
























PUREIMSFOR INTERESTED VC FUNDS
Series A Investment Round
January 2021
DISCLAIMER

























This presentation was prepared by PureIMS B.V. (‘PureIMS’) for informational purposes only. This presentation is incomplete without reference to, and should be viewed solely in conjunction with, the oral briefing provided by PureIMS. In preparing this presentation, we have relied upon and assumed, without independent verification, the accuracy, completeness and correctness of any information available from public sources. No statutory audit has been performed by PureIMS either with respect to the information included in this report or any other information supplied. PureIMS expressly disclaims any and all responsibility for the accuracy, completeness or correctness of the information contained in this report. Taking into account the uncertainty inherent to prospective information, our analysis neither warrants nor confirms the realization of the future financial developments as presented. Opinions expressed herein reflect the judgment of PureIMS as of the date of this presentation and may be subject to change without notice if PureIMS becomes aware of any information, which may have a material impact on any such opinions.
PUREIMS INVESTMENT CASE

























Executive summary
*€23.8 M incl. fee reduction FDA/EMA 1st NDA application
Clinical stage pharmaceutical and medication systems company
Proprietary inhalation device for rapid and controlled drug delivery:
allowing easy-to-use rescue therapy with very fast onset-of action
Focus on therapeutic areas with “acute” unmet need
One program marketed (Colistin Cyclops™ - cystic fibrosis)
- Compassionate use regimen
- Reimbursed by payers
Funds raised to date: €3.3M (equity)
Current investment round: €27.9 M*
Use of proceeds by mid 2023
- Bring 2 lead clinical programs to submission/approval in the US and EU
- Advance 7 additional programs to Phase II/III clinical development
OUR STORY - PUREIMS

























- Broad pipeline across key therapeutic areas with significant unmet need covering commercial and exploratory programs
• Colistin Cyclops™ application (for cystic fibrosis-related infectious disease) marketed in the Netherlands under compassionate use regimen,
reimbursed by payers
• Lead programs Levodopa Cyclops™ (for neurology) and Epinephrine Cyclops™ (for allergy) pursuing accelerated regulatory routes to market in US and EU
• Clinical-stage follow-on programs exploring applicability in respiratory indications with high unmet need such as COVID-19
Therapeutics programs based on Cyclops™ technology addressing unmet medical need
Therapeutic programs for Series A Investment Round are selected on the basis of:
- Potential to solving an ‘acute’ medical need (i.e. ‘rescue medication’)
- Market attractiveness
- Possibility to obtain marketing authorization via accelerated regulatory pathway, e.g. US 505(b)(2) route
- Opportunity for early exit: Series A funding covers lead programs until marketing authorization in initial marketing regions within 2-2.5 years
Company information
- HQ: Roden/Groningen (the Netherlands)
- Clinical stage pharmaceutical and medication systems
company developing inhaled dry powder products - Spin-off 2014 from University of Groningen and from
medical device manufacturer IMDS - Funds raised: €3.3M equity
- Cyclops™ technology
• Proprietary dry powder inhaler
• Design and functionality patent protected until 2034
• CE marked medical device
- IP in-licensed from University of Groningen
Cyclops™ technology – IP and regulatory
MANAGEMENT TEAM

























Seasoned Life Sciences Executives



Bram van Dijck, MBA – CEO
- Bram van Dijck held senior Commercial, Portfolio and General Management
- Positions at Grünenthal Group, Hospira, Teva Pharmaceuticals, Organon
and Abbott Laboratories. - He is also Adviser to several Pharma and MedTech companies.
Jeroen Tonnaer, PhD, CLP – CBDO
- Jeroen Tonnaer was previously CBO of Cristal Therapeutics, Executive Director at Merck/MSD, and held various prior BD&L and R&D positions at
Schering-Plough and Organon. - He is also member of the Advisory Board of Protinhi Therapeutics.
Reinier Schwietert, PhD – CSO
- Reinier Schwietert received his PhD degree (cum laude) in Pharmacology
from the University of Amsterdam and thereafter fulfilled different
(management) roles and responsibilities within the pharmaceutical industry. Since 2017 he also acts as an independent drug development consultant for small biotech companies.
SCIENTIFIC ADVISORY BOARD

























