Nuclear Physics
The Atomic Nucleus, Radioactivity, Fission and Fussion
M. Rocha
Physics 2B
The Atomic Nucleus
Rutherford Scattering
The Rutherford scattering experiment (1911) demonstrated that atoms must have a core (Nucleus)
The Atomic Nucleus

-10
-14
-15
-18
-18
Today we know the atomic nucleus is made out of protons and neutrons, which subsequently are made out of quarks
Nuclear Forces
Electric Force
Strong Force
vs.
Electric force pushes protons apart
But the strong force pulls the quarks inside the nucleons together
The electric force has a long range
The strong force has a short range
The strong force is stronger than the electric force only when the nucleons are very close together (short distances)


Heavy Nuclei
As nuclei get heavier, they need more neutrons than protons in order for the attractive strong force to dominate over the repulsive electric force

Unstable Nuclei


The lightest unstable atom is Technetium
Radioactivity
Isotopes Review


= Protons + Neutrons
= Number of Protons
Hydrogen-1
Hydrogen-2
Hydrogen-3
Checkpoint 1
Carbon-14 is a radioactive Carbon isotope with 6 protons. Carbon-14 decays into Nitrogen-14 by one of its neutrons decaying into a proton. How many neutrons does Nitrogen-14 has?
Nitrogen-14 has 7 neutrons and 7 protons
Neutron Decay
Neutrons are unstable by themselves but stable in nuclei.
A high neutron/proton ratio however makes nuclei unstable, causing the neutrons to decay into protons
Neutrons decay into a proton, an electron and an antineutrino

Radioactive Decay
Unstable atoms undergo radioactive decay in order to find stability
Beta Decay: A neutron decays into a proton emitting an electron (Beta Particle)
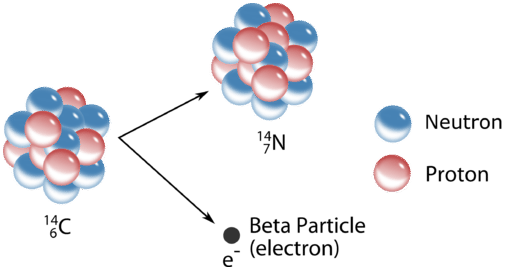
Alpha Decay: A Helium atom (alpha particle) is ejected from the parent nucleus

Checkpoint 2
If an atom decays emitting alpha radiation, would the decay product (daughter atom) be an isotope of the original atom, or a new element?
A new element because the number of protons changes
Gamma Decay
Gamma decay is the emission of a gamma photon from the transition of a nucleus in an excited energy state to a lower (relaxed) energy state
There is no change in the atomic or mass number of the nucleus after the gamma decay
X-Rays

X-Rays are photons with a lot less energy than gamma rays. X-Rays originate from high energy electronic energy transitions, not from nuclear radioactive decays
X-Rays penetrate through skin but are stopped by bones


Radiation Penetration Power
- Alpha particles have the most charge and the least speed, so they can be easily stopped. A few centimeters of air or a sheet of paper is enough to stop alpha rays.
- Beta particles travel faster and have less charge, so they are harder to stop. Aluminum foil or any other thin metal sheet will stop them.
- You need a thick layer of lead to stop gamma rays.

Radioactive Ray Dispersion
You can separate the alpha, beta and gamma rays from a sample with a magnetic field


