Towards Atomistic Modeling of Complex Environments with Many-Body Machine Learning Potentials
Alex M. Maldonado
September 1, 2022
aalexmmaldonado

Keith Group
Clean energy is growing, but slowly
Advancing energy technologies
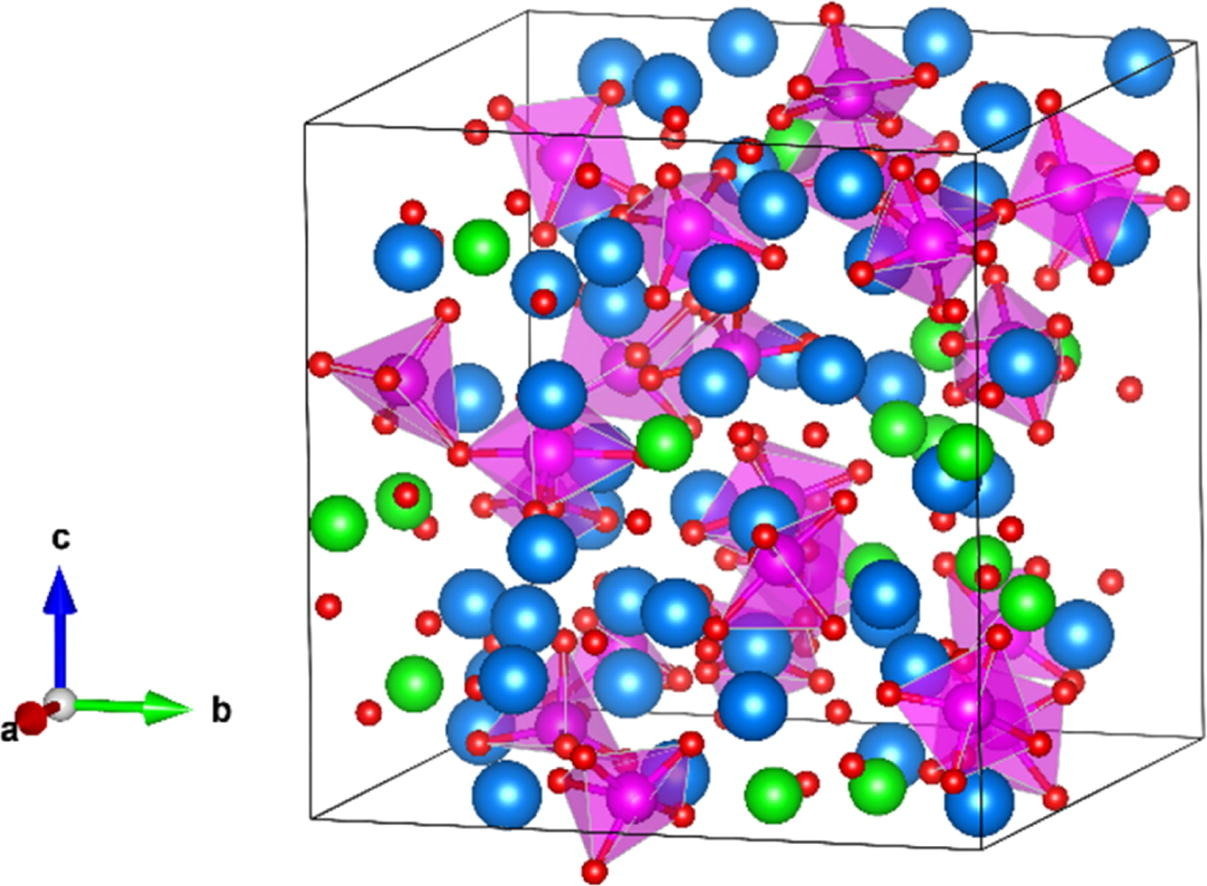
Nuclear power
Molten salts
Nuclear Reactor by Olena Panasovska from Noun Project
Batteries


Electrolyte by M. Oki Orlando from Noun Project
Charge carriers and electrolytes
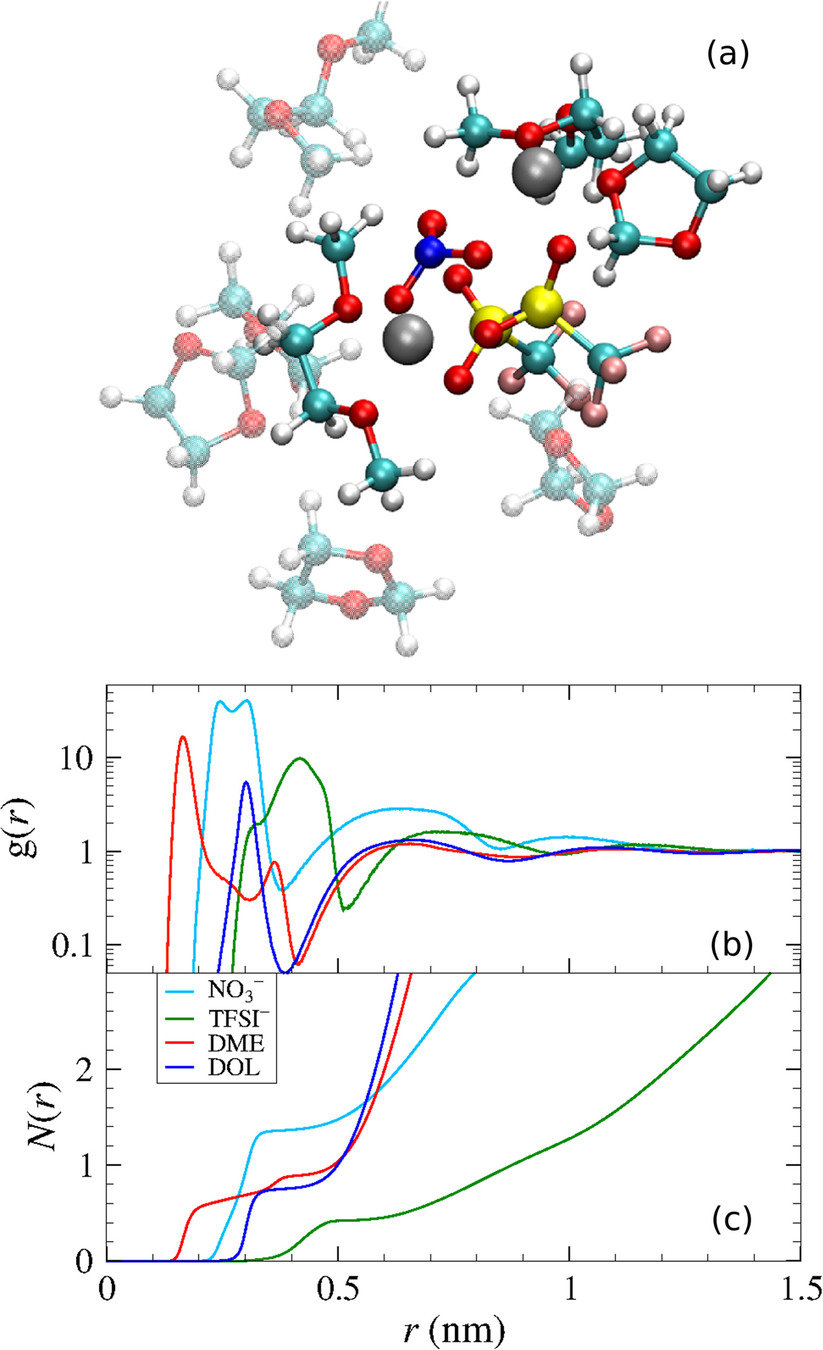
Park, C.; et al. J. Power Sources 2018, 373, 70-78. DOI: 10.1016/j.jpowsour.2017.10.081
Lv, X.; et al. Chem. Phys. Lett. 2018, 706, 237-242. DOI: 10.1016/j.cplett.2018.06.005
Fuel production
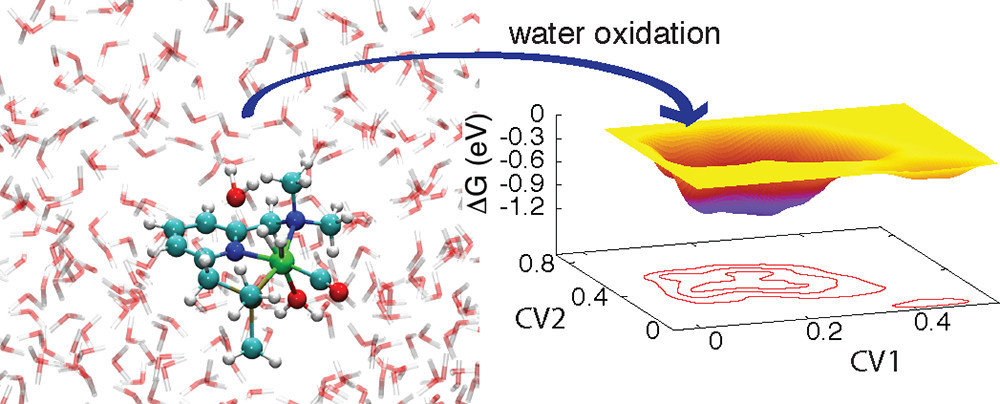
Solvation plays an important role
Catalysts
Ma, C.; et al. ACS Catal. 2012, 373, 1500-1506. DOI: 10.1021/cs300350b
Accelerating exploration with solvents
Computational modeling
Cost

