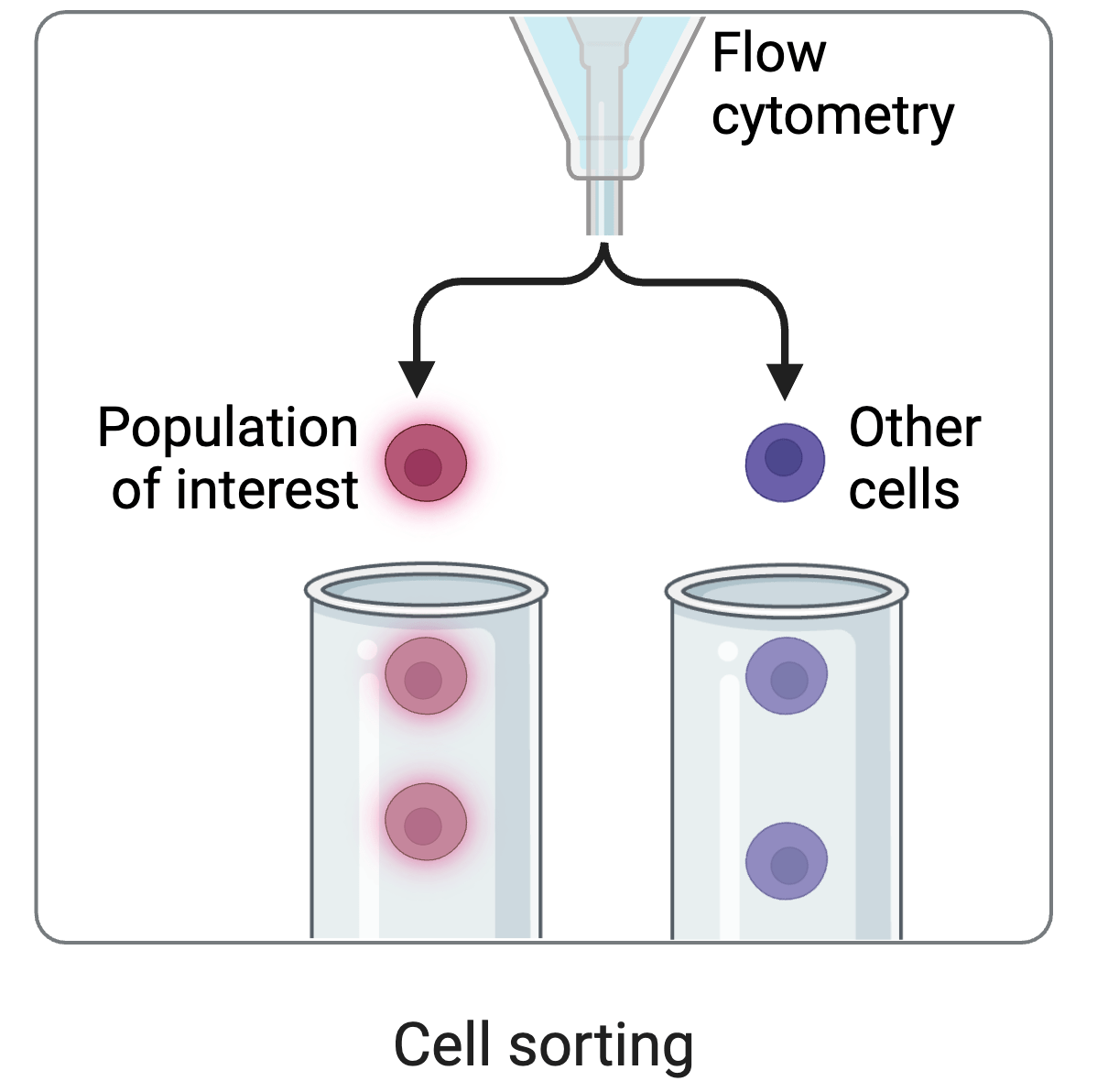
cell sorting
RBD
fluorescent ACE2
yeast
fluorescent tag on RBD
Yeast display allows us to separate RBD mutants by how strongly they bind ACE2
In 2020, we identified N501Y as affinity-enhancing mutation; by 2021 it was widespread in SARS-CoV-2
RBD
fluorescently labeled antibody
yeast