Summary of Nuclear Radioactive Decays
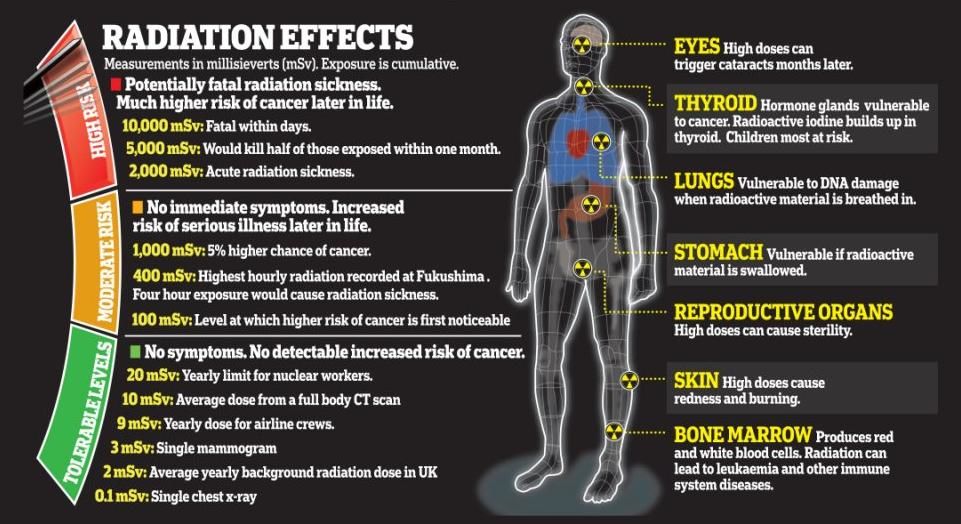
Radiation Exposure
Units of Radiation Exposure
RADS (Radiation Absorbed Dose): Is a unit of radiation dosage that measures the amount of energy in Joules absorbed per 1 kg of tissue
REMS (Roentgen Equivalent Man) and Sieverts: Units of radiation potential damage. They correlate the absorbed dose of any radiation to the biological effect of that radiation. Since not all radiation has the same biological effect, the dosage is multiplied by a "quality factor" Q.
Q = 1 for X, Gamma and Beta radiation
Q = 10 for Alpha radiation
,
(SI unit)
Radon Gas
We all get about a 100-150 millirem radiation dose per year from radon gas
Radiation Exposure
Radiation exposure/dose per year depends on life choices—however the average, healthy US citizen receives about 360 millirem of dose per year.

1 millisievert (mSv) = 100 millirem

1 millisievert (mSv) = 100 millirem
Extreme radiation exposure can damage DNA molecules in cells and produce mutations
Radiation Detection
Scintillators
Scintillators produce photons from the fluorescence of a gas or a phosphor as the radiation excites electrons in the atoms
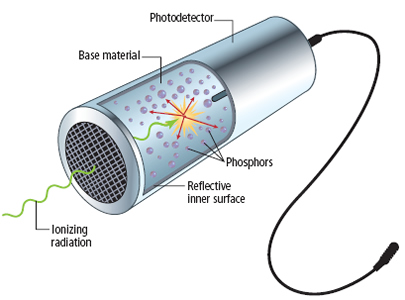
Scintillators are more sensitive to gamma rays
Geiger Counters
Geiger Counters produce currents from the ionization of a gas as the radiation knocks some electrons off from the gas atoms
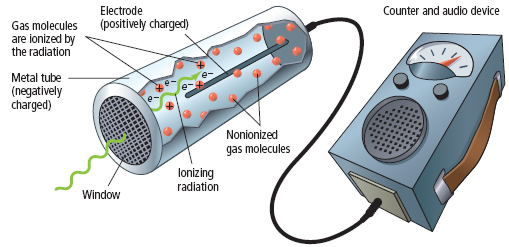
Radioactive Half-Life
The half-life of a radioactive material is the time needed for half of its mass to decay
Since some radioactive nuclei are more stable than others, different radioactive materials have different half-lifes
For example Uranium-238 has a half life of 4.5 billion years, whereas Helium-6 has a half-life of ~1 second
Environmental conditions do not affect the rate of decay of radioactive nuclei. Decay rates are always constant
Checkpoint 1
If a 10 kg sample of Carbon-14 reduces in weight by 1 g per yr, what would be the half life of Carbon-14?
I would take 5000 years for it to reduce to 5 kg
Radioactive Half-Life
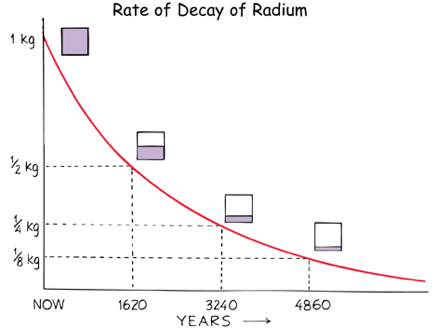
Radium-226 has a half-life of 1620 years
An initial 1 Kg Radium-226 sample will decay to 1/2 Kg in 1620 years. In another 1620 years there will be only 1/4 Kg left
Radiometric Dating
Carbon Dating
- Organic material emits radiation due to Carbon-14 decay
- When living organism die they stop taking Carbon-14 and the radiation rates go down with time
- Radiation rates tell us how long ago organic material died


