Temperature & Thermodynamics
Thermodynamics is the study of general properties of macroscopic physical systems in states of thermal equilibrium, and the transformation between such states.
To start with, we would like to
- Define Temperature and describe it qualitatively
- Define Thermal equilibrium
- State the zeroth law of thermodynamics
- Get familiar with common thermometers and temperature scales
- Understand the law of thermal expansion
Thermodynamics
Temperature
Objectives
How is temperature defined?
- Operationally: Temperature is what you measure with a thermometer!
Thermodynamics
Temperature
Definition
What is a thermometer?
- It's an instrument that exploits some specific thermometric property to measure temperature to a calibrated temperature scale.

A Thermometric Property is ...
- a physical property that varies with temperature.
Thermodynamics
Temperature
Thermometric properties
Examples of thermometric properties:
- The volume of a gas or liquid under constant pressure.
- The pressure of a constant volume of gas.
- The electrical resistance of a conductor.
- The color of black-body radiation.
- Thermoelectric effect.
- ...
Liquid in Glass
Thermometer
Thermodynamics
Temperature
Examples of thermometers

Non-contact
Thermometer
Thermodynamics
Temperature
Examples of thermometers
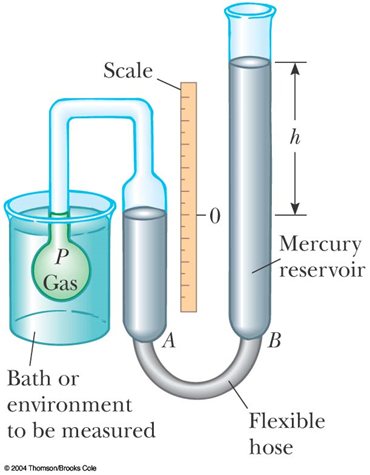
Constant-Volume Gas Thermometer
Thermodynamics
Temperature
Examples of thermometers
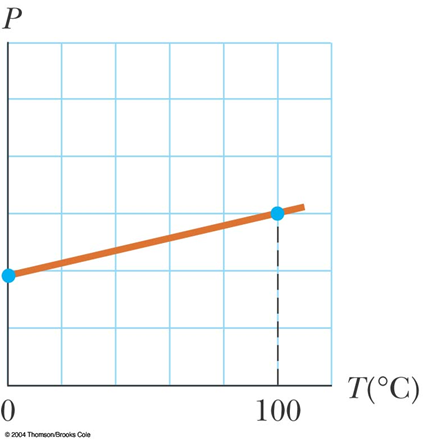
The Pressure
(of a constant-volume of gas)
varies linearly
with Temperature.