AIMD
Classical MD
Implicit/explicit
Implicit
Confidence
Cost

Screening
approach
Promising candidates
Search space
without experimental data
Solvation treatments
Confidence requires explicit (MD) methods
Goal
Explicit solvation

Lv, X.; et al. Chem. Phys. Lett. 2018, 706, 237-242. DOI: 10.1016/j.cplett.2018.06.005
Let's model a molten salt
DFT
MP2
CCSD(T)

Pro: Fast
Con: Parameters
Classical potential
Quantum chemistry
Pro: Accurate
Con: Cost
We need a new method
Confidence
2x atoms
128x cost
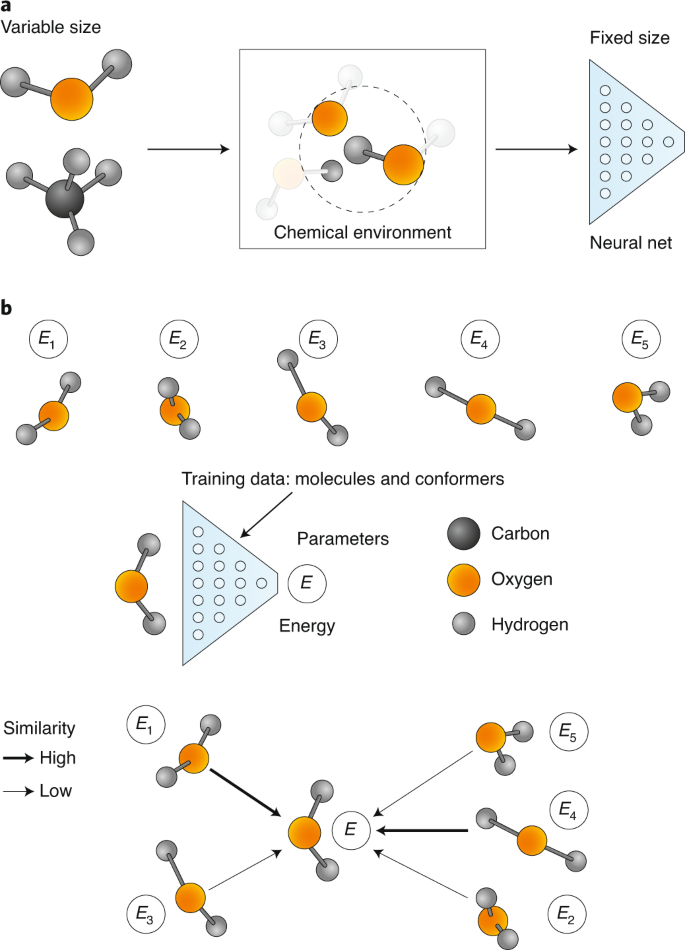
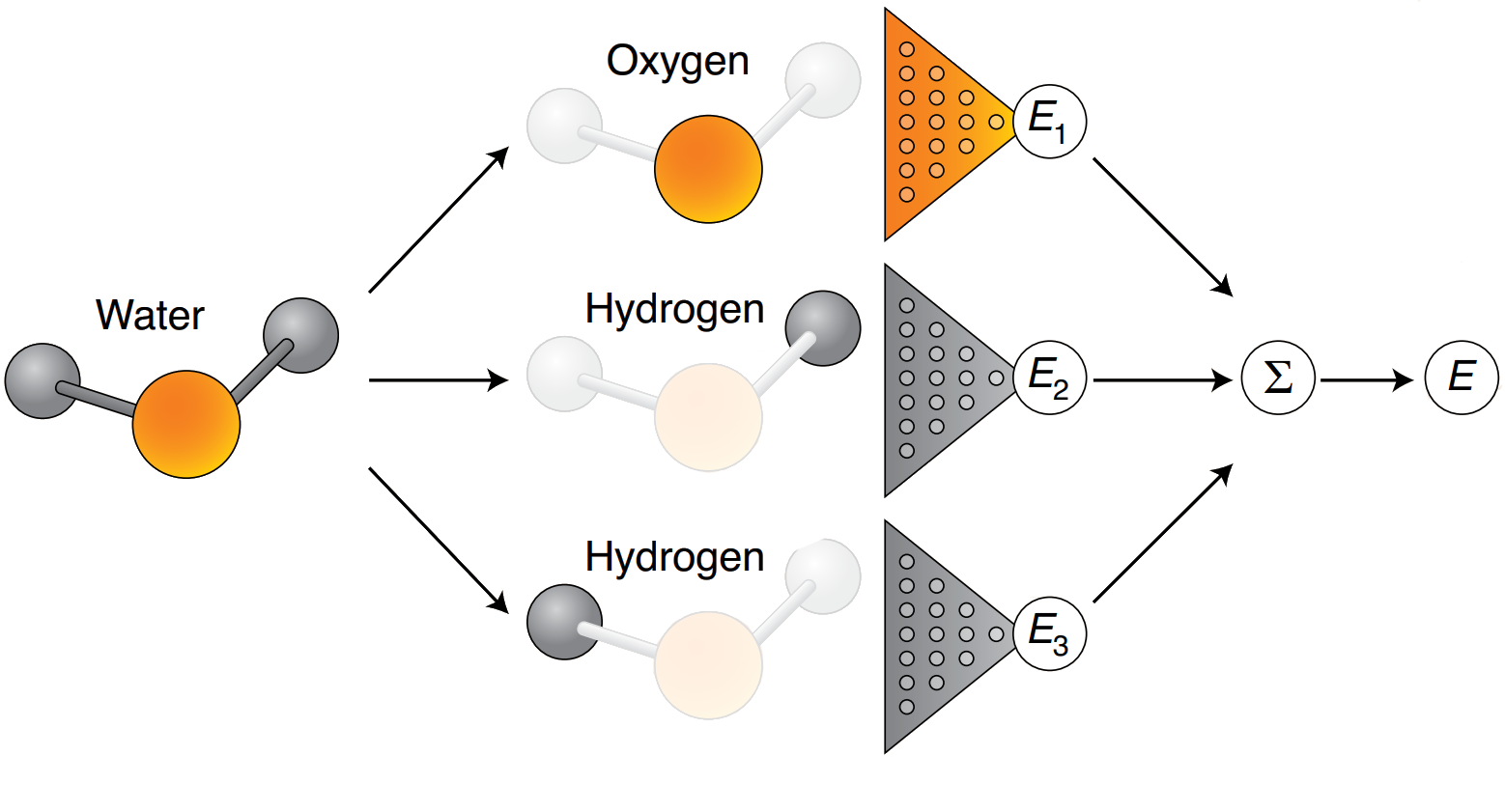
Machine learning potentials