Carbon Dating

The radioactive carbon isotopes in the skeleton diminish by one half every 5730 years. The red arrows symbolize relative amounts of carbon-14
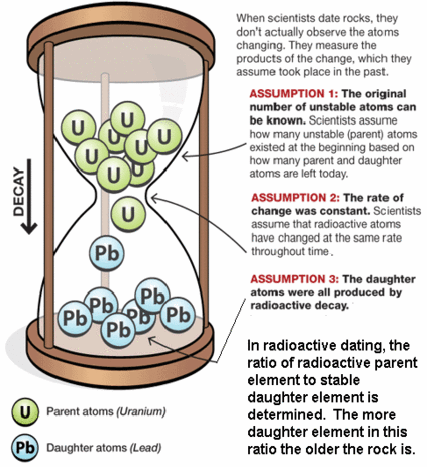
Uranium Dating
The current ratio of lead to uranium in a mineral can be used to determine its age

Nuclear Fission and Fusion
Nuclear Binding Energy
The mass of a nucleus is always less than the sum of the individual masses of the protons and neutrons which constitute it
The difference is a measure of the nuclear binding energy which holds the nucleus together
Nuclear binding energy = Δmc^2

Nuclear Binding Energy
Another way to see the same concept is by looking at the mass per nucleon
The higher the binding energy per nucleon the lower the mass per nucleon

Nuclear Fusion

In nuclear fusion, energy is released when light nuclei fuse together
Nuclear Binding Energy
The binding energy per nucleon increases from Hydrogen to Iron, but then decreases as the repulsive electric force between protons starts to dominate in heavy elements

Checkpoint 2
If there are 10^21 atoms in 1 gram of iron, with about 500 Mev of binding energy per atom. For how many hours can you keep a 60 Watt light bulb on with the binding energy of 1 gram piece of iron?
5 x 10^10 J /10^5 J = 3 x 10^5 = 500000 hours
Binding energy in 1 gram = 500 x 10^21 x 10^(-13) Joules
= 500 x 10^8 Joules = 5 x 10^10 J
Binding energy in 1 gram / 60 WattHour =
Nuclear Fission


Nuclear fission is the splitting of a heavy nucleus into lighter nuclei plus energy
Nuclear Fission

Nuclear deformation leads to fission when repelling electrical forces dominate over attracting nuclear forces
Nuclear Fission
The absorption of a neutron by a uranium nucleus supplies enough energy to cause such an elongation

One neutron starts the fission of the uranium atom and three more neutrons are produced when the uranium fissions
Chain Reaction

If there are other fissionable uranium atoms around a chain reaction starts, and a fission explosion occurs
The fission of one U-235 atom releases about seven million times the energy released by the explosion of one TNT molecule

Chain reactions do not occur in uranium deposits because only the rare isotope U-235 is fissionable by absorption of a neutron
Only 0.7% or 1 part in 140 of uranium is U-235. The prevalent isotope, U-238, absorbs neutrons but does not undergo fission
U-235 vs U-238

Critical Mass
a. If the piece of uranium is too small, a neutron is likely to escape through the surface before it “finds” another nucleus and the chain reaction dies out.
b. For there to be a sustained chain reaction a