Bi-metallic strip
Thermodynamics
Temperature
Examples of thermometers



Thermocouples
Thermodynamics
Temperature
Examples of thermometers
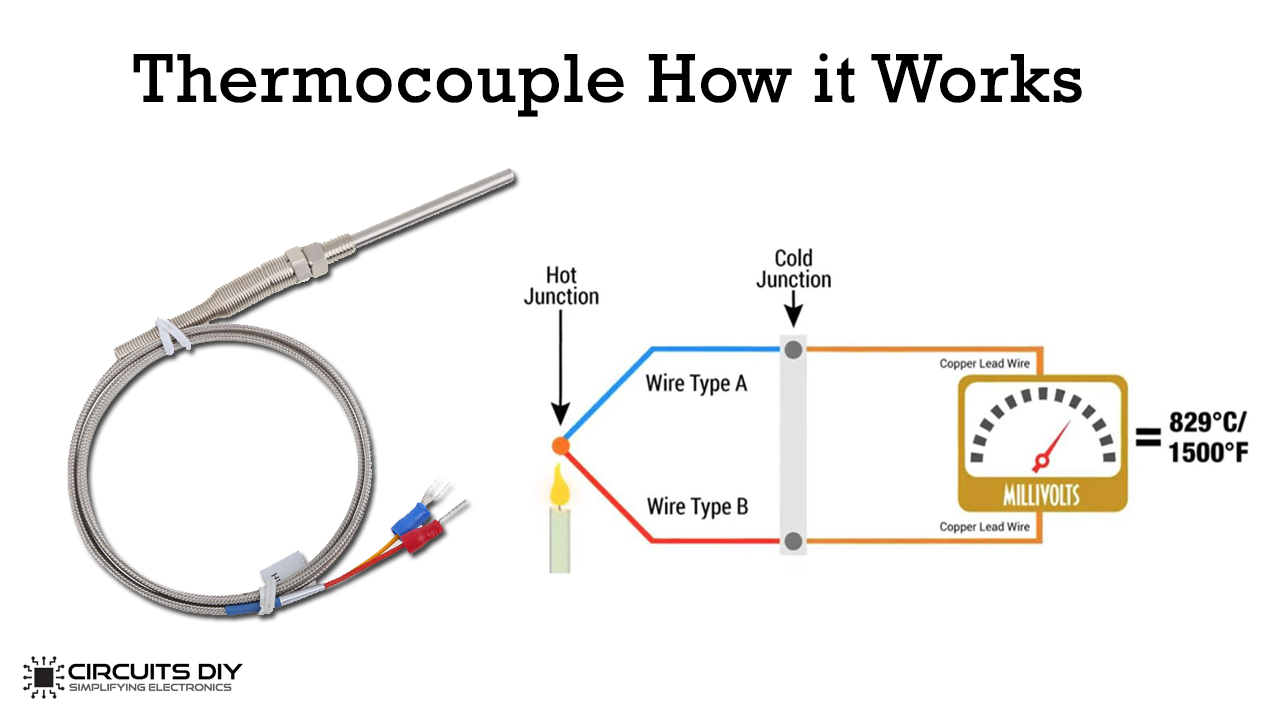
Others
Thermodynamics
Temperature
Examples of thermometers
Common Temperature Scales
Thermodynamics
Temperature
Temperature scales
Common Temperature Scales
Thermodynamics
Temperature
Temperature scales
Fahernheit
Common Temperature Scales
Thermodynamics
Temperature
Temperature scales
Celsius
Common Temperature Scales
Thermodynamics
Temperature
Temperature scales
Kelvin
Common Temperature Scales
Thermodynamics
Temperature
Temperature scales
Rankine
Thermodynamics
Temperature
Converting between scales
Thermodynamics
Temperature
Converting between scales
Thermodynamics
Temperature
Converting between scales
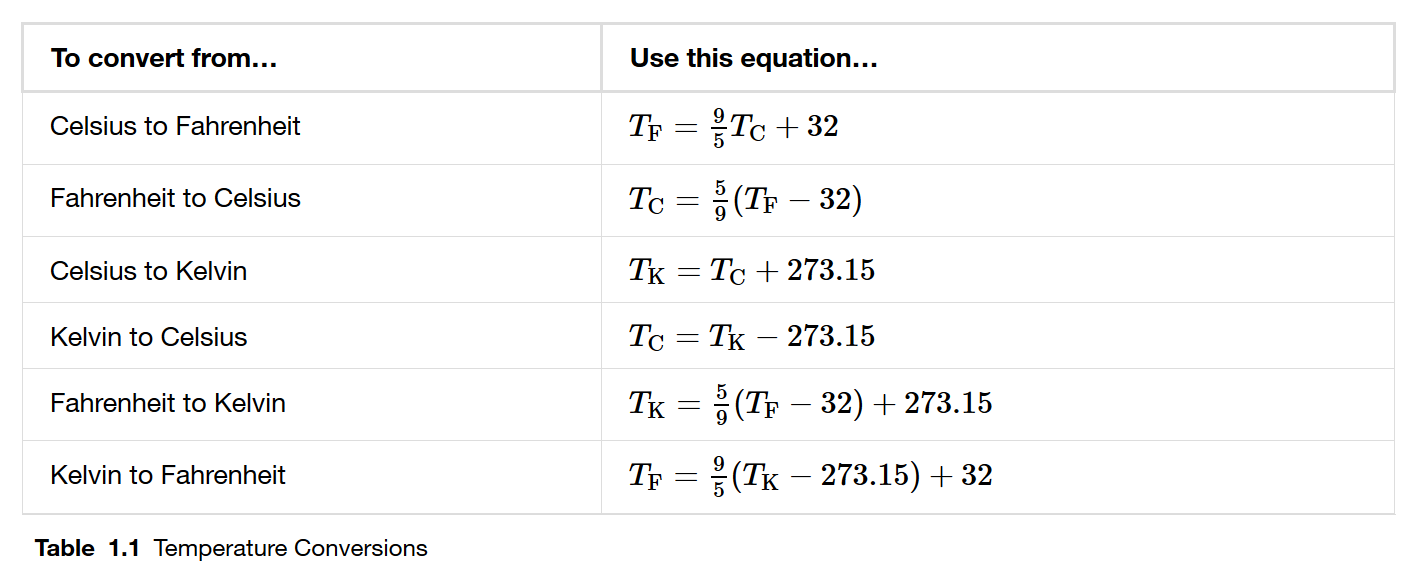
Thermodynamics
Temperature
[activity] Converting between scales
Thermodynamics
Temperature
The Kelvin scale



Make up your own scale:
Thermodynamics
Temperature
Temperature scales
I want to make up my own scale for temperature, which I will call the M-scale.
I want to use my own body temperature (37 C) as the zero for the M-scale; and since I do not like the heat very much, anything that is cooler than me is positive on the M-scale, and anything that is hotter than me is negative. I am most comfortable at 20 C, so I'm going to call that a 10 on the M-scale.
Find the equations that convert from the M-scale to the K-scale and vice versa.

Thermodynamics
Temperature
The scale of temperatures in the universe
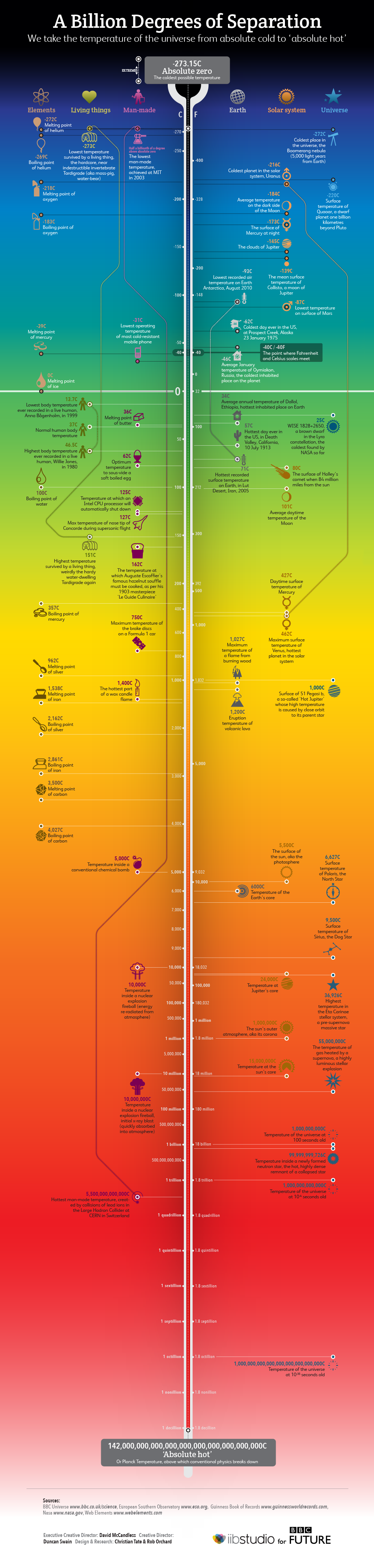
Thermodynamics
Temperature
The scale of temperatures in the universe
Thermodynamics
Temperature
A word on temperature & color