fluorescent tag on RBD
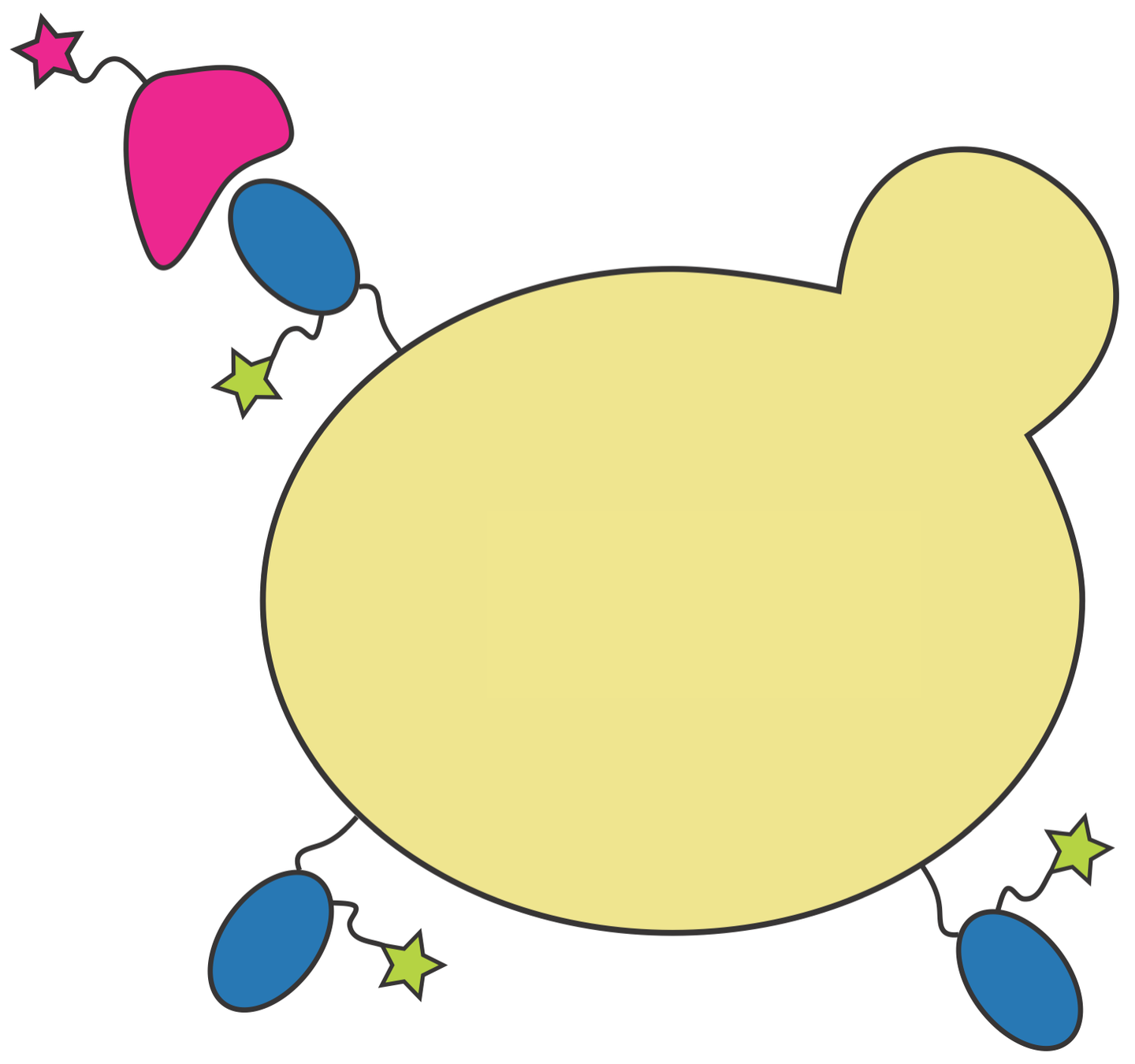
We also used yeast display to measure how all RBD mutations affect antibody binding
See https://jbloomlab.github.io/SARS2-RBD-escape-calc/ for interactive version of this antibody escape calculator (data from our lab and Yunlong Cao's), which has been used >100,000 times

