Melva Mitchell Fort Worth Regulations for publishing a clinical trial state that all treatment results, whether positive or adverse, must be reported. If no adverse effects are found, the investigators should state this in the report.
"Complete information on incidence, severity, duration, frequency and method of reporting adverse effects was only included in one study," the researchers note.
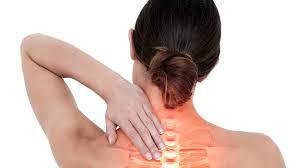
The conclusion, they add, is that "adverse effects are poorly reported in recent randomized clinical trials of chiropractic manipulations." This lack of information is creating a false image about the safety of these treatments.
"Most people believe that alternative therapies are safe"