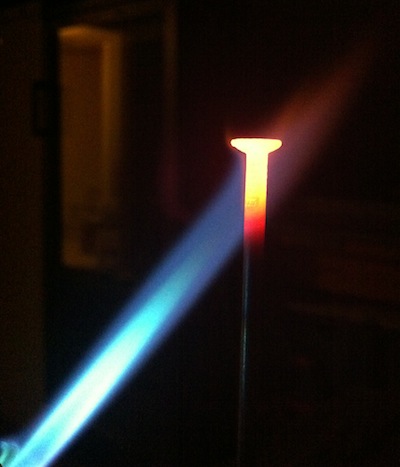
What color signifies an object at a higher temperature?
Thermodynamics
Temperature
Thermal Expansion






Thermodynamics
Temperature
Thermal Expansion
Linear expansion



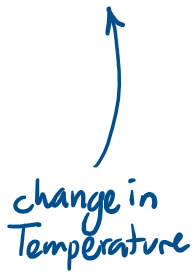
Volume expansion
Next,
- Define Thermal Contact and Thermal Equilibrium.
- Explain phenomena involving heat transfer.
- Solve problems involving heat transfer.
- Distinguish Temperature changes vs Phase changes.
Thermodynamics
Heat Transfer
Objectives
Thermal Contact
- Two objects are in thermal contact with each other if energy can be exchanged between them.
- The exchanges we will focus on will be in the form of heat or electromagnetic radiation (we'll get to that later).
- Thermal contact does not have to also be physical contact.
- Energy will be exchanged only if there is a temperature difference.
Thermodynamics
Temperature
Thermal contact and Thermal equilibrium
Thermodynamics
Heat transfer
Mechanisms of Heat Transfer
Convection
Convection is the process in which heat is carried from one place to another by the bulk movement of a fluid.
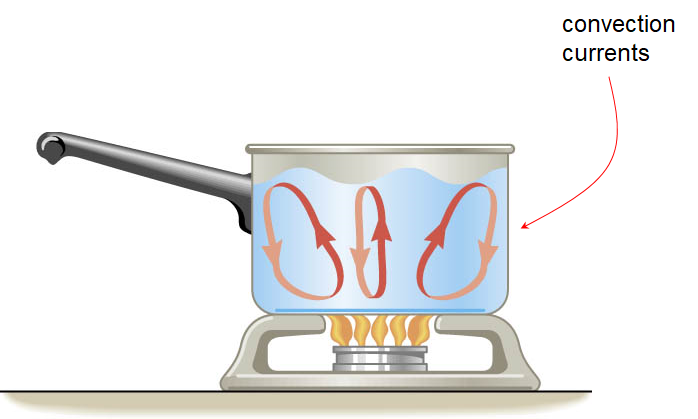
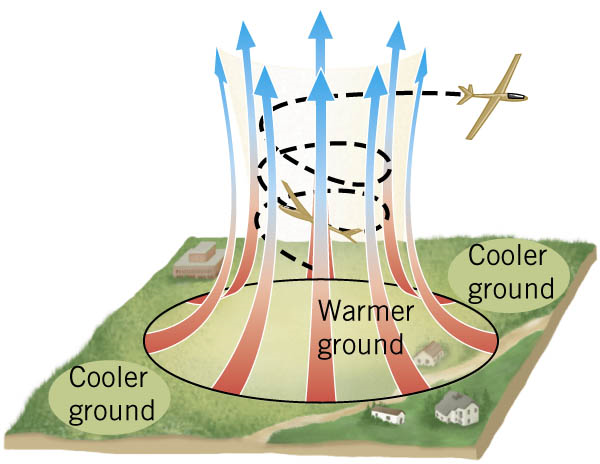
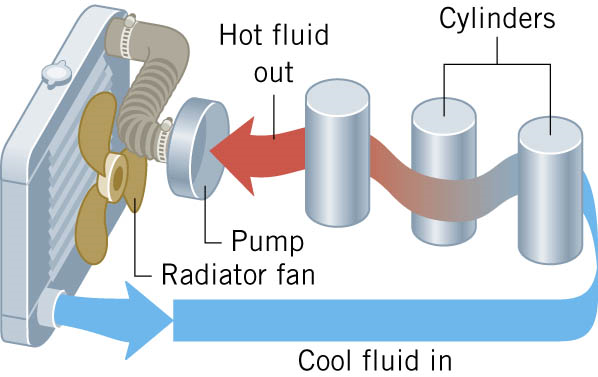
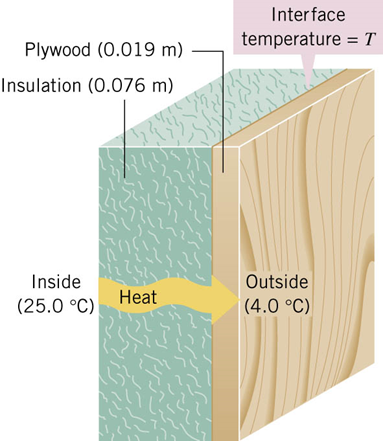
Conduction
Conduction is the process whereby heat is transferred directly through a material, with any bulk motion of the material playing no role in the
transfer.
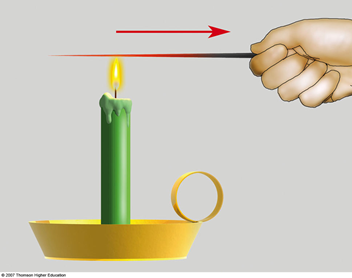
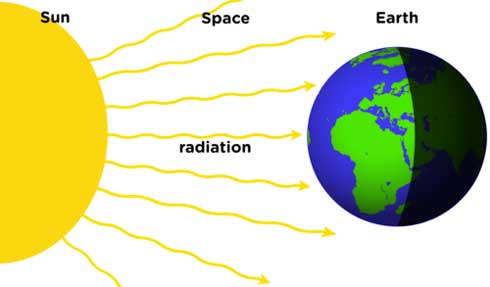
Radiation
Radiation is the process in which energy is transferred by means of electromagnetic waves.
Thermodynamics
Heat transfer
Mechanisms of Heat Transfer
Convection
Convection is the process in which heat is carried from one place to another by the bulk movement of a fluid.
Radiation
Radiation is the process in which energy is transferred by means of electromagnetic waves.
Conduction
Conduction is the process whereby heat is transferred directly through a material, with any bulk motion of the material playing no role in the
transfer.