Inhaler development
Colistin Cyclops™– Cystic Fibrosis
Harry Heijerman, PhD, Professor – University Medical Center Utrecht (UMCU)
Head of Department of Pulmonary Diseases
Anne de Boer, PhD – University of Groningen (retired)
Project Leader Inhalation Group Department of Pharmaceutical Technology and Biopharmacy
Gerard Koppelman, PhD, Professor – Beatrix Children’s Hospital (UMCG)
Head of Pediatric Pulmonology and Pediatric Allergology
Daan Touw, PhD, Professor – University Medical Center Groningen (UMCG)
Head of Clinical Pharmacological Laboratory
Levodopa Cyclops™ – Parkinson’s Disease
Teus van Laar, PhD, Professor – University Medical Center Groningen (UMCG)
Medical Director of the Parkinson Expertise Center in Groningen
Geert Jan Groeneveld, PhD, Professor – Centre for Human Drug Research University of Leiden
Professor of Clinical Neuropharmacology
Bert Tuk, PhD – Tuk Management
Clinical Pharmacologist
PROPRIETARY DRY POWDER INHALATION DEVICE

























Applicable to a broad spectrum of drugs
Use
Cyclops™ Device
Drug Delivery
through Inhalation
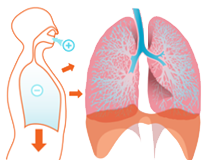
- Non-invasive
- Easy to use
- Deep lung deposition
- Local application or rapid drug absorption into circulation

- User friendly, pre-loaded, disposable, credit card-size inhalation device
- Overcomes current inhaler limitations
✔ poor drug deposition
✔ dose limitation - Poor motor skills don't affect use
- Cost-effective manufacturing
- CE marked – patent protected
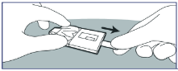
1. Remove cover foil
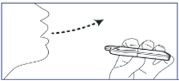
2. Exhale
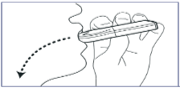
3. Inhale
4. Check delivery
JANUARY 2021: 6 PRODUCTS IN CLINICAL DEVELOPMENT

























Candidate
Preclinical
Phase I
Phase II
Phase III
Submitted
Marketed
Lead programs
Levodopa Cyclops™
Epinephrine Cyclops™
Colistin Cyclops™
Tobramycin Cyclops™
Amikacin Cyclops™
Explorative programs
HCQ Cyclops™
Mannitol Cyclops™
* Bronchial challenge testing
Inbrija™
Follow-on programs

Parkinson’s Disease
Anaphylaxis
Cystic Fibrosis
Cystic Fibrosis
Tuberculosis
*
Marketed under compassionate use and reimbursed
Lead programs pursuing advanced registration pathway
Lead programs qualify for regulatory routes allowing advanced marketing authorization
Other programs explore respiratory indications of significant unmet need
COVID-19
PARKINSON'S DISEASE FACTS

























Worldwide between 6 and 10 million have PD and between 1996 to 2016 the global prevalence increased 145%.

PD is the second-most-common neurodegenerative disorder in the world.


REFERENCES
2. Poewe et al. Parkinson disease.
Nat Rev Dis Primers.
2017;3:17013
3. Feigin et al. GBD 2016
Neurology Collaborators.
Global, regional, and national
burden of neurological
disorders, 1990-2016: a
systematic analysis for the
Global Burden of Disease Study.
Lancet Neurol, 2019. 18(5):
p. 459-480.
2
3
PARKINSON'S DISEASE FACTS

























