Microenvironmental regulation of stem cells in intestinal homeostasis and cancer

Chakshu Tandon
chakshu.tandon@rutgers.edu
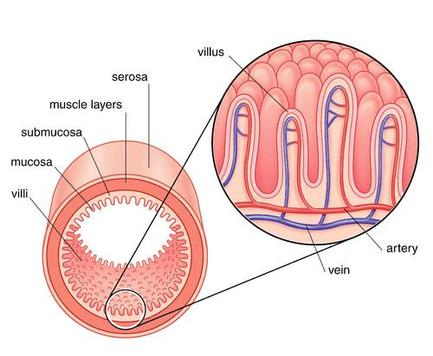
The function of the intestines makes them incredibly prone to damage.
The epithelial cells lining the stomach and intestine are replaced every 4-5 days.
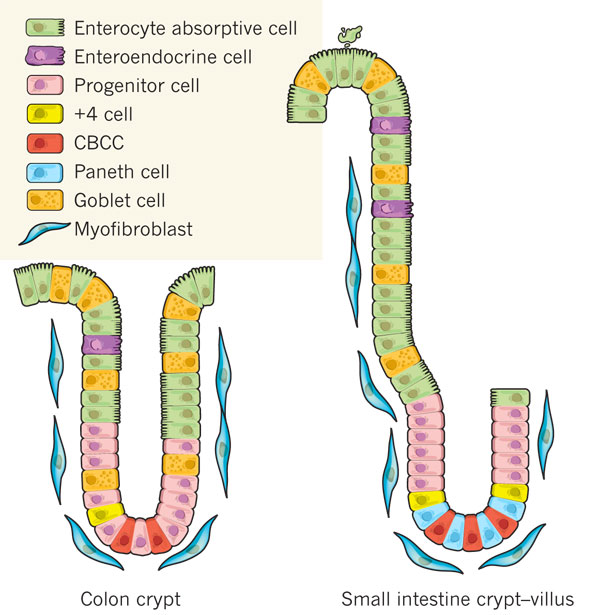

- Enterocyte cell - Absorbtion
- Enteroendocrine cell - Secretion of Hormones
- Progenitor cell - Can further differentiate into other intestinal cells
- +4 cell - Back up stem cells (divide slowly)
- CBCC - Active intestinal stem cells
- Paneth cell - Secrete antimicrobial peptides that provide immunity
- Goblet cell - Secrete mucus to provide gastrointestinal barrier
- Myofibroblast - Provide muscle-like flexibility
Video
Time
Homeostasis is maintained in the intestine by the interaction of morphogenetic pathways in the microenvironment of the cyrpt.
What-tadi-what?
Morphogenetic Pathways?
Microenvironment?
Homeostasis?
INTESTINAL STEM CELLS (ISC)
CANCER STEM CELLS (CSC)
COLORECTAL CANCER (CRC)
Most common human cancer
Approximately 1.2 million new cases every year
FINDING
ISCs
Present
Past
ISCs were a rather elusive entity at the bottom of the intestinal crypt and could be identified only by indirect measurements.
Different biological markers can help identify cells within the intestinal crypt including ISCs
Most of the exciting stem cell action happens at the bottom of the crypt where there are fewer shear stresses and toxic hazards. Cells are created and pushed upward where they further differentiate into enterocyte and enteroendocrine cells.
Back to the pathways
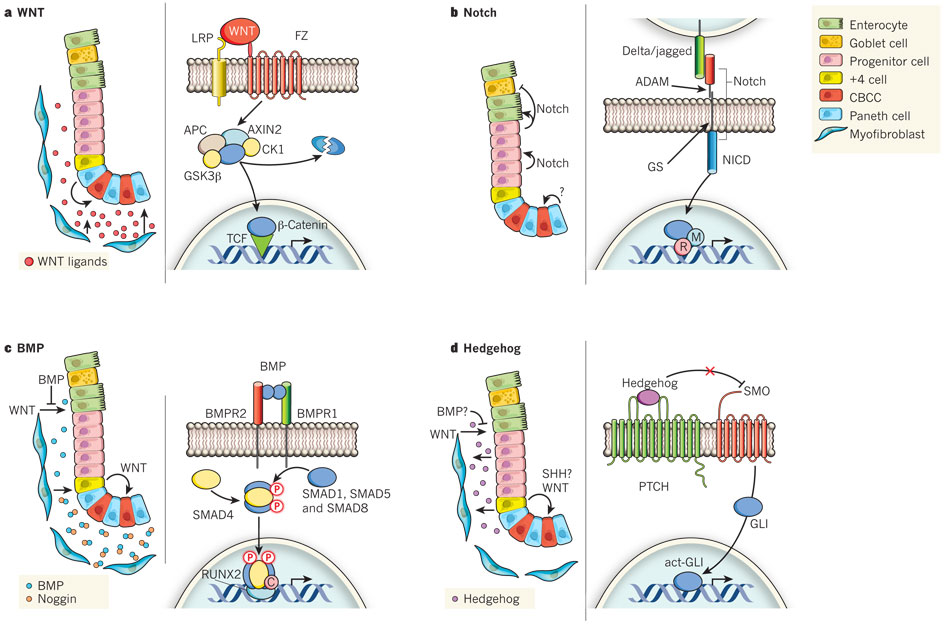
WNT
Proliferation and Maintainance of ISCs
For instance, inactivating mutations in [Apc] or activating mutations in B-catenin - both key WNT signaling factors - drive intestinal hyperplasia (the enlargement of an organ, also an early stage of cancer)
Paneth cells also have the function of releasing WNT(3a).
Deletion of paneth cells decreases the number of ISCs in the crypt.
Notch
Notch cooperates with WNT to drive proliferation and is involved in lineage-fate decisions.
Notch activity determines lineage decisions between enterocyte and secretory cell differentiation
Inhibition of the Notch pathway results in a massive increase in goblet cells, whereas its activation results in goblet-cell depletition
When WNT product is high,
Notch
ISC Proliferation
When WNT product is low,
Notch
Enterocyte Differentiation
Bone Morphogenic Proteins (BMP)
Counteract WNT signals to avoid excessive proliferation, especially at the top of the crypt.
They favor lineage fate towards secretory cells (i.e. Goblet and Enteroendocrine)
Hedgehog (IHH/SHH)
The role of the Hedgehog, which acts through the membrane proteins smoothened and patched, in intestinal homeostasis is more confusing.