site in RBD
antibody escape
We identified E484 and K417 as key sites of antibody escape; by 2021 mutations at both sites were common in SARS-CoV-2
484
417
These anecdotes suggest experiments can help predict viral evolution

But we wanted more relevant experiments: yeast display only measures binding


cell sorting
Viral infection is more biologically relevant measure of mutation effects

However, we are cognizant of biosafety concerns of mutating actual virus

actual SARS-CoV-2 virion: pathogen capable of spread in humans
pseudotyped lentiviral particle: not a pathogen, cannot spread in humans

actual SARS-CoV-2 virion: pathogen capable of spread in humans
pseudotyped lentiviral particle: not a pathogen, cannot spread in humans
Therefore we use pseudoviruses rather than actual replicative SARS-CoV-2
Pseudoviruses are modified viruses that can only undergo single round of infection


We need to link phenotype (spike mutant) and genotype (sequence encoded by virus)
To link genotype to phenotype link, we encode spike in viral genome with barcode
We then create genotype-phenotype linked libraries by two-step process
Result is library of spike mutant pseudoviruses with identifying barcodes
- Captures full cell-entry function of spike
- Each mutant identified by short barcode
- Pseudoviruses safe at biosafety-level 2
- Broadly applicable to viral entry proteins
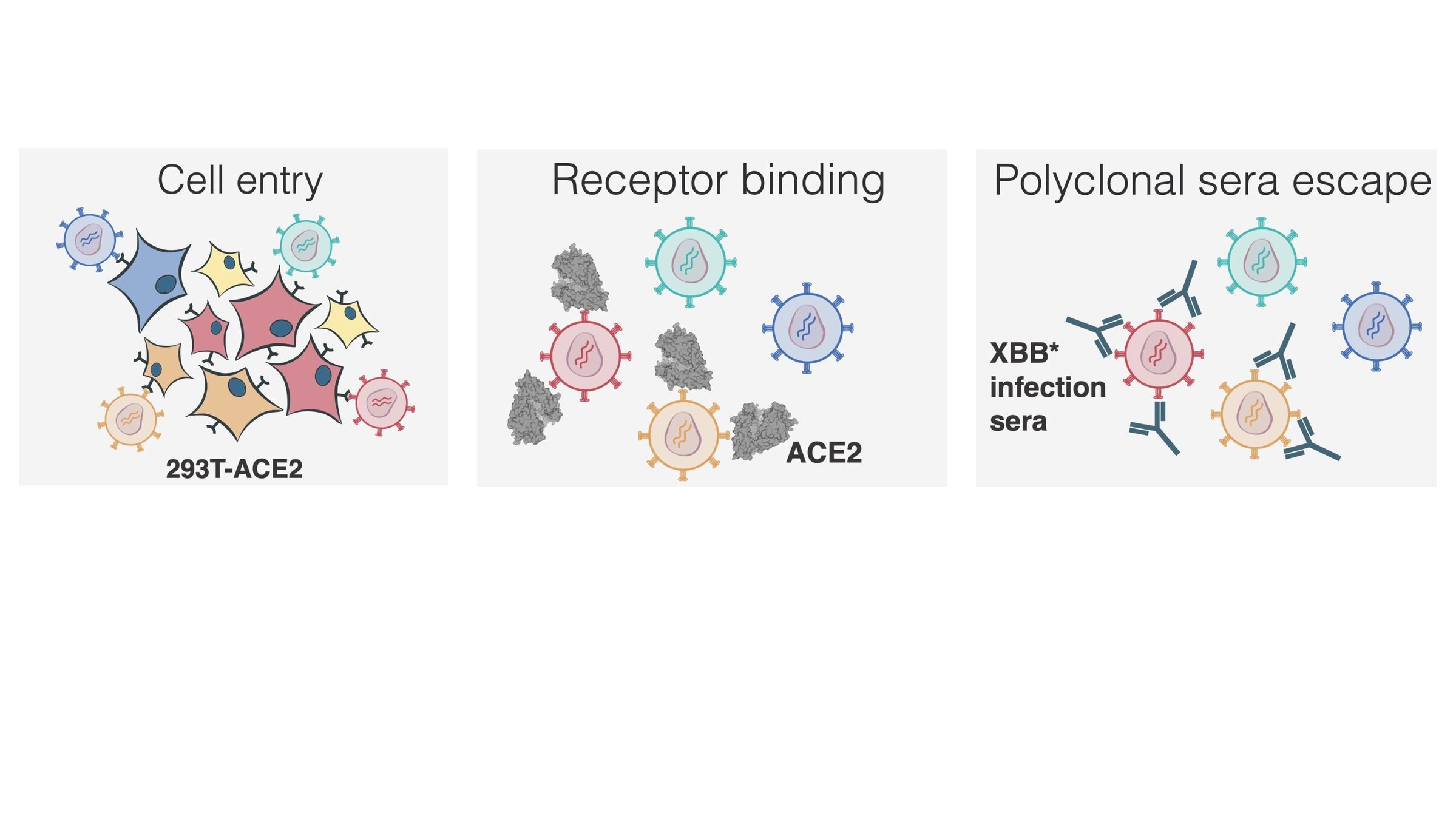
Measured how spike mutations to XBB.1.5 spike affect three molecular phenotypes