REFERENCES
1. Ahlskog etal. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord, 2001. 16(3): p.448–458.
2. Poewe et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013
4. Tambasco et al. Levodopa in Parkinson's Disease: Current Status and Future Developments. Curr Neuropharmacol, 2018. 16(8): p. 1239-1252.
5. Obeso et al. Levodopa motor complications in Parkinson's disease. Trends Neurosci, 2000. 23(10 Suppl): p. S2-7.
6. Olanow et al. Apomorphine sublingual film for off episodes in Parkinson's disease: a randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurol, 2020. 19(2): p, 135-144.
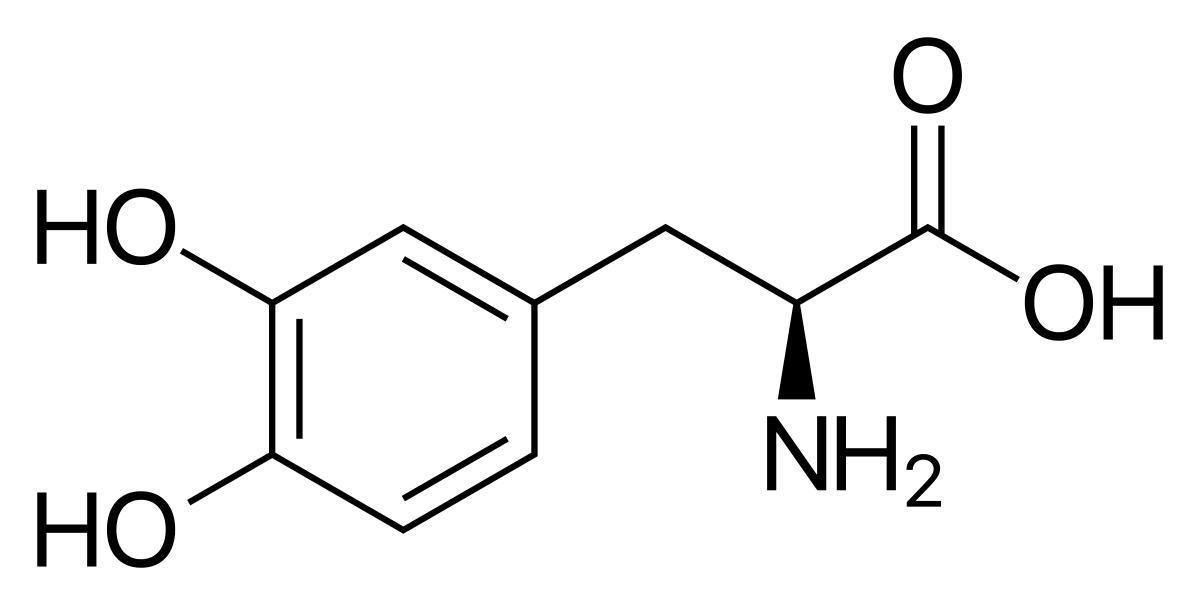



Levodopa is the cornerstone in the treatment of PD disease for well over 50 years.
It is the most effective, safe and well tolerated drug available to this end.
70%
Approximately
of patients experience off episodes after they have been taking levodopa for 9 or more years.
Approximately
40%
of patients experience off episodes after they have been taking levodopa for 4 to 6 years.
90%
Approximately
of young onset patients (less than 40 years old) develop off episodes after 5 years of taking levodopa.
However, patients suffering from PD will encounter a major unmet medical need as the disease progresses:
Available medication has a slow or unpredictable onset of action or is poorly tolerated and uncomfortable to use.
4
4
6
1
1
2
5
PARKINSON'S DISEASE FACTS




























Studies showed that PD patients are able to readily use Levodopa Cyclops™ during an OFF episode.
Levodopa Cyclops™ results in peak plasma concentrations within minutes and its absorption is less variable than that of oral levodopa, both of which are prerequisites for the rapid and predictable relief of OFF episodes.
Levodopa Cyclops™ offers PD patients easier and more convenient handling, and a faster and more predictable onset of action to counteract OFF episodes.
LEVODOPA CYCLOPS™ –
MEDICAL NEEDS AND USPs

