Thermodynamics
Temperature
Thermal contact and Thermal equilibrium
Thermal Equilibrium
-
Thermal equilibrium is the state whereby objects in thermal contact do not exchange energy.
Another definition of Temperature
-
Temperature is the quantity that determines whether objects are in thermal equilibrium (i.e. whether heat energy will flow between them.)
Heat is
Thermodynamics
Heat Transfer
Definitions
a form of energy flow

In units of joules (or calories.)
Internal Energy of a system
Thermodynamics
Heat Transfer
Definitions
sum of the mechanical energies of constituents
is the
proportional to its temperature
andis
It is possible to change the internal energy of a system
by
transferring heat to/from the system.
Thermodynamics
Heat Transfer
Definitions
Thermodynamics
Heat Transfer
Heat transfer and Temperature change






For a system of mass m and specific heat capacity c, the amount of heat transferred into or out of the system associated with a change in its temperature is given by
Where the sign of the heat transferred matches the sign of the temperature change.
Thermodynamics
Heat Transfer
Heat transfer and Temperature change (example)
Suppose that you want to make tea with oat-milk in a ceramic mug.
You add 40g of cold oat-milk, which was in thermal equilibrium with the refrigerator environment at 5C; to 300g of water just below boiling point, at 94 C. You find that if the mug started at room temperature, 71.6 F, then when it equilibrates with its contents, its temperature becomes 77.5 C
Take the mass of the ceramic mug to be 200g, and its specific heat capacity to be 804 J/kg K. Take the specific heat of oat milk to be 4000 J/kg K.
How much heat is gained/lost by each of the elements?
Example
Thermodynamics
Heat Transfer
Heat transfer and Temperature change (example)
| mass (kg) | specific heat J/(kg K) | initial T (C) | final T (C) | heat (J) | |
|---|---|---|---|---|---|
| water | 0.3 | 4186 | 94 | 77.5 | -20720.7 |
| milk | 0.04 | 4000 | 5 | 77.5 | 11600 |
| mug | 0.2 | 804 | 22 | 77.5 | 8924.4 |
| Total | -196.3 |
Suppose that you want to make tea with oat-milk in a ceramic mug.
You add 40g of cold oat-milk, which was in thermal equilibrium with the refrigerator environment at 5C; to 300g of water just below boiling point, at 94 C. You find that if the mug started at room temperature, 71.6 F, then when it equilibrates with its contents, its temperature becomes 77.5 C
Take the mass of the ceramic mug to be 200g, and its specific heat capacity to be 804 J/kg K. Take the specific heat of oat milk to be 4000 J/kg K.
How much heat is gained/lost by each of the elements?
Example
Thermodynamics
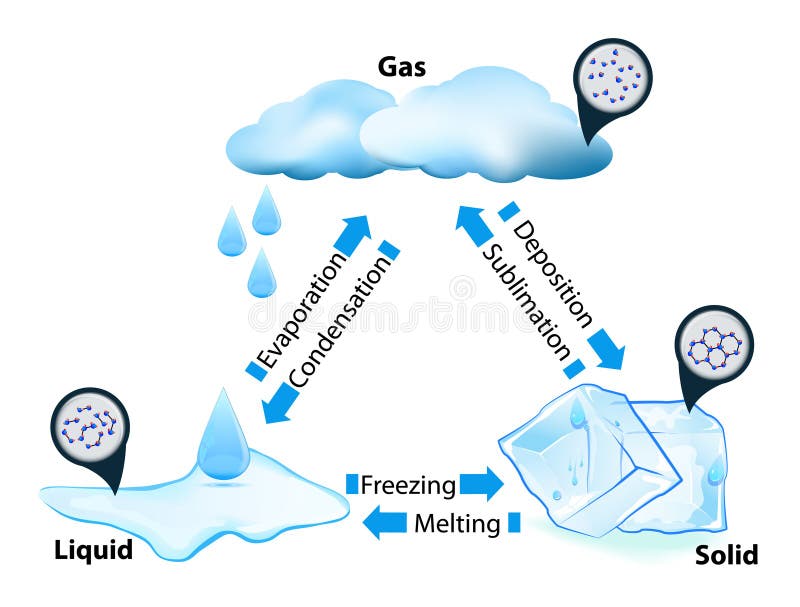
Heat transfer
States (phases) of matter
There are many distinguishable states of matter
Typically distinguished by some order parameter
The classical states of matter are:
Solid,
Liquid,
Gas,
Plasma.
Thermodynamics
Heat transfer
States (phases) of matter