Measured how spike mutations to XBB.1.5 spike affect three molecular phenotypes

Measured how spike mutations to XBB.1.5 spike affect three molecular phenotypes
Neutralization by soluble ACE2 is proportional to ACE2 binding affinity
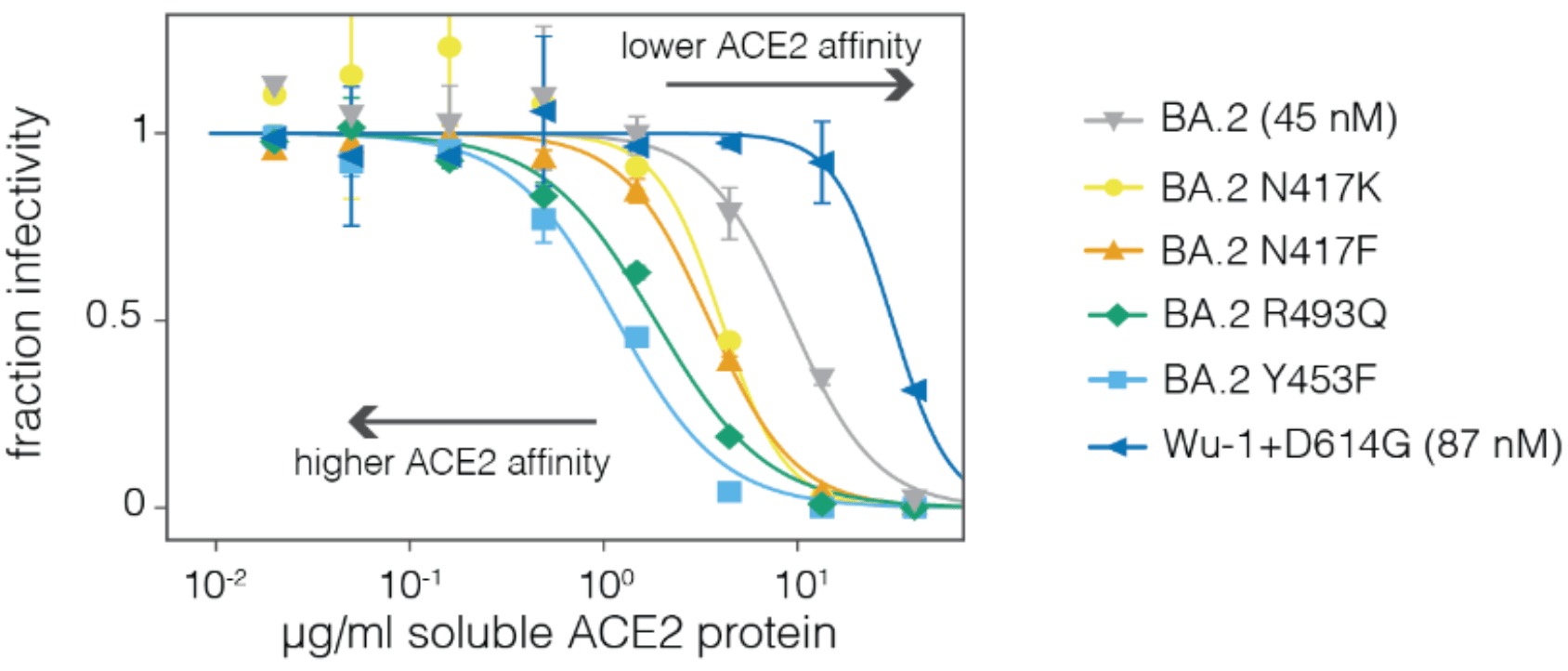
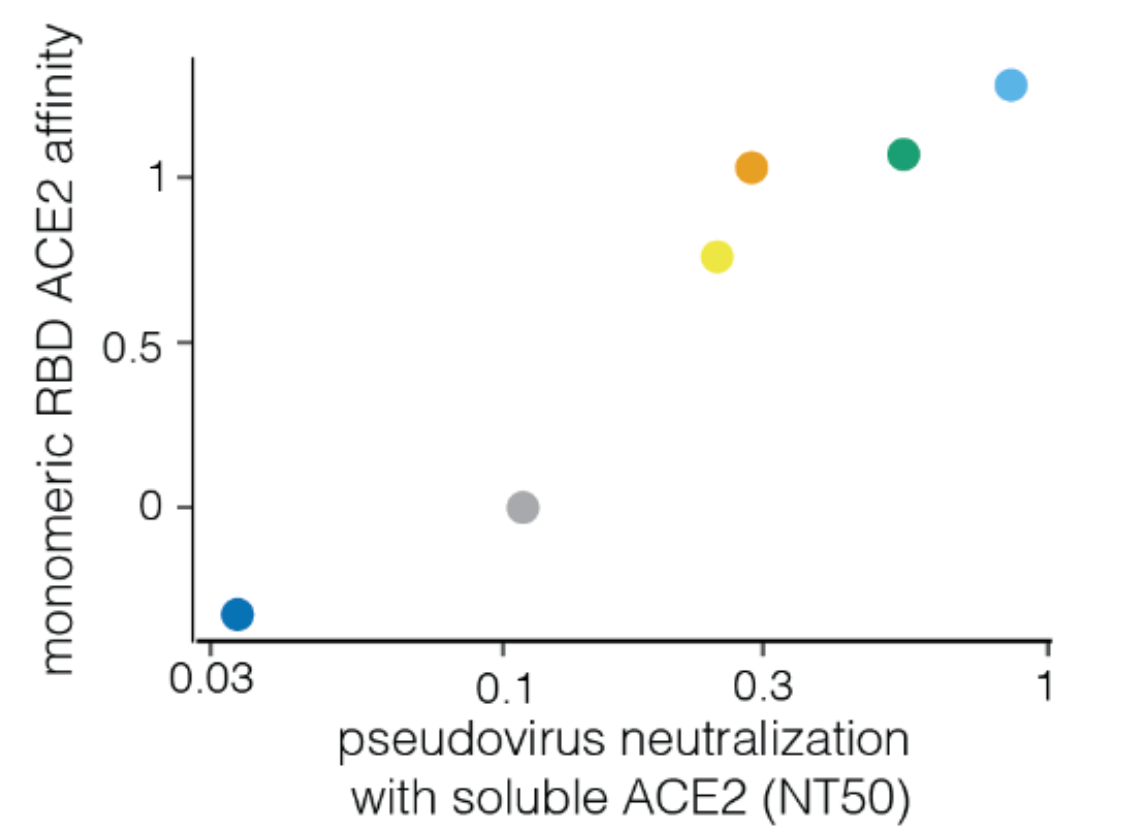