Structure
ML potential
Energy and forces

Quantum
chemistry
Machine learning potentials
Calculate total energies with QC
Training a typical ML potential
Sample tens of thousands of configurations





Approximation: atomic contributions can reproduce total energy
Examples: DeePMD, GAP, SchNet, PhysNet, ANI, . . .
Known
Learned
with a local descriptor
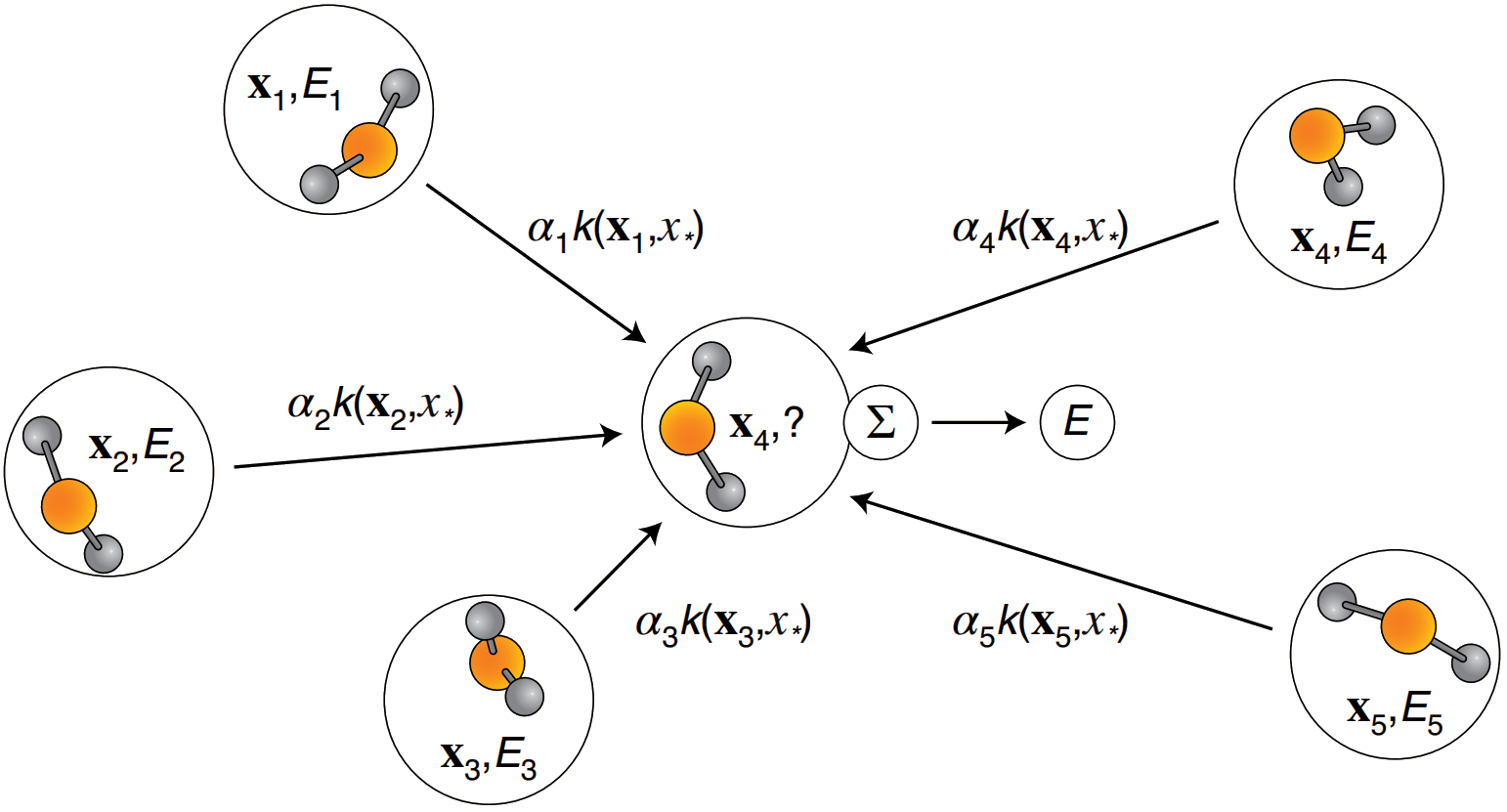
Machine learning potentials