Thermodynamics
Heat transfer
States (phases) of matter
Thermodynamics
Heat transfer
Heat transfer and phase change
Heat energy required for a system of mass m
to change phases.
Where
L is the latent heat associated
with
that transition
for
that material
Thermodynamics
Heat transfer
Heat transfer and phase change (example)
How much energy does it take to turn 1.0 Ton of ice to water vapor?

btw, 1 Ton of ice, volume wise, is roughly the size of a medium washer or dryer.
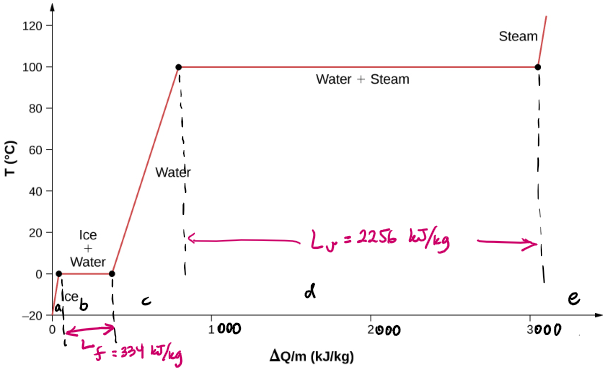
Thermodynamics
Heat transfer
Heat transfer and phase change (example)
How much energy does it take to turn 1.0 Ton of ice to water vapor?
| Sub process | What is changing? | Initial Temperature | Final Temperature | Required Heat per unit mass (J/kg) | Total required Heat (J) |
|---|---|---|---|---|---|
| Ice heating up | temperature | -20 | 0 | 2090 /degree | |
| Ice melting to water | phase | 0 | 0 | 334,000 | |
| Water heating up | temperature | 0 | 100 | 4186 /degree | |
| Water vaporizing | phase | 100 | 100 | 2,256,000 |


btw, 1 Ton of ice, volume wise, is roughly the size of a medium washer or dryer.
Thermodynamics
Heat transfer
Heat transfer and phase change (example)
Example:
A 0.0500-kg ice cube at -30 C is placed in 0.400 kg of 35 C water in a very well insulated container: what is the temperature after all elements have reached thermal equilibrium?
Next,
- Explain the macroscopic properties of gases in terms of the microscopic picture.
- Explain and Apply the ideal gas law
Thermodynamics
The Kinetic Theory of Gases
Objectives
Thermodynamics
The Kinetic Theory of Gases
Macroscopic properties of ideal gases
How do the macroscopic properties --P, V, and T--
relate to
each other?
How do the macroscopic properties --P, V, and T--
relate to the microscopic picture?
What is an _ideal_ gas?
Thermodynamics
The Kinetic Theory of Gases
Macroscopic properties of ideal gases
- A collection of molecules that are widely separated.
- The interactions between the individual molecules are mostly negligible.
- The motion of the molecules are independent of each other (except for the rare collision, where the momentum exchange contributes to maintaining thermal equilibrium.)
Thermodynamics
The Kinetic Theory of Gases
Macroscopic properties of ideal gases
How do the macroscopic properties --P, V, and T--
relate to the microscopic picture?
- is proportional to the internal energy of the system,
- which is the sum of the mechanical energies of the microscopic constituents. (mathematical expression coming soon.)
- Note that temperature is not defined for a single atom or molecule.
Temperature
- The volume of a gas is the volume of the container of the gas.
- The molecules of a gas fills the space it occupies.
- Note that volume is not really defined for a single atom or molecule.
Volume
Thermodynamics
The Kinetic Theory of Gases
Macroscopic properties of ideal gases
How do the macroscopic properties --P, V, and T--
relate to the microscopic picture?
- is a property that describes the average normal force per unit area that is exerted on any surface by the molecules of the gas.


Pressure
- pressure is in all directions. (think Newton's 3rd)
- the SI unit of pressure is the Pascal.
- Atmospheric pressure
- Gauge pressure = Absolute pressure - 1 Atm
Thermodynamics
The Kinetic Theory of Gases
Macroscopic properties of ideal gases
Joseph Wright’s An Experiment on a Bird in the Air Pump (1768) housed at the National Gallery, London.
How do the macroscopic properties --P, V, and T--
relate to
each other?