Measured how spike mutations to XBB.1.5 spike affect three molecular phenotypes
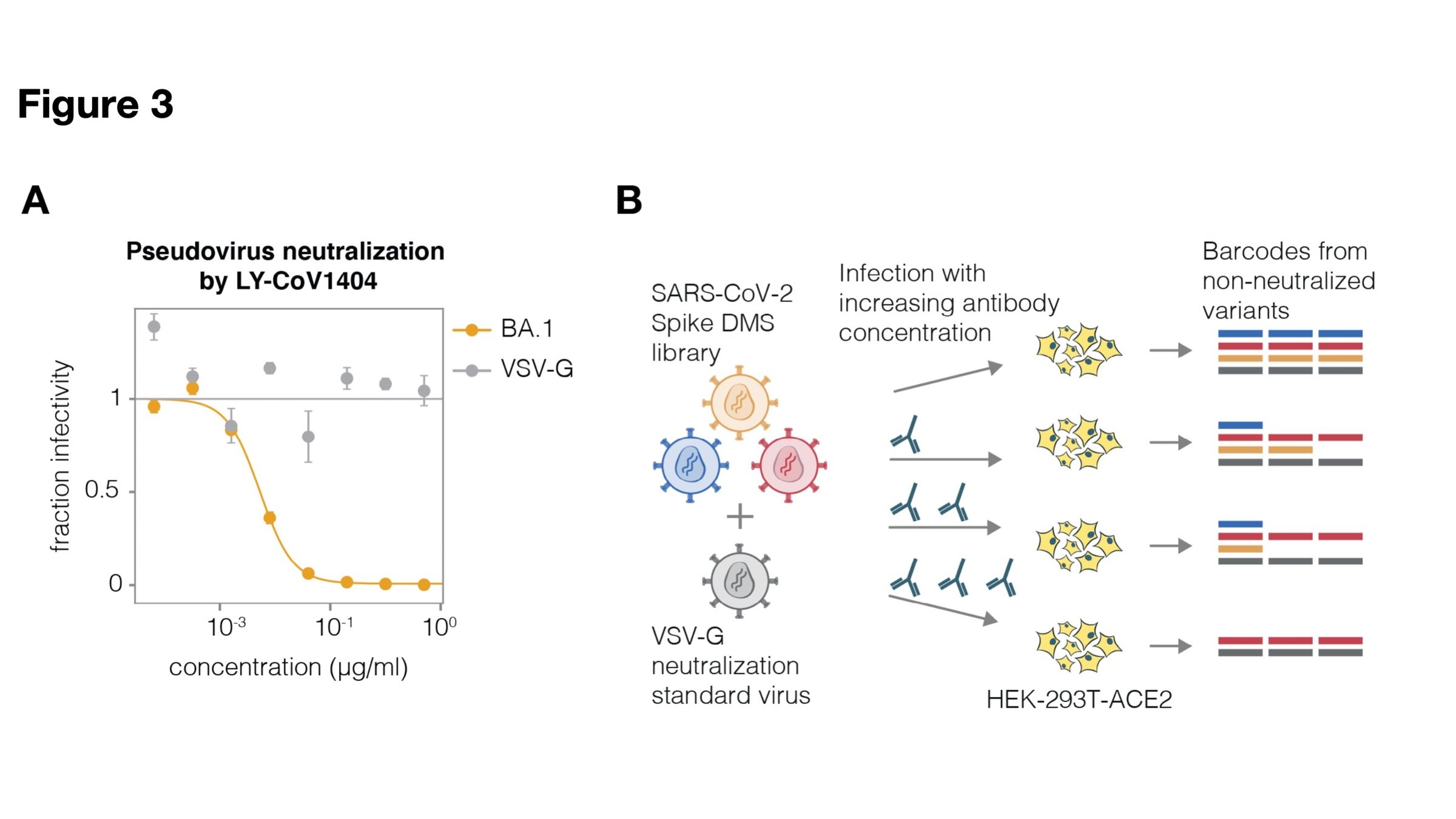
Full workflow for measure effects of mutations on antibody neutralization
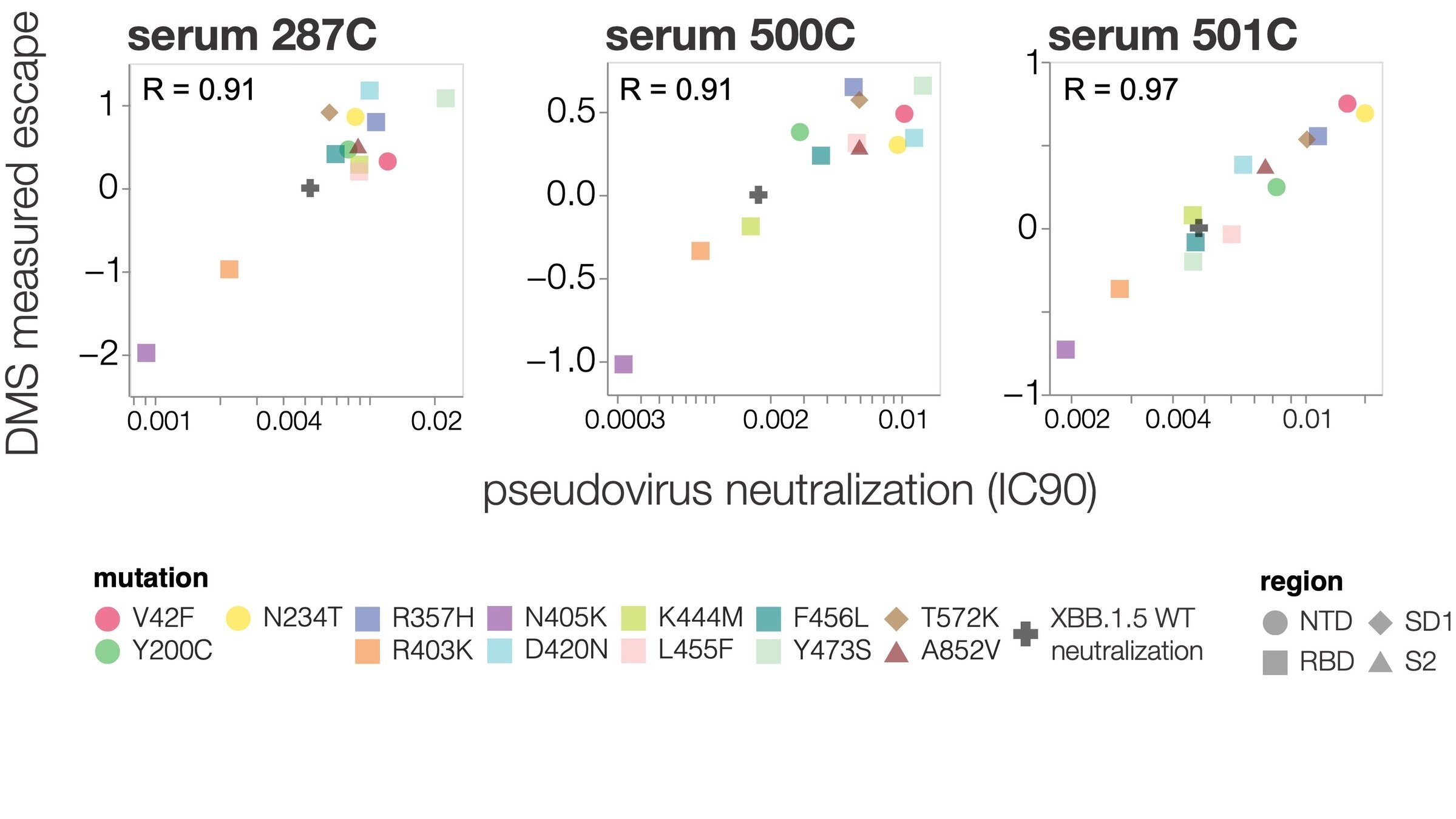
Deep mutational scanning correlates well with traditional neutralization assays
We next estimated actual fitness (growth rate) of SARS-CoV-2 variants in humans
Fitness from multinomial logistic regression
With Trevor Bedford & Ben Murrell
Fitness from multinomial logistic regression