Training with a global descriptor
Local
Global

Encodes each
atom
Encodes entire
structure
increased data efficiency
Many descriptors and parameters
Single descriptor
Machine learning potentials
Training on forces provides more information about the geometry and energy relationship
Chimiela, S. ; et al. Sci. Adv. 2017, 3 (5), e1603015. DOI: 10.1126/sciadv.1603015
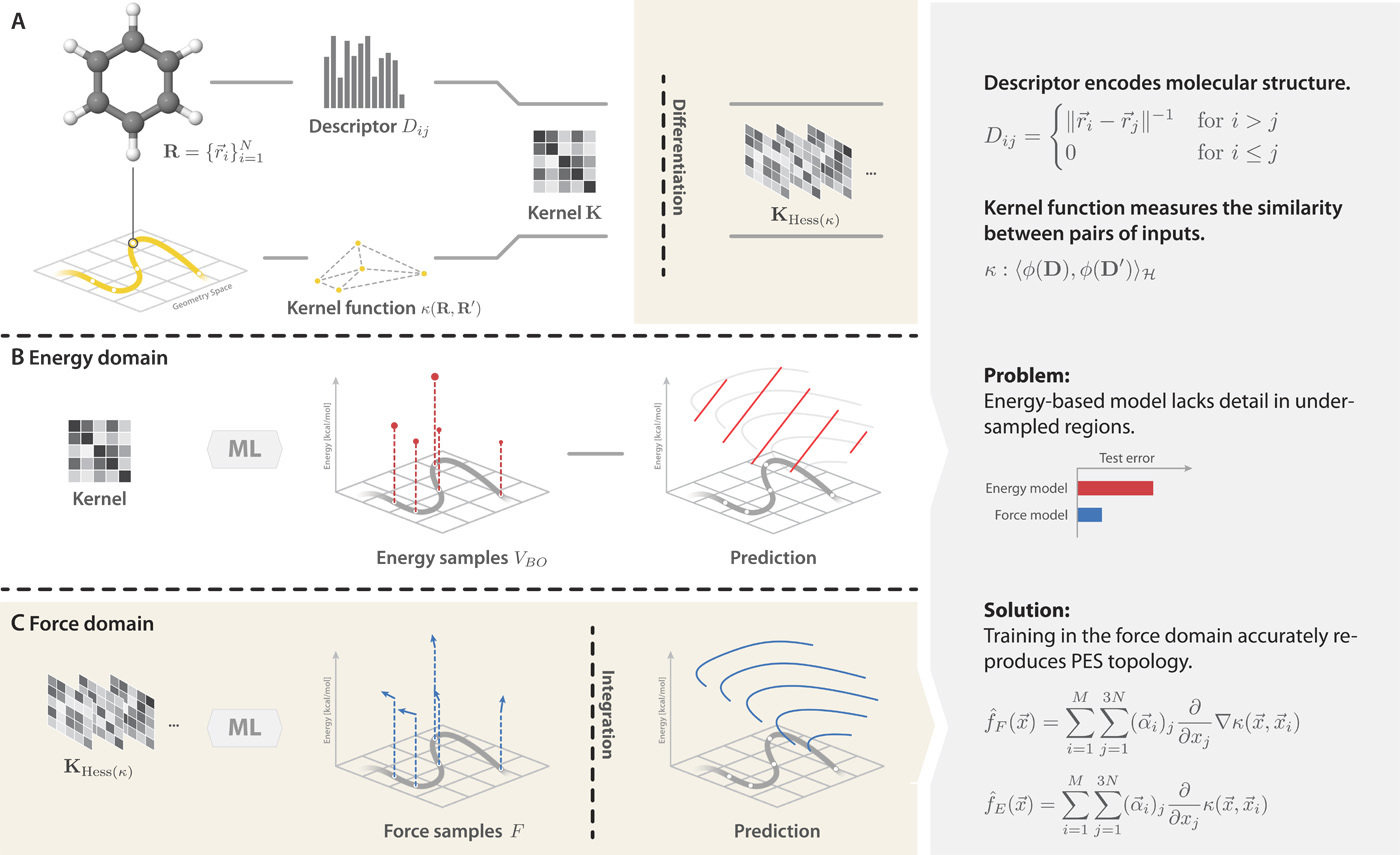

Gradient-domain machine learning (GDML)
Better interpolation
Global descriptor
Training on forces
+
requires 1 000 structures instead of 10 000+
=
Machine learning potentials
System size is still a limiting factor
Global
Fewer structures enables higher levels of theory
CCSD(T)
CCSD(T)
Local
No
Tons of sampling
CCSD(T)
What we want
What we can afford
Descriptor
Size transferable?
CCSD(T)
How can we make GDML potentials size transferable?
Transferability with n-body interactions





-76.31270
-76.31251
-76.31273
-228.96298
-0.00831
-0.00705
-0.00700
-228.93794
(-0.02504)
-228.96031
(-0.00267)




1 body
1+2 body
3-body
+
+
=
+
+
=
Add energy
Remove energy
All energies are in Hartrees
Many-body expansion (MBE)
MBE: the total energy of a system is equal to the sum of all n-body interactions

Truncate
Unlocks size transferability for highly accurate methods
CCSD(T)
Many-body expansion (MBE)
Training a many-body GDML (mbGDML) potential
Sample a thousand
configurations




Calculate n-body energy (+ forces) with QC
Calculate total energies with QC
Known
Known
Learned




Reproduce physical n-body energies
Approximation: atomic contributions can reproduce total energy
Incorporates more physics into our ML potentials
Sample tens of thousands of configurations
Our innovation
- Less training data
- Use higher levels of theory
- Easy to parallelize

Many-body expansion framework accelerated with GDML
Unique opportunity with GDML accuracy and efficiency
If successful



Case study
Modeling three common solvents
Water (H2O)
Acetonitrile (MeCN)
Methanol (MeOH)
Training set
1 000 structures
(instead of 10 000+)
Sampling
n-body structures from GFN2-xTB simulations
Level of theory
MP2/def2-TZVP
ORCA v4.2.0
Case study - Results
Isomer rankings
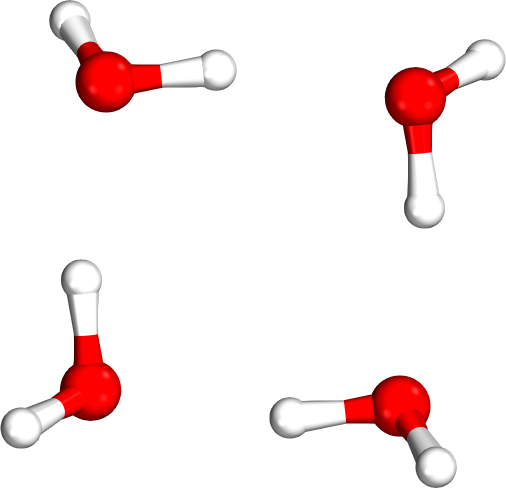
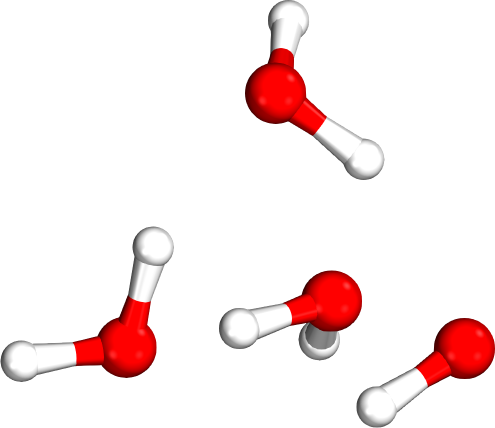
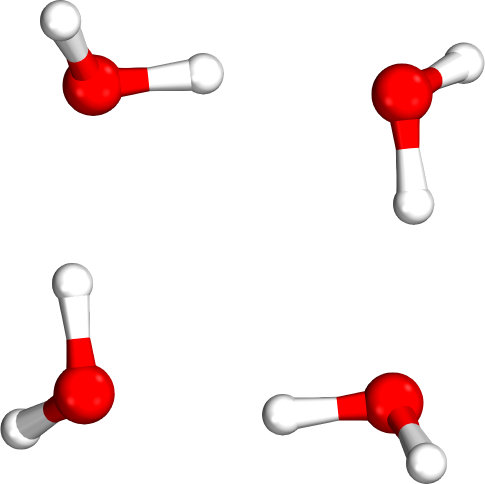
Which tetramer (4mer) has the lowest energy (i.e., global minimum)?
Requires accurate relative energies
Isomer #1
Isomer #2
Isomer #3
Case study - Results
Tetramer rankings