Major unmet medical needs Parkinson’s Disease patients
Advantages Levodopa Cyclops™ over conventional therapies
Patients in mid- and late-stage of disease suffer from variable therapeutic
efficacy resulting OFF-episodes
Currently available medication insufficient to effectively offer rescue
with fast onset of action
Rapid onset of action
Good control over levodopa plasma levels
Optimal flexibility regarding individual dose titration, ensuring better
efficacy and avoidance of dyskinesias
Text
LEVODOPA CYCLOPS™ - CLINICAL PK DATA

























Clinical PK data Levodopa Cyclops™

Phase II studies show fast and reproducible absorption of levodopa
Levodopa Cyclops™ can offer fast and reliable rescue treatment for Parkinson’s patients suffering from OFF-episodes





Plasma levels upon inhaled (Levodopa Cyclops™) or oral dosing levodopa
L-DOPA plasma levels (ng/mL)
Dose
Levodopa Cyclops™ 30 mg
Levodopa Cyclops™ 60 mg
Levodopa Cyclops™ 90 mg
Oral 100 mg
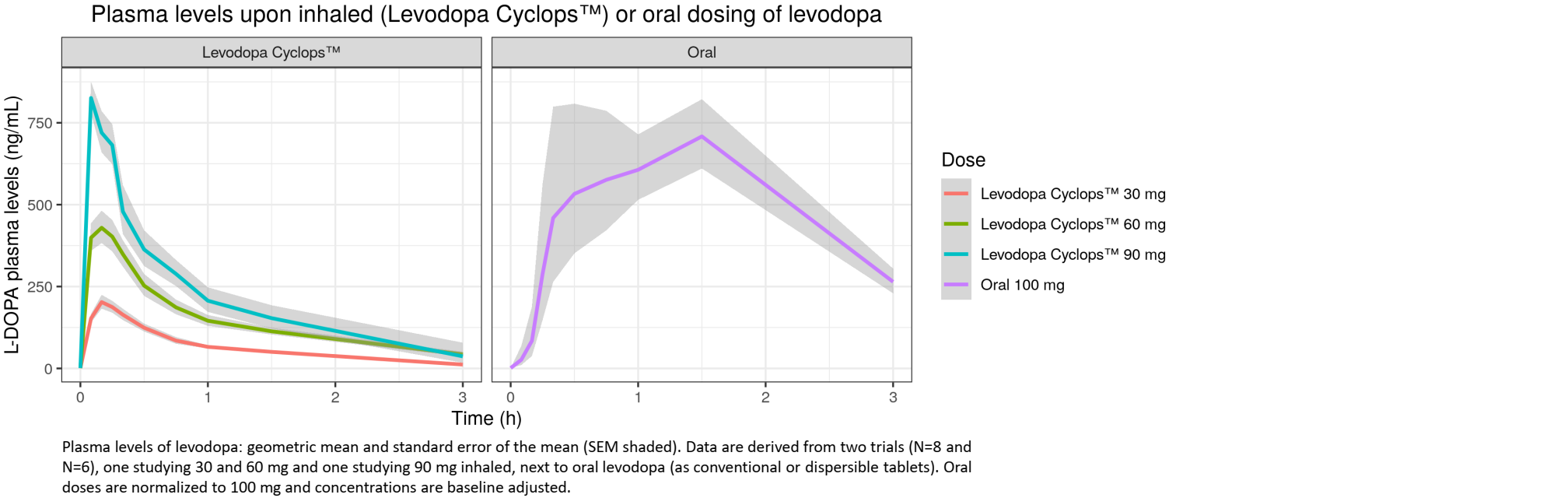
Levodopa Cyclops™
Oral
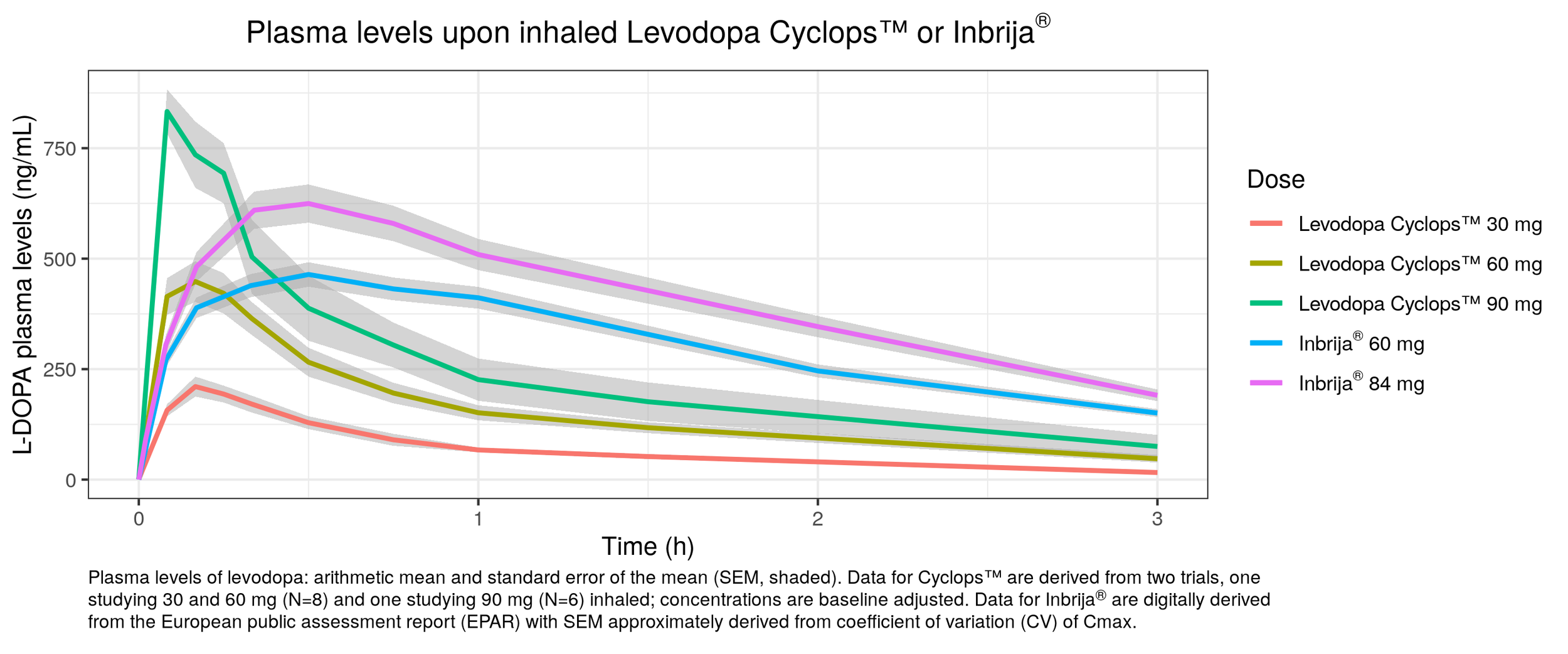
LEVODOPA CYCLOPS™ - CLINICAL PK DATA

