With Trevor Bedford & Ben Murrell
We analyze changes in clade growth rather than raw clade growth
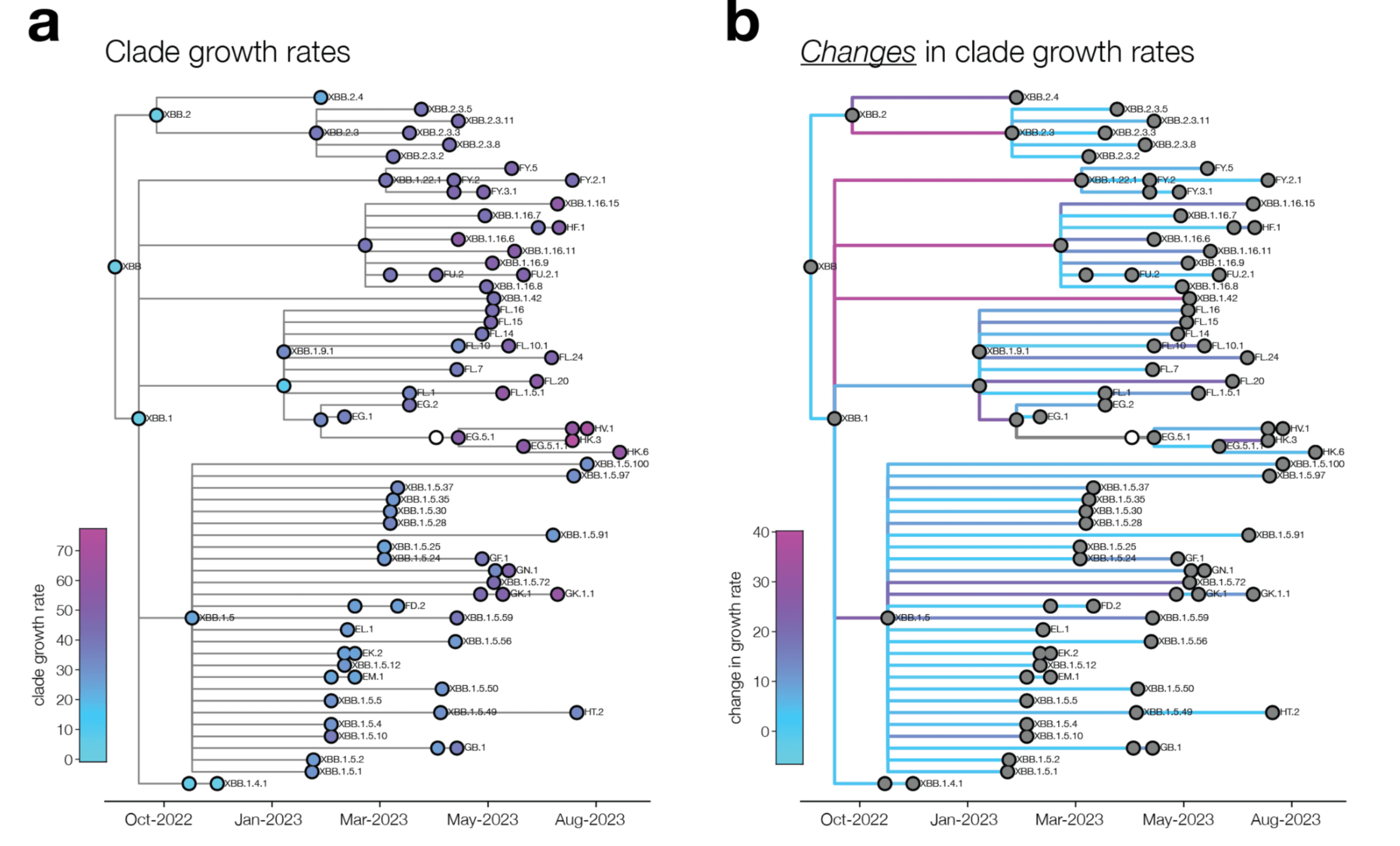
change in clade growth
clade growth
Deep mutational scanning measurements correlate with SARS-CoV-2 variant growth
Combining phenotypes offers best predictions (because mutations can involve tradeoffs)
Can partially predict evolution in real-world from experiments!
Extending approach to other viruses
- SARS-CoV-2 spike
- influenza hemagglutinin (HA)
- HIV envelope protein
- Lassa virus glycoprotein
- Nipah virus RBP and F proteins
- RSV G and F proteins
- Rabies G protein
- Chikungunya virus E proteins
All enveloped viruses have entry proteins
- SARS-CoV-2 spike
- influenza hemagglutinin (HA)
- HIV envelope protein
- Lassa virus glycoprotein
- Nipah virus RBP and F proteins
- RSV G and F proteins
- Rabies G protein
- Chikungunya virus E proteins
All enveloped viruses have entry proteins
Pseudovirus of clade 2.3.4.4b H5 HA
HA molecular phenotypes relevant to pandemic risk

