VANADIUM
What is Vanadium?
Vanadium is a rare, soft and ductile metal
It is the 23rd element on the periodic table
It is a transition metal
It has a melting point of 1910 °C
and a boiling point of 3407 °C
History
Vanadium was discovered in 1801 by Andres Manuel de Rio.
It was rediscovered by Nils Sefstrom in 1831, who named it after the Norse god of beauty Vanadis.

Applications of Vanadium
Vanadium is mostly mixed into alloys. It is used in titanium and aluminium alloys to make jet engines, rockets, and nuclear reactor parts. Due to its high melting point it can be used in drill bits and nuclear reactors
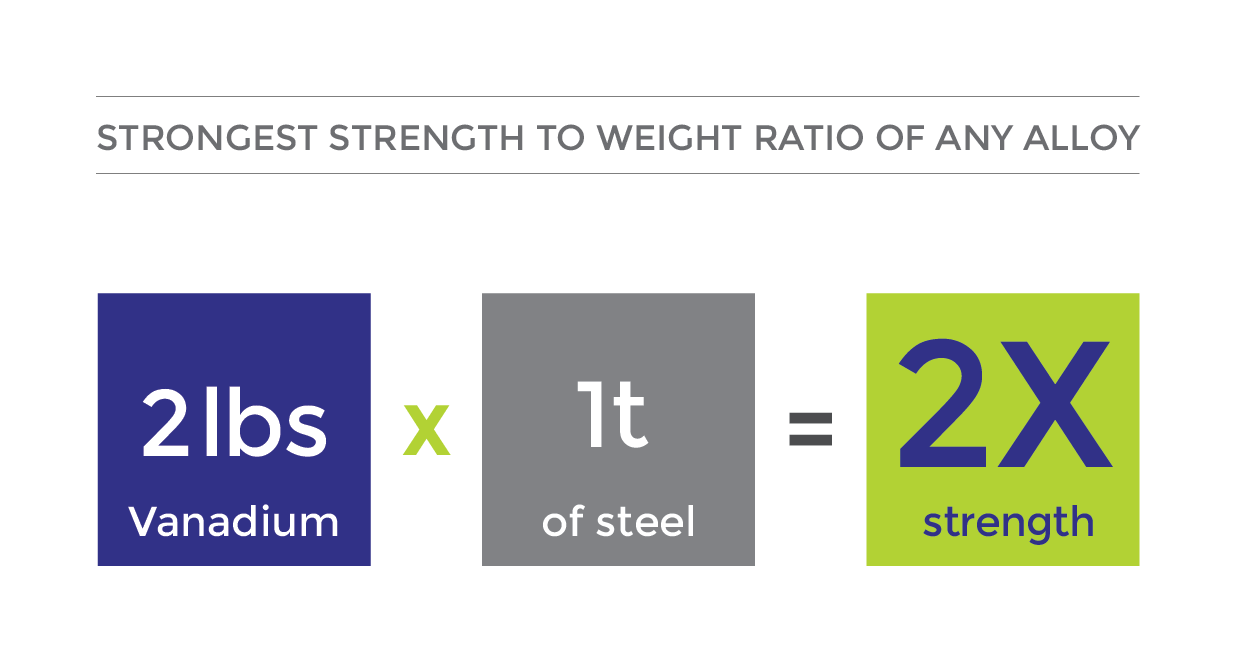
When added to a steel alloy, vanadium strengthens the steel and makes it more resilient. 90% of vanadium mined is used in steel alloys.

Vanadium is also used as a catalyst for sulfric acid.
Vanadium has four oxidation stages.
Due to the ease at which Vanadium gains and looses electrons, it can be used as a redox battery.
A Vanadium battery would last decades, and would have the capacity to recharge at least 20 thousand times.
Finding Vanadium
Vanadium is never found on its own in nature. It is always mined along with other metals.
Vanadium is found in 65 different minerals, as well as carbon containing deposits.
The largest resources of Vanadium are in China, South Africa and Russia.
Some naturally occuring enzymes contain Vanadium, especially vanadium nutrogenase which is found in microorganisms.
Vanadium is an essential micronutrient for animals, but toxic in large dosages
Thanks for watching!