Thermodynamics
The Kinetic Theory of Gases
Macroscopic properties of ideal gases
Boyle's Law
Constant N and T
Charles's Law
Constant N and P
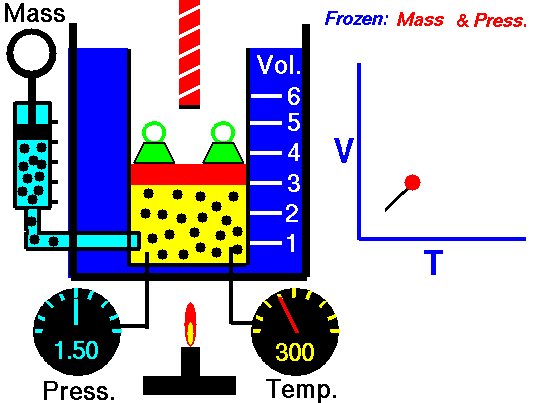
P-T Law
Constant N and V
New Experiments Physico-Mechanical, Touching the Spring of the Air and Its Effects (1660)

How do the macroscopic properties --P, V, and T--
relate to
each other?
Thermodynamics
The Kinetic Theory of Gases
Macroscopic properties of ideal gases
Ideal Gas Law
How do the macroscopic properties --P, V, and T--
relate to
each other?

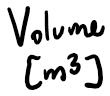
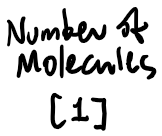