Deletion of patched, a negative regulator of the pathway, inhibits WNT in the small intestine and leads to premature enterocyte differentiation.
Physiologically, it seems that Indian Hedgehog (IHH), which is mainly expressed by differentiated epithelial cells, signals to the mesenchyme, where it is thought to induce BMP secretion.
The complex interaction of these pathways makes it incredibly difficult to model intestinal behavior in vitro.
When Things Go Wrong

The deregulation of morphogenetic pathways can trigger cancerous growth
Environmental
Genetic
- Western Diet
- History of inflammatory bowel disease
- Predispositions such as familial adenomatous polyposis (FAP)
- Hereditary non-polyposis CRC (HNPCC)
FAP
Patients with FAP develop hundreds of colonic polyps early on in life, and their lifetime risk of developing CRC is almost 100%
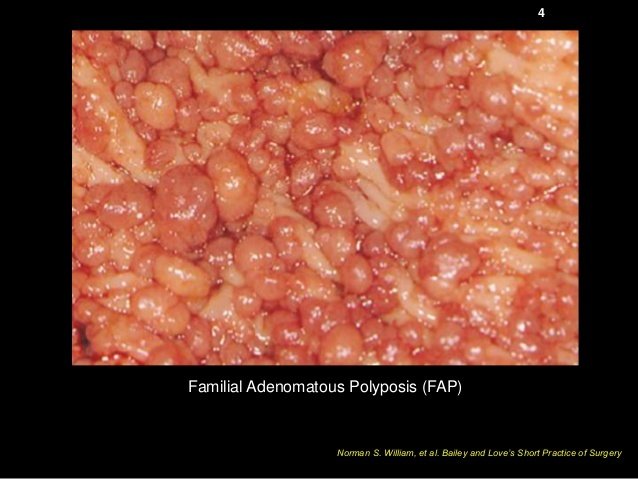
The syndrome is caused by a mutation in the APC gene which is a "crucial negative regulator of the WNT pathway," causing uncontrolled tissue growth.
First, tumorigencity induced by mutations is proposed to be different when these are introduced in ISCs compared with transit-amplifying or differentiated cells, which has led to the idea that ISCs are the cell of origin in cancer.
Other cancers such as chronic myeloid leukaemia, prostate carcinoma, and glioblastoma have similar processes that initiate tumor proliferation.
In humans, tumerogenisis occurs exclusively in the colon citing differences in structure and morphogenetic pathways
My brain hurts
There is so much more detail in the paper that going deeper would just add summary. Instead lets just review some of the basic details about stem cells in the intestine.
- There are four pathways in intestinal epithelial cells that govern growth and differentiation. They are namely, WNT, Notch, BMP, and Hedgehog.
- Of these, WNT plays a critical role in the proliferation of cells. An overactive WNT pathway can lead to CRC.
- BMP counteracts WNT to prevent tumor formation.
- CSCs are colon cancer cells that have the ability to self-renew, expand, and differentiate.
- Their flexibility and capacity to differentiate makes it incredibly challenging to target CSCs.

Chakshu Tandon
chakshu.tandon@rutgers.edu
Microenvironmental regulation of stem cells in intestinal homeostasis and cancer
By Chakshu Tandon
Microenvironmental regulation of stem cells in intestinal homeostasis and cancer
- 929