(per monomer)
| System | Energy RMSE [kcal/mol] | Force RMSE [kcal/(mol A)] |
|---|---|---|
| (H2O)4 | 0.82 (0.20) |
0.78 (0.07) |
| (MeCN)4 | 0.29 (0.07) | 0.16 (0.01) |
| (MeOH)4 | 1.37 (0.34) | 1.49 (0.06) |
(per atom)
Many-body GDML accurately captures relative energies


Info: We desire methods with less than 2 kcal/mol error
Does mbGDML scale to larger systems?
| System | Energy Error [kcal/mol] | Force RMSE [kcal/(mol A)] |
|---|---|---|
| (H2O)16 | 4.01 (0.25) | 1.12 (0.02) |
| (MeCN)16 | 0.28 (0.02) | 0.35 (0.004) |
| (MeOH)16 | 5.56 (0.35) | 1.79 (0.02) |
Case study - Results
Consistent normalized errors
Size transferable
Case study - Results
Radial distribution function (rdf)

What we want
r
g(r)

Tells us if we are getting the correct liquid structure
Case study - Results
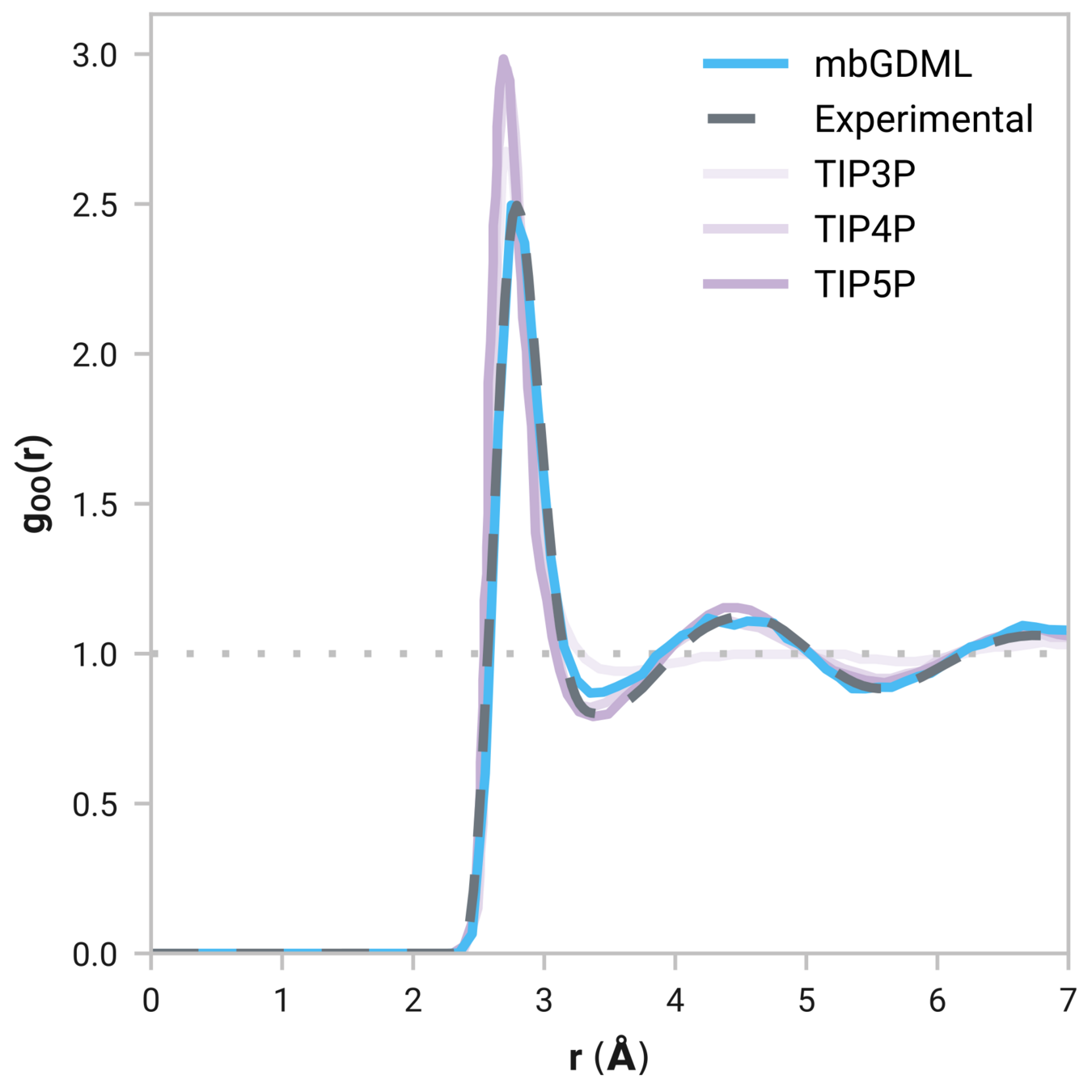
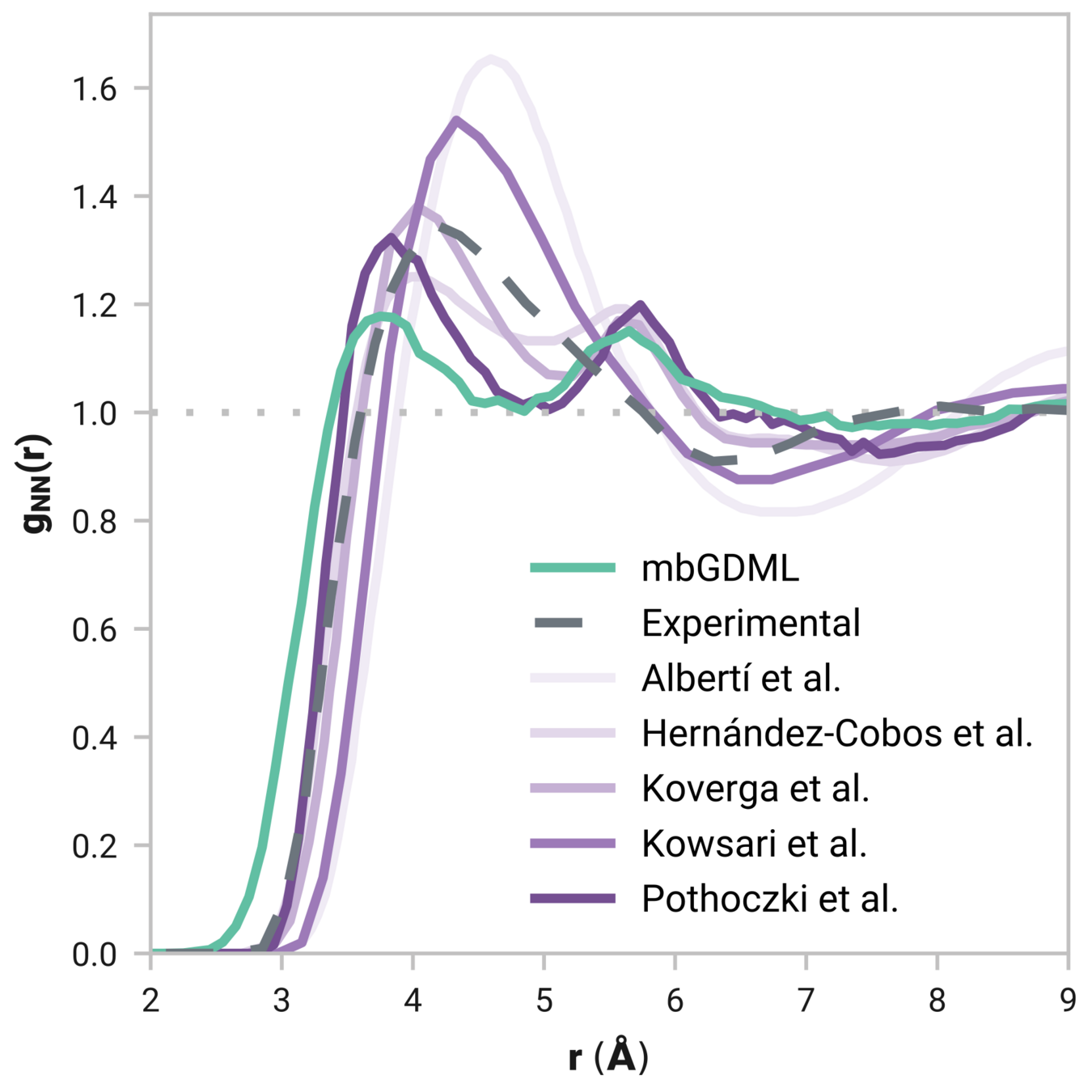
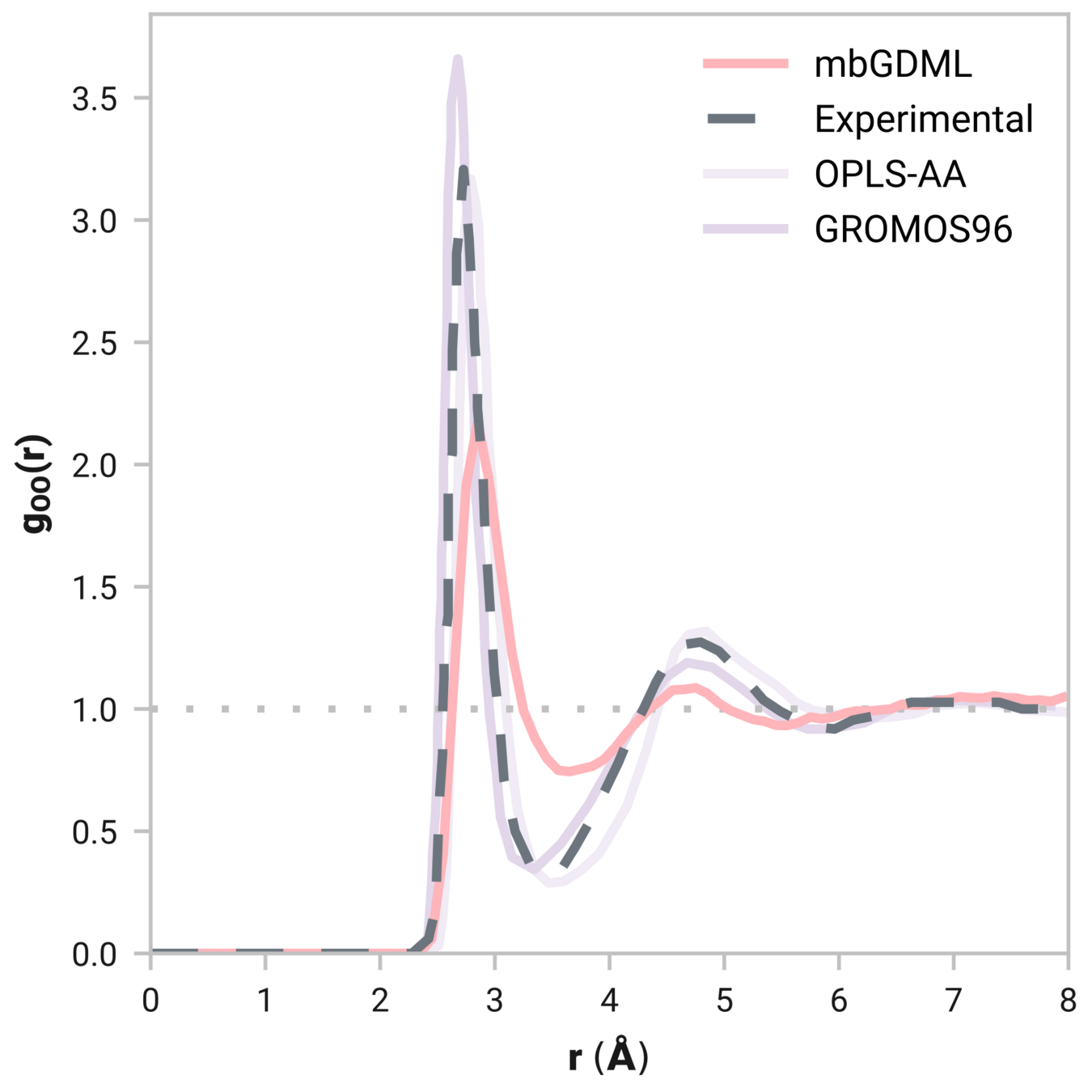
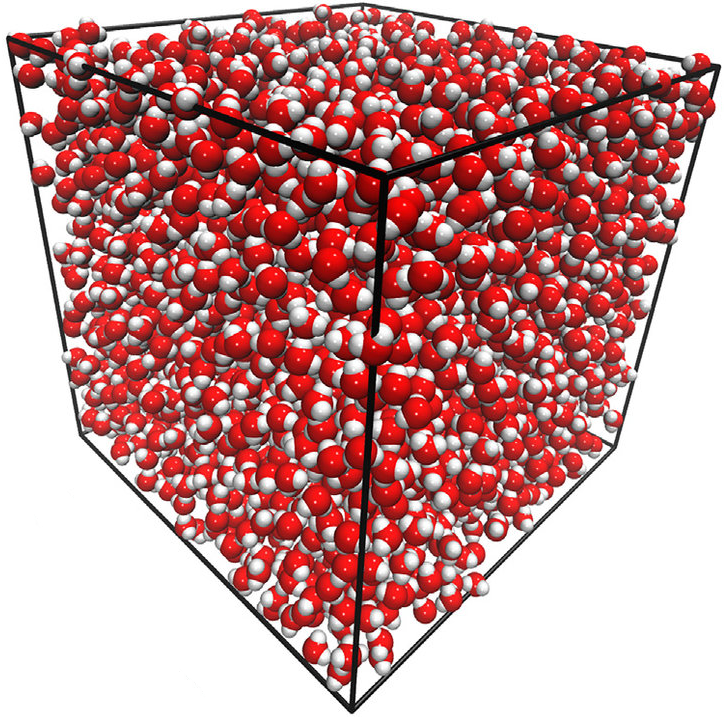
Many-body GDML accurately captures liquid structure
137 H2O molecules
67 MeCN molecules
61 MeOH molecules
Reminder: We have only trained on clusters with up to three molecules
Time for 10 ps MeCN simulation:
mbGDML 19 hours
MP2 23 762 years
20 ps NVT MD simulations; 1 fs time step; Berendsen thermostat at 298 K
Conclusions
Explicit solvent modeling without experimental data
Classical
Ab initio
ML
mbGDML
Training