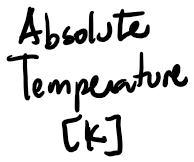




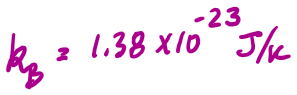



Thermodynamics
The Kinetic Theory of Gases
Macroscopic properties of ideal gases
Suppose 9.00g of water is vaporized inside a 2.00L container, and heated to 500 C -- what is the gauge pressure inside the container?
Example
Thermodynamics
The Kinetic Theory of Gases
Macroscopic properties of ideal gases
Suppose a tire is inflated to a gauge pressure of 32 Psi. If the compressed air inside the tire escapes to the outside atmosphere, what would its volume be compared to its volume inside the tire?
Example
Lastly, we would like to
- Explain the first law of thermodynamics in terms of conservation of energy
- Explain the second law of thermodynamics and the concept of Entropy
Thermodynamics
The Laws of Thermodynamics
Objectives
First, a word about work
- Technically, Work is done by a _force_
Thermodynamics
The Laws of Thermodynamics
Work done by a thermodynamic system
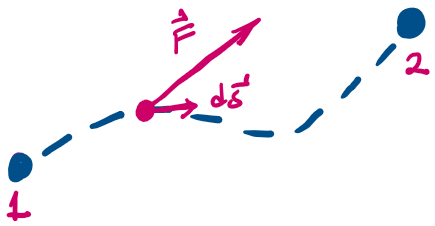
- Work happens as the system transitions from one state to another.
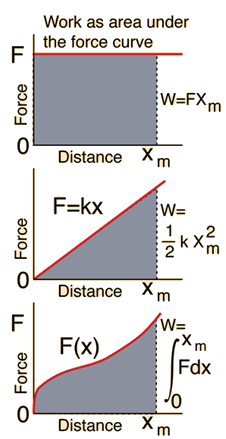
- The work done by the force F as the system displaces along a prescribed path s from state 1 to state 2 is given by the integral along the path:
Notes
- The work can be represented by the area "under" the force v. displacement curve.
Work done "by" a "thermodynamic system" as it expands from V_1 to V_2
Thermodynamics
The Laws of Thermodynamics
Work done by a thermodynamic system
Work done "by" a "thermodynamic system" as it expands from V_1 to V_2
Thermodynamics
The Laws of Thermodynamics
Work done by a thermodynamic system
Does the work depend on the path?
(Analysis on next slide)
Let's classify some common processes
(but, first)
No heat transfer
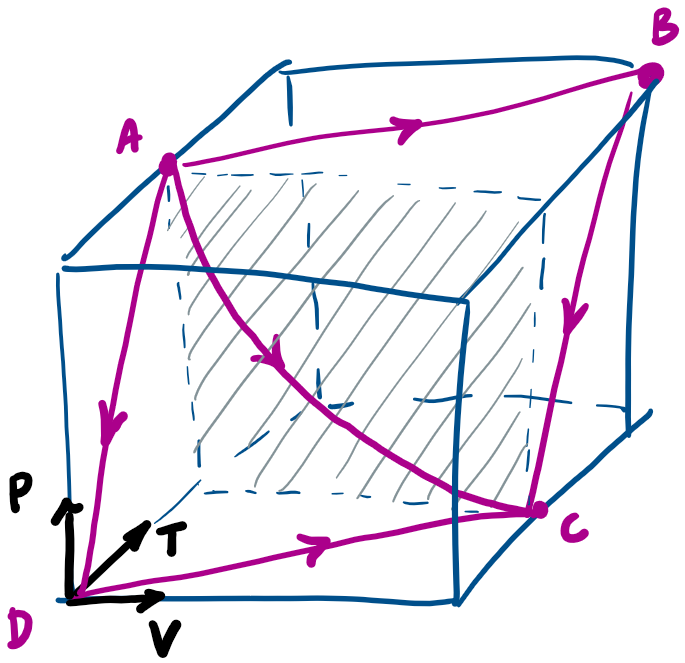
Work done "by" a "thermodynamic system" as it expands from V_1 to V_2
Thermodynamics
The Laws of Thermodynamics
Work done by a thermodynamic system
Thermodynamics
The Laws of Thermodynamics
Temperature as a measure of internal energy
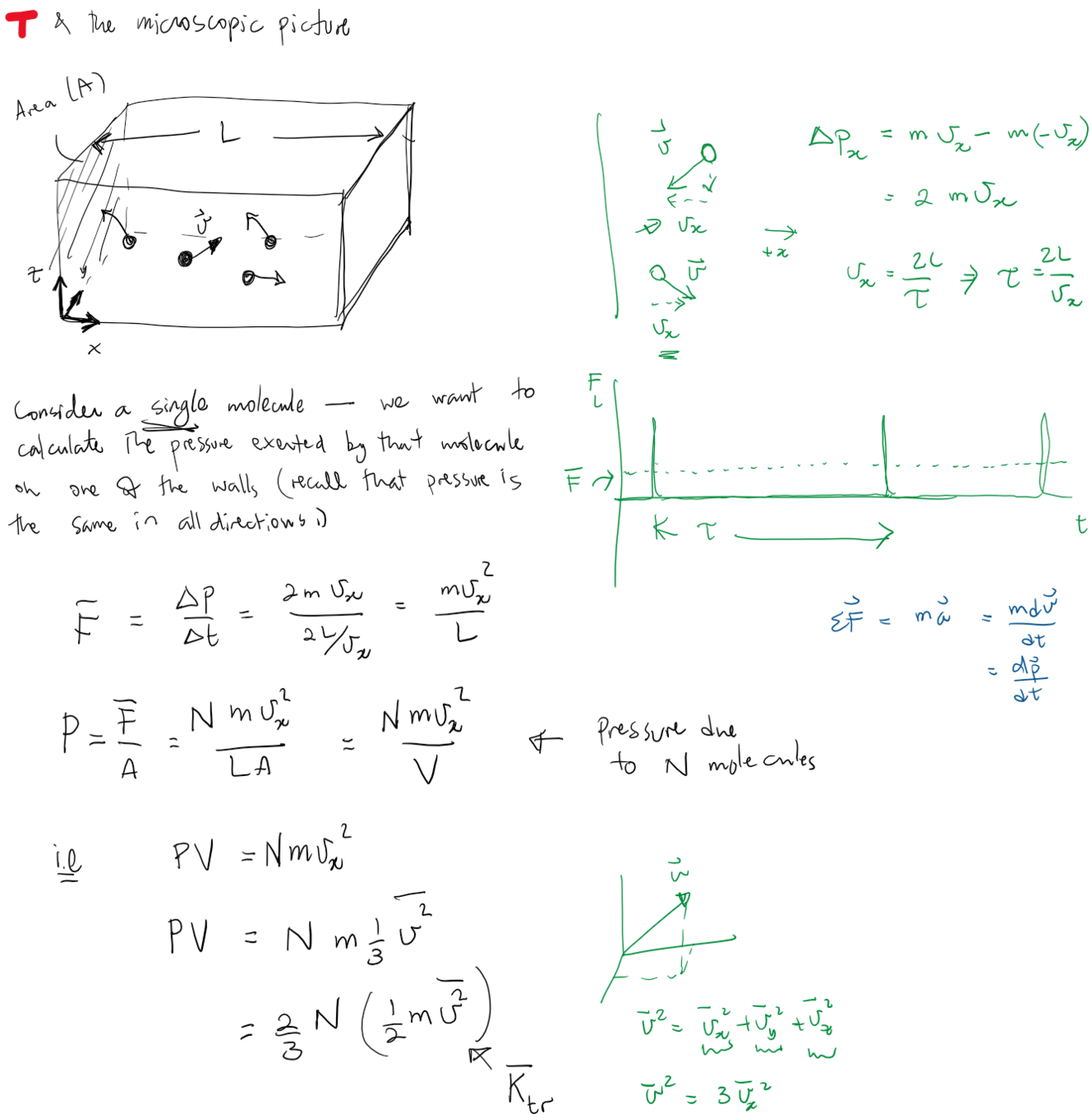
Consider an ideal gas of molecules in some thermodynamic state (P, V, T)
We wish to write an expression of the pressure exerted on the left wall by a single molecule undergoing elastic collisions with the walls.
The impulse is given by the change in momentum,
And the time between successive contacts is given by:
putting it together
Thermodynamics
The Laws of Thermodynamics
Temperature as a measure of internal energy