Clinical PK data Levodopa Cyclops™
Phase II studies show faster absorption of levodopa compared to Inbrija®
The higher levodopa elimination rate following the use of Levodopa Cyclops™ is caused by the absence of a decarboxylase inhibitor during the studies
Plasma levels upon inhaled Levodopa Cyclops™ or Inbrija®
L-DOPA plasma levels (ng/mL)
Levodopa Cyclops™ 30 mg
Levodopa Cyclops™ 60 mg
Levodopa Cyclops™ 90 mg
Inbrija® 60 mg
Inbrija® 84 mg







LEVODOPA CYCLOPS™ -
FAST REGULATORY ROUTE

























USPs Levodopa Cyclops™ over competitor Inbrija®
Market potential
505(b)(2) trajectory offering fast US marketing authorization for Levodopa Cyclops™

*Being finetuned on the basis of FDA’s feedback on package proposed in pre-IND application
- FDA confirmed applicability of 505(b)(2) regulatory pathway
- Quick route to market – marketing authorization expected ≥2023
- Few (pre)clinical activities to be conducted for marketing authorization*:
• Limited preclinical safety package L-Leucine excipient
• Human factor study
• Relative bioavailability study vs. reference listed drug Inbrija®
- Reference to dossier of reference listed drug Inbrija® possible as of December 2021
- Anticipated peak sales ~€300M (US only) -
€700M (US+EU)
- Levodopa Cyclops™ substantially easier in use than Inbrija®
• Better compliance
• Better efficacy
- Business case indicates Levodopa Cyclops™ outperforms Inbrija® on price
• Larger market share
• Higher reimbursement rates
Fast trajectory to US market Levodopa Cyclops™
Epinephrine Cyclops™

























1. Allergy 2013;68:1353–61
2. JACI 2014;133:461-7
3. JACI 2000;106:1184–9
4. AAAI 2010;104:172–7
5. J Asthma Allergy 2018;11:143-151
6. JRSM Open 2015;6:2054270415593443
7. FAERS Public Dashboard. June 30, 2018





Anaphylaxis is on the rise, with the lifetime prevalence rates estimated at 0.3% in Europe and at least 1.6% in
the US
1
Anyone who has ever had a severe allergic reaction or anaphylaxis is at risk of this happening again and is prescribed an epinephrine autoinjector for first-line rescue treatment
3
Anaphylaxis is a severe systemic allergic reaction, which if not treated promptly, may result in respiratory or cardiovascular shock and ultimately even death.
2
Less than 50% of persons at risk of anaphylaxis know how to use their autoinjector correctly.
What’s more, many people are reluctant to use their autoinjector, putting them at risk of untimely treatment. When they do use their autoinjector, there is a real risk of accidental exposure or device
failure.
4
Epinephrine Cyclops offers the solution: an easy-to-use, non-invasive device with an immediate onset of effect
5


- Immediate onset of action
- Short exposure allowing for repeated treatment without dose stacking
- Effective local treatment of symptoms of the airways and oropharyngeal region
- Easy to use
2
3,4
5,6
7
1
EPINEPHRINE CYCLOPS™ –
MEDICAL NEEDS AND USPs

























Major unmet medical needs allergic subjects at risk of anaphylaxis
Advantages Epinephrine Cyclops™ over conventional emergency treatment
Allergic subjects are reluctant to use available invasive autoinjector devices (EpiPen) putting them at risk of untimely treatment of an anaphylactic reaction
Less than 50% of subjects at risk of anaphylaxis can use their autoinjector
device correctly
Non-invasive & easy to use
Immediate onset of action
Effective local treatment of symptoms of the airways and
oropharyngeal region
Text
Short exposure allowing for repeated treatment without dose stacking
LEVODOPA CYCLOPS™ - CONCLUSIONS OF COMPETITIVE ANALYSIS

























Active substance
Mode of administration
Tmax
Clinical onset of action*
Efficacy
Side effect profile
*
**
***
****
as defined by a significant (p<0.05) improvement in UPDRS III motors score compared to placebo post-dose
LeWitt et al. Safety and efficacy of CVT-301 (levodopa inhalation powder) on motor function during off periods in patients with Parkinson’s disease:
a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet Neurology (2019) 19: 145 - 154
Olanow et al. Apomorphine sublingual film for off episodes in Parkinson’s disease: a randomized, double-blind, placebo-controlled phase 3 study. The Lancet Neurology (2020) 19: 135 - 144
Pfeiffer et al. The APO302 Study Investigators. Continued efficacy and safety of subcutaneous apomorphine in patients with advanced Parkinson's disease. Parkinsonism & related disorders (2007) 13: 93-100
Levodopa Cyclops™
Levodopa
Inhalation
10 min
< 10 min
(based on PK)
Better than Inbrija™ (based on PK)
Mild cough
+++++
Inbrija™
Levodopa
Inhalation
30 min
30 min**
Moderate
Cough
+
Kynmobi™
Apomorphine
Sublingual
51 min
15 min***
Good
Nausea and oral lesions (30% of PD patients do not tolerate treatment)
++
Apokyn™
Apomorphine
SQ Injection
23 min
10 min****
Good
Nausea and injection site reactions
-
Ease of self-administration
EPINEPHINE CYCLOPS™ - CLINICAL PK DATA

