Speed
Accuracy


Scaling
















Poor
Excellent


Acknowledgments
InSiDe ChEMS group
Dr. John Keith
Dr. Yasemin Basdogan
Dr. Charles Griego
Dr. Emily Eikey
Lingyan Zhao
Barbaro Zulueta
Chinmay Mhatre
Dominick Filonowich
Funding






Office of the Provost
Solvent effects
Modeling in the absence of experimental data

Implicit
Explicit
Implicit/Explicit
Explicit modeling is required for confident predictions
Maldonado, A. M. ; et al. J. Phys. Chem. A. 2021, 125 (1), 154-164. DOI: 10.1021/acs.jpca.0c08961
Global descriptor: inverse pairwise distances
GDML is a highly accurate and efficient ML potential
Gradient-domain machine learning (GDML)
Learns: relationship between structure and atomic forces
Chimiela, S. ; et al. Sci. Adv. 2017, 3 (5), e1603015. DOI: 10.1126/sciadv.1603015


Performance: sub kcal/mol accuracy on 1,000 training structure
No symmetries
Symmetries
Chmiela, S. ; et al. Nat. Commun. 2018, 9 (1), 3887. DOI: 10.1038/s41467-018-06169-2
Machine learning potentials
Closing the gap between ab initio accuracy and classical efficiency
Ab initio accurate

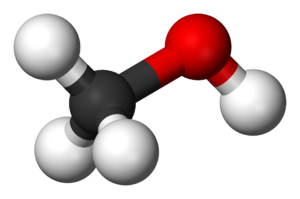

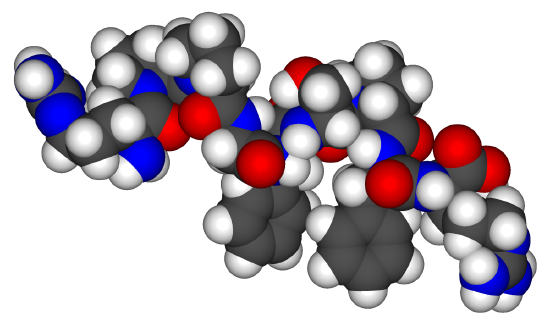
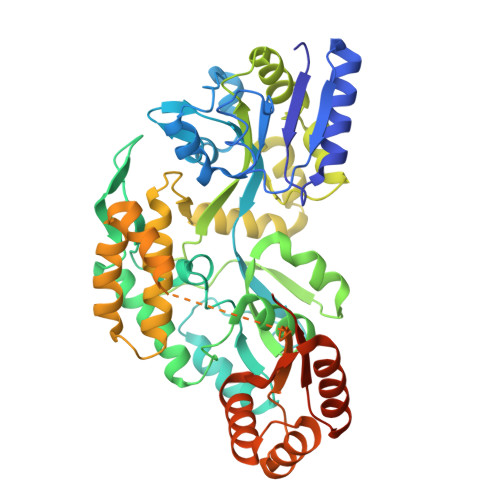
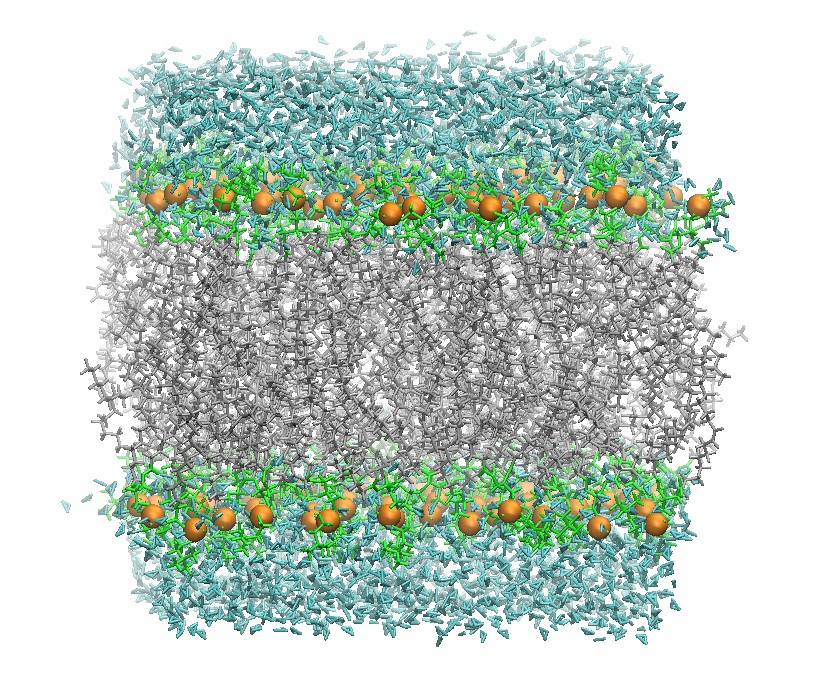

Classical efficient
Machine learning
Many-body GDML
Specifying many-body system

entity_ids: integers that assign an atom to an entity (i.e., molecule, material, functional group)
z: Atomic numbers of all atoms in the system
R: Cartesian coordinates of all atoms
[8, 1, 1, 8, 1, 6, 1, 1, 1]H2O
MeOH
comp_ids: labels that assigned to each entity for model identification
[[ -0.93996, -0.73423, -0.91605],
-0.64198, -0.63683, 0.00659],
-0.40197, -0.12512, -1.34936],
9.26385, -2.64576, -0.31342],
9.47745, -1.80746, 0.18550],
7.82122, -2.82878, -0.46631],
7.21722, -1.88002, -0.42174],
7.43501, -3.56731, 0.30104],
7.54895, -3.05498, -1.46330]]H2O
MeOH
['h2o', 'meoh']H2O
MeOH
[0, 0, 0, 1, 1, 1, 1, 1]H2O
MeOH
Many-body GDML
Driving many-body predictions

Model
n-body energy & force predictions
Parallel workers
Predictor
Customizable
n-body
predictions
+
n+1
n-body combinations generator
Total energy and forces


Model
n-body energy & force predictions
Parallel workers
Predictor
Customizable
n-body
predictions
+
n+1
n-body combinations generator
Total energy and forces