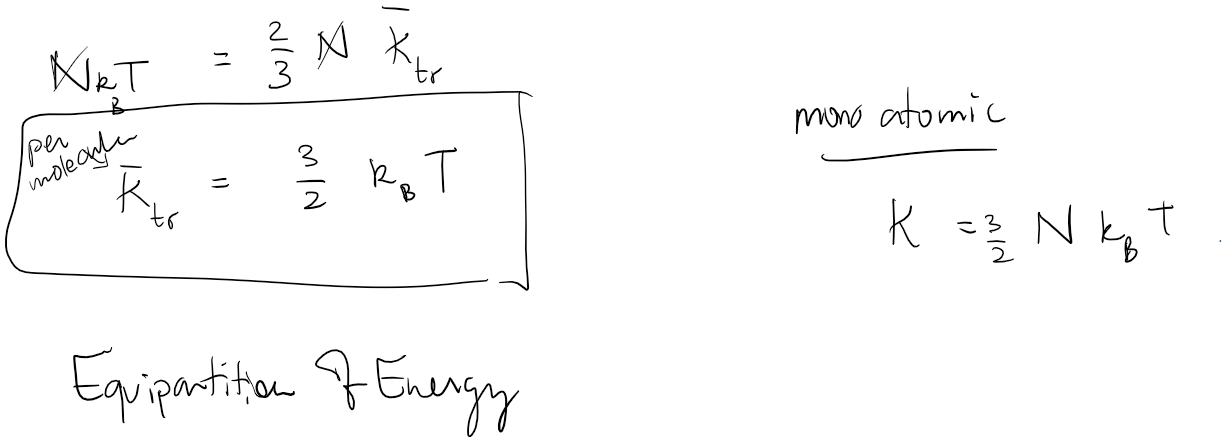
Thermodynamics
The Laws of Thermodynamics
First Law of Thermodynamics
Ling .... (3.7)
Thornton .. (1.8)
Essentially, 1st law is a statement about the conservation of Energy
For any transition between states of thermal equilibrium
Heat transferred
_to_
the system
The change in the internal energy of a system
Work done _by_
the
system
?
Thermodynamics
The Laws of Thermodynamics
First Law of Thermodynamics
Heat transferred
_to_
the system
The change in the internal energy of a system
Work done _by_
the
system
| process | ||||||
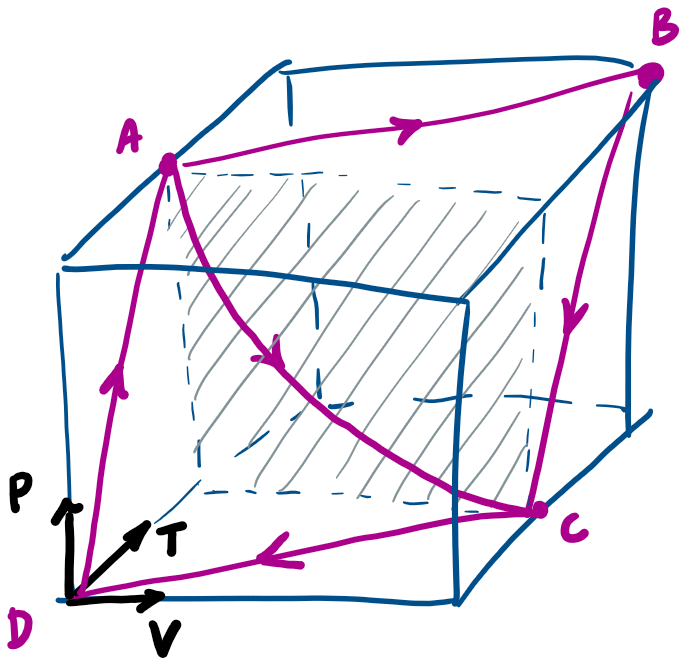
Thermodynamics
The Laws of Thermodynamics
First Law of Thermodynamics
Heat transferred
_to_
the system
The change in the internal energy of a system
Work done _by_
the
system
| process | ||||||

| isobaric | isochoric | isobaric | isochoric | isotherm | ||
| + | - | - | + | 0 | ||
| + | - | - | + | + | ||
| + | 0 | - | 0 | + |
| isobaric | isochoric | isobaric | isochoric | isotherm | isotherm | |
| + | - | - | + | 0 | 0 | |
| + | - | - | + | + | - | |
| + | 0 | - | 0 | + | - |
Thermodynamics
The Laws of Thermodynamics
motivating 2nd law: Apparent symmetry of heat and work
Heat transferred
_to_
the system
The change in the internal energy of a system
Work done _by_
the
system
Q: Suppose I look at the class of all thermodynamic processes (or combinations thereof) such that the final temperature is the same as the initial temperature...
Does that mean that I can change all heat to work and all work to heat?
Thermodynamics
The Laws of Thermodynamics
motivating 2nd law: The direction of heat flow

same action
opposite outcomes

Blowing
warms
cold hands

Blowing
cools
hot drinks
The implication is that there is a natural direction for thermodynamic evolution!
Thermodynamics
The Laws of Thermodynamics
motivating 2nd law: Reversibility
Q: Is it possible to reverse a thermodynamic process?
Q: What does it mean to reverse a process?
Examples from newtonian mechanics to illustrate the difference between reversible and irreversible processes:
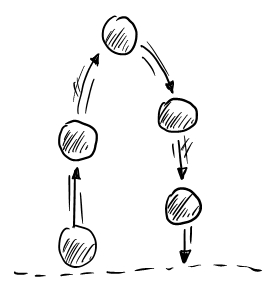
Thermodynamics
The Laws of Thermodynamics
motivating 2nd law: spelling it out
Suppose a block is sitting on a table, in thermal equilibrium with the surroundings.
The work you do on the system is converted into internal energy of the system and the block-table system becomes warmer → the system is no longer in thermal equilibrium with its surroundings → the system will transfer energy as heat to its surroundings until it returns to the thermal equilibrium
Suppose you spend two minutes pushing the block along the tabletop in a closed path, finally leaving the block in its initial position.
Because the final and initial states of the system are the same, the 1-st Law of TD dictates that the energy transferred to the environment as heat equals to the work done on the system.
The reverse process never occurs – a block and table that are warm will never spontaneously cool by converting their internal energy into the work that causes the block to push your hand around the table!
Thermodynamics
The Laws of Thermodynamics
motivating 2nd law: spelling it out
There is a lack of symmetry in the roles played by heat and work that is not evident from the first law. This lack of symmetry is related to the fact that some processes are naturally irreversible.
Thermodynamics
The Laws of Thermodynamics
Statements of the second law