Clinical PK data Epinephrine Cyclops™
Phase I study shows immediate and reproducible absorption of epinephrine
Epinephrine Cyclops™ can offer fast and reliable rescue treatment for
subjects at risk of anaphylaxis
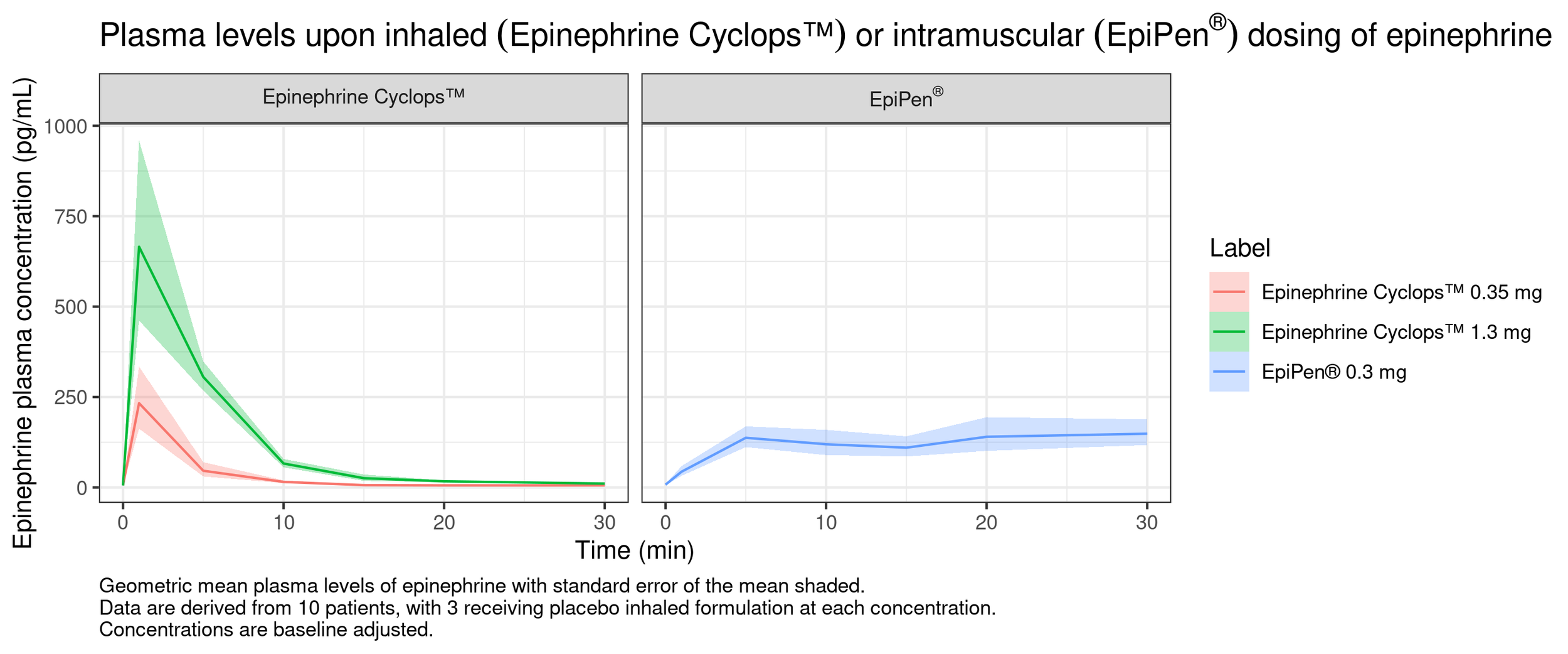