HA molecular phenotypes relevant to pandemic risk

HA molecular phenotypes relevant to pandemic risk

HA molecular phenotypes relevant to pandemic risk

HA molecular phenotypes relevant to pandemic risk
These data can inform surveillance of H5 influenza viruses spreading in animals
We prospectively identified A160T as strongly reducing neutralization by sera elicited by candidate vaccine strains

(L122Q, A160T, T199I)
(L122Q, P162Q, T199I)
A160T in recent human case in Missouri
Conclusions
For human endemic (SARS-CoV-2) and potential pandemic (H5N1) viruses, we can safely measure how mutations to entry proteins affect key molecular phenotypes.
For SARS-CoV-2, these measurements can help predict success of variants in humans.
For H5N1, these measurements can help inform surveillance of viral evolution.
Bloom lab
Bernadeta Dadonaite
Kate Crawford
Caelan Radford
Tyler Starr
Allie Greaney
Rachel Eguia
William Hannon
Jenny Ahn
Fred Hutch Cancer Center
Trevor Bedford
John Huddleston
University of Washington
Helen Chu and HAARVI cohort
Neil King
David Veesler




Thanks


Pirbright Institute
Thomas Peacock
University of Pennsylvania
Scott Hensley
Louise Moncla
Jordan Ort
St Jude Children's Hospital
Richard Webby