Case study - Methods
MBE distance-based screening
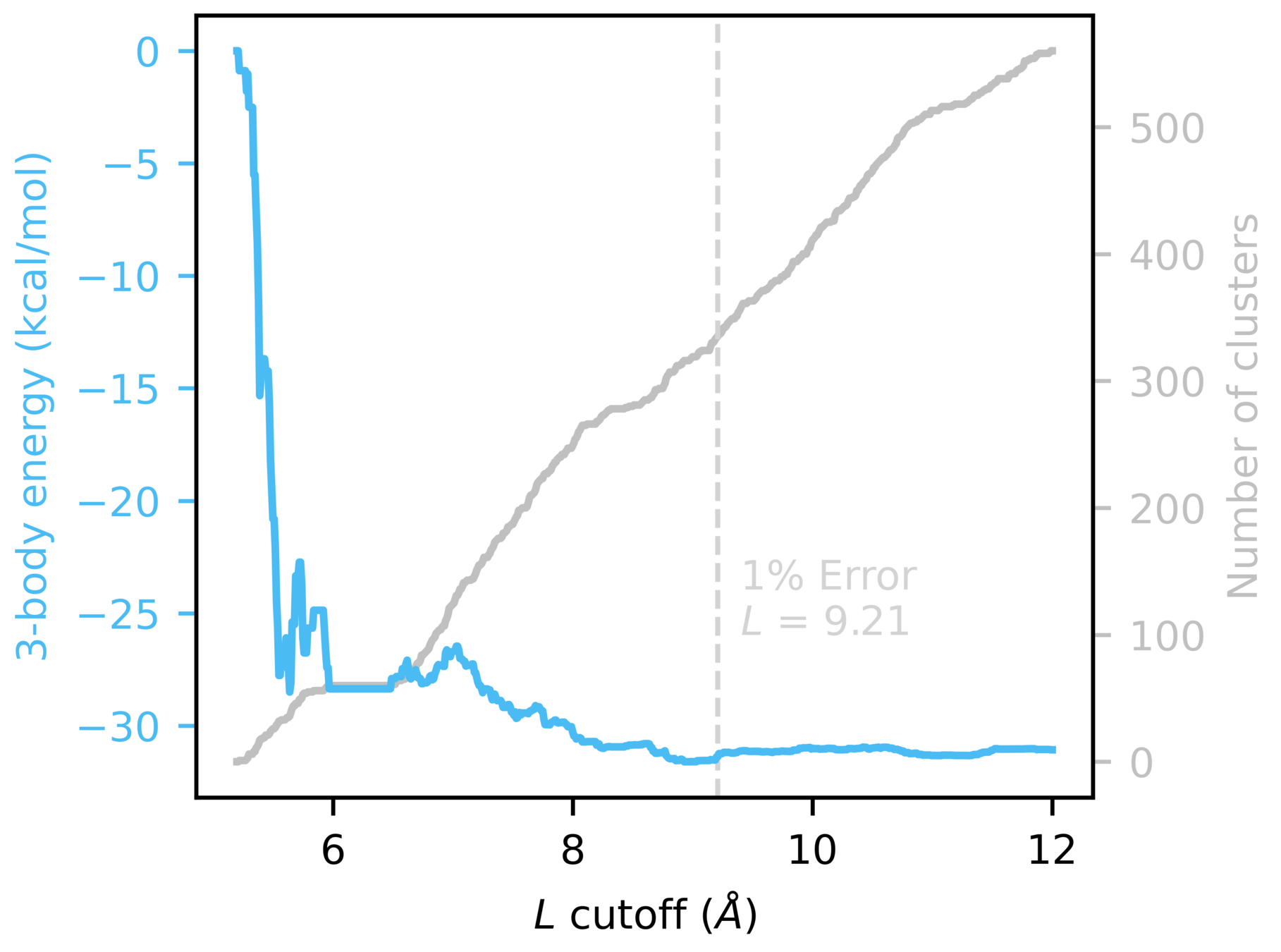
Focus predictions on clusters with meaningful n-body contributions
Training ML potentials
Methods:
GDML
Training set:
1,000 structures

Used 16mer to determine 2- and 3-body cutoffs
Many-body GDML
Developed a Python package for creating, using, and analyzing many-body ML potentials
Supported ML potentials:
GDML, SchNet, GAP
Interfaced with ASE
Parallelized with Ray

Archived on Zenodo

Motivation
Solvated reaction mechanisms
For example, reduction of carbon dioxide with sodium borohydride
Explicit local environments are necessary for accurate predictions
Typical for our systems of interest


Maldonado, A. M.; et al. J. Phys. Chem. A 2021, 125 (1), 154-164. DOI: 10.1021/acs.jpca.0c08961
Local descriptor
Machine learning potentials
ML potential descriptors require thousands of total energy (+ force) evaluations to train
Global descriptor
Friederich, P. ; et al. Nat. Mater. 2021, 20 (6), 750-761. DOI: 10.1038/s41563-020-0777-6
Case study - ML potential
Gradient-domain machine learning (GDML) learns the relationship between atomic structure and forces


Chimiela, S. ; et al. Sci. Adv. 2017, 3 (5), e1603015. DOI: 10.1126/sciadv.1603015
Training set
1,000 structures
(~10x less than most ML potentials)
Validation set
2,000 structures
Sampling
Semiempirical QC MD simulations
Why not train ML potential on total energies of small clusters?
| System Size | Many-body Energy MAE | Standard Energy MAE |
|---|---|---|
| (MeOH)4 | 2.59 (0.65) | 11.00 (2.75) |
| (MeOH)5 | 3.30 (0.66) | 18.78 (3.76) |
| (MeOH)6 | 3.75 (0.63) | 22.81 (3.80) |
| (MeOH)16 | 3.42 (0.21) | 53.36 (3.34) |
Case study - Results

Train SchNet potential on 1mer, 2mer, and 3mer energies



There are limitations on size transferabilities of local descriptors
Avoids combinatorics of many-body expansions
(per monomer)
SOAP descriptor
Smooth overlap of atomic positions

Neural network potential
High-dimensional neural network potential

Case study - Results
Isomer rankings

(per monomer)
| Size | Energy RMSE [kcal/mol] | Force RMSE [kcal/(mol A)] |
|---|---|---|
| 4mer | 0.82 (0.20) | 0.78 (0.07) |
| 5mer | 1.12 (0.22) | 0.91 (0.06) |
| 6mer | 1.80 (0.30) | 1.26 (0.07) |
(per atom)
mbGDML accurately predicts global minimum for water isomers
Case study - Results

Isomer rankings
(per monomer)
| Size | Energy RMSE [kcal/mol] | Force RMSE [kcal/(mol A)] |
|---|---|---|
| 4mer | 0.29 (0.07) | 0.16 (0.01) |
| 5mer | 0.33 (0.07) | 0.22 (0.01) |
| 6mer | 0.34 (0.06) | 0.24 (0.01) |
(per atom)
mbGDML is only as good as many-body expansions
Several low-lying isomers within 1 kcal/mol
Isomer rankings

Case study - Results
(per monomer)
| Size | Energy RMSE [kcal/mol] | Force RMSE [kcal/(mol A)] |
|---|---|---|
| 4mer | 1.37 (0.34) | 1.49 (0.06) |
| 5mer | 1.87 (0.37) | 1.77 (0.06) |
| 6mer | 2.29 (0.38) | 1.73 (0.05) |
(per atom)
Higher-order contributions mainly impact absolute energies
Pitt ChemE Research Day 2022
By aalexmmaldonado
Pitt ChemE Research Day 2022
Presentation for Research Day in the Chemical and Petroleum Engineering department at the University of Pittsburgh.
- 358