Plasma levels upon inhaled (Epinephrine Cyclops™)
or intramuscal (EpiPen®) dosing of epineprihne
Epinephine plasma concentration (pg/mL)
Label
Levodopa Cyclops™ 35 mg
Levodopa Cyclops™ 1.3 mg
EpiPen® 0.3 mg
Epinephrine Cyclops™
EpiPen®

EPINEPHRINE CYCLOPS™ -
FAST REGULATORY ROUTE

























USPs Epinephrine Cyclops™ over competitor EpiPen®
Market potential
Hybrid route offering fast EU marketing authorization for Epinephrine Cyclops™
*Being finetuned on basis of Swedish Authorities' feedback on package proposed in pre-IND application
- Decentralized procedure with Sweden as reference member state
- Swedish Authority to confirm applicability of hybrid regulatory pathway
- Quick route to market – marketing authorization expected ≤2023
- Few clinical activities to be conducted for marketing authorization*:
• Phase II efficacy study in 40 subjects
• Human factor study - Reference to dossier of reference listed drug EpiPen®
- Current sales competitor EpiPen ~$200M
- Total addressable market €2.3B
- Epinephrine Cyclops™ offers a needle-free substitute for EpiPen® and other autoinjectors
- Epinephrine Cyclops™ is substantially easier in use than EpiPen®
Fast trajectory to EU market Epinephrine Cyclops™
SUMMARY INVESTMENT OPPORTUNITY

























Lead programs: exit at marketing authorization within 2-2.5 years
Market potential Levodopa Cyclops™ (Parkinson) & Epinephrine Cyclops™ (Anaphylactic reaction)
*FDA and EMA may grant €4.1M fee reduction for first NDA application by SME, reducing capital need to €23.8M
Prioritized strategic development programs
- Levodopa Cyclops™: €11.6M – US market
- Epinephrine Cyclops™: €2.3M – EU market
- Capital need prioritized programs: €13.9M
Optional expansion programs
- Levodopa Cyclops™: €9.7M – expansion to EU market
- Epinephrine Cyclops™: €4.3M – expansion to US market
- Capital need optional expansion programs: €14.0M
→ Capital need for both programs: €27.9M*
- Levodopa Cyclops™: anticipated peak sales ~€300M (US only) - €700M (US+EU)
- Epinephrine Cyclops™: current sales competitor EpiPen ~$200M; total addressable market €2.3B
MID 2023: PORTFOLIO READY FOR EXIT

























Candidate
Preclinical
Phase I
Phase II
Phase III
Submitted
Marketed
Lead programs
Levodopa Cyclops™
Epinephrine Cyclops™
Colistin Cyclops™
Tobramycin Cyclops™
Amikacin Cyclops™
Explorative programs
HCQ Cyclops™
Mannitol Cyclops™
Inbrija™
Follow-on programs

Marketed under compassionate use and reimbursed
Lead programs pursuing advanced registration pathway
2 programs approved / under approval
7 additional programs in Phase II/III
Parkinson’s Disease
Anaphylaxis
Cystic Fibrosis
Cystic Fibrosis
Tuberculosis
COVID-19
Bronchial challenge testing








































































JEROEN TONNAER
Chief Business Development Officer
jtonnaer@pureims.com
+31 6 534 249 85
www.pureims.com
BRAM VAN DIJCK
Chief Executive Officer
bvandijck@pureims.com
+31 6 302 181 91
www.pureims.com
REINIER SCHWIETERT
Chief Scientific Officer
rschwietert@pureims.com
+31 6 511 483 08
www.pureims.com
CONTACT DETAILS
CONTACT US
Ceintuurbaan Noord 152
9301 NZ Roden
The Netherlands
T: +31 (0)50 – 2053 325
F: +31 (0)50 – 2053 326
E: info@pureims.com

Thank you for your interest and attention
END OF PRESENTATION