Science summative task
By: Marcela Salini, Facundo Reyes, Luca Mercy & Joaquin Araneta
〞
How do the chosen gasses (CH4, NO2, CO2, H2O) affect the temperature (ºC) of air in our temperature?
Theorical background
Mol size
Molecule size is inherently related to the amount of radiation a molecule takes in, as the molecule’s bonds’ (Absorption bands; “bands” of electromagnetic absorption between particles) and Dipole Moments (Product from slices of time when positive and negative charges in a molecule split, letting radiation get absorbed by molecules a process of vibration.
gwp
GWP (Global Warming Potential) is a concept conceived by scientists where they measure the energy absorbed by greenhouse gasses over a period of time, and it’s base value is described by CO2 measures.
GH Gases
- CH4
- NO2
- H2O
- CO2


Aim and problem to be solved
- To evaluate and compare the impact of distinct greenhouse gasses (CH4, NO2, CO2, H2O) on temperature changes within a controlled environment
- To compare and contrast the heating capacities of CH4, NO2, CO2, and H2O by monitoring temperature changes, providing insights into the differential impacts of these gasses on the greenhouse effect
- To examine the varying abilities of CH4, H2O, CO2 and NO2 to retain heat within a closed environment, aiming to determine the differential impact of these gasses on temperature alterations attributed to the greenhouse effect.
- To investigate the differential heating effects caused by CH4, CO2, N2O to perceive their unique roles in altering temperatures within enclosed settings.
Given their respective Global Warming Potentials (GWPs), methane (CH4) will likely contribute most significantly to the increased global temperature in the short term due to its higher potency as a greenhouse gas than NO2 and CO2 and lastly H2O, despite its lower atmospheric concentration compared to carbon dioxide (CO2) over a specific timeframe.
Hypothesis

Variables
I.V: Gasses within the bottle (CH4, NO2, CO2, H2O)
D.V: Temperature (ºC) of the air after 1h
To control
- Wattage on the Lamp
- Distance of the Lamp from the Bottles: 14cm
- Amount of Water: 250ml of water in one bottle
- GH Gasses within the Bottle
- Amount of tablets: 0,5gr in one bottle
- Type of Bottle (Container):
- Thermometer:
Equipement
-
Personal Equipment:
- Laboratory Glasses
- Laboratory Coat
- Laboratory Gloves (Latex)
-
Laboratory made Gasses (CH4 - N2O - H2O)
-
Plastics Bottles 500ml (1/per Gas)
-
Thermometer (Alcohol T° & Electronic T°)
-
Rubber Stoppers (Bottles)
Reagents
-
Laboratory Lamp (100W)
-
Gas Effervescents (CaCO3)
-
Chronometer (Electronic)
Equipement and reagents required

Personal and enviromental safety measures

methane (Ch4)


Signal
Danger
GHS Hazard Statements
H220: Extremely flammable gas [Danger Flammable gases]
Molar mass
16.043 g·mol−1
Appearance
Colorless gas
Odor
Odorless
*
*
*1. Compressed gas 2. Flammable
Nitrous oxide (N2O)

Signal
Danger
GHS Hazard Statements
H270 (100%): May cause or intensify fire; oxidizer [Danger Oxidizing gases]
H280 (64.27%): Contains gas under pressure; may explode if heated [Warning Gases under pressure]
H281 (32.61%): Contains refrigerated gas; may cause cryogenic burns or injury [Warning Gases under pressure]
Molar mass
44.013 g/mol
Appearance
Colorless gas
Odor
Odorless
*
*
*1. Compressed gas 2. Oxidizer

Carbon dioxide (CO2)

Signal
Warning
GHS Hazard Statements
H280 (81.38%): Contains gas under pressure; may explode if heated [Warning Gases under pressure]
H281 (26.69%): Contains refrigerated gas; may cause cryogenic burns or injury [Warning Gases under pressure]
Molar mass
44.009 g/mol
Appearance
Colorless gas
Odor
At low concentrations, the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor.
*
*Compressed gas
-
Prepare the glass containers, ensuring they're clean and dry.
-
Label each container to indicate the gas to be tested (CH4, CO2, N2O) and set up a control container.
-
Place a thermometer inside each container without touching the sides.
-
Position the containers equidistant from the light source in a controlled environment.
-
Introduce the specific gas into the labeled containers in measured quantities.
-
Leave one container empty (control) without adding any additional gasses.
-
Record initial temperatures of all containers, including the control, before exposing them to the light source. Note the starting time.
-
Turn on the light source and ensure consistent heat distribution among the containers.
-
Regularly monitor and record temperatures in each container at set intervals (every 5 minutes) for a specific duration (an hour).
-
Stop the experiment after the set duration and record final temperatures for each container.
-
Compare temperature changes between containers with different gasses and the control group.
-
Analyze the data to determine which gas caused the most significant temperature increase within the given timeframe.
-
Draw conclusions based on observed temperature changes, noting the impact of different gasses on temperature rise due to the greenhouse effect.
-
Ensure safety precautions when handling gasses and experimental materials throughout the procedure.
Procedure

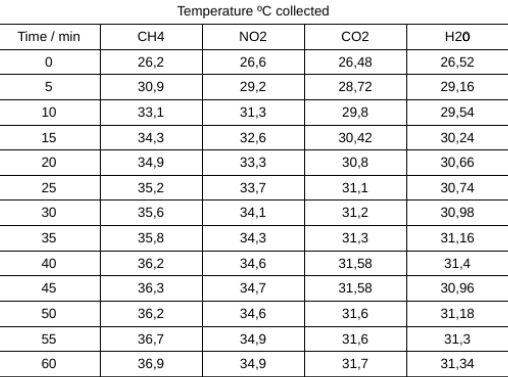
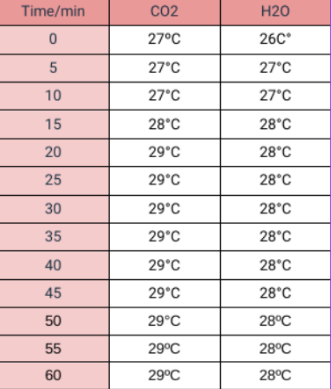
Tables
Table 1: contains info from the whole class
Table 2: Contains the info we recolected during our experiment
Graph
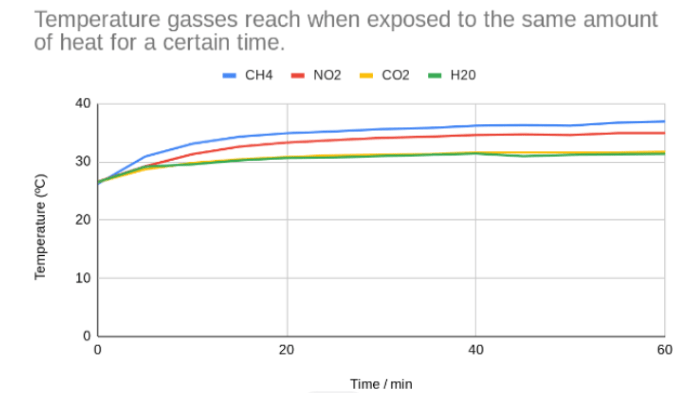

Conclusion
In this practical the aim was To evaluate and compare the impact of distinct greenhouse gasses (CH4, N2O, CO2, H2O) on temperature changes within a controlled environment such as to compare and contrast the heating capacities of CH4, N2O, CO2, and H2O by monitoring temperature changes, providing insights into the differential impacts of these gasses on the greenhouse effect also to examine the varying abilities of CH4, H2O, CO2 and N2O to retain heat within a closed environment, aiming to determine the differential impact of these gasses on temperature alterations attributed to the greenhouse effect and to investigate the differential heating effects caused by CH4, CO2, N2O to perceive their unique roles in altering temperatures within enclosed settings. Our results were more Accurate than precise as they showed a latent difference to other groups’ results varying in small degrees.
Potential difficulties would be the main interference of a class-set chronometer interrupting the group’s chronometer with around a half minute difference, and inherently a potential improvement to our investigation would be better confidence and precision whilst writing down measurements along with consistency with decisions.
OUR COMMITMENT
What we want to incentivate and spread is the consciousness of naturally emitted gasses and their impact, as our lives are constantly reliant on what they do to our planet.
What I mean with this is the constant relation of our greenhouse gasses with our ecosystem as constant emissions are something we can not control, but because as a society we exploit our planet's natural limits for profit which is product for our slow, slow demise.
BIBLIOGRAPHY
References
\/. (2023, June 16). YouTube. Retrieved November 30, 2023, from https://www.sciencedirect.com/topics/engineering/dipole-moment
The Atmosphere. (2023, July 28). National Oceanic and Atmospheric Administration. Retrieved December 7, 2023, from https://www.noaa.gov/jetstream/atmosphere
Atmospheric Methane. (2005, February 21). NASA Earth Observatory. Retrieved December 7, 2023, from https://earthobservatory.nasa.gov/images/5270/atmospheric-methane
Carbon Dioxide | CO2 | CID 280. (n.d.). PubChem. Retrieved December 7, 2023, from https://pubchem.ncbi.nlm.nih.gov/compound/Carbon-Dioxide
Glossary:Global-warming potential (GWP) - Statistics Explained. (n.d.). European Commission. Retrieved November 30, 2023, from https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary:Global-warming_potential_(GWP)
Herring, D. (2020, October 29). Doesn't carbon dioxide in the atmosphere come from natural sources? Climate.gov. Retrieved December 7, 2023, from https://www.climate.gov/news-features/climate-qa/doesnt-carbon-dioxide-atmosphere-come-natural-sources
Lindsey, R. (n.d.). Climate Change: Atmospheric Carbon Dioxide | NOAA Climate.gov. Climate.gov. Retrieved December 7, 2023, from https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide
Methane | CH4 | CID 297. (n.d.). PubChem. Retrieved December 7, 2023, from https://pubchem.ncbi.nlm.nih.gov/compound/Methane#section=Physical-Description
Nitrogen Dioxide | NO2 | CID 3032552. (n.d.). PubChem. Retrieved December 7, 2023, from https://pubchem.ncbi.nlm.nih.gov/compound/Nitrogen-Dioxide
Overview of Greenhouse Gases | US EPA. (2023, October 10). Environmental Protection Agency. Retrieved December 7, 2023, from https://www.epa.gov/ghgemissions/overview-greenhouse-gases
Water | H2O | CID 962. (n.d.). PubChem. Retrieved December 7, 2023, from https://pubchem.ncbi.nlm.nih.gov/compound/Water
Water Vapor. (n.d.). NASA Earth Observatory. Retrieved December 7, 2023, from https://earthobservatory.nasa.gov/global-maps/MYDAL2_M_SKY_WV
thank you!
Science Summative Task
By marsalinii
Science Summative Task
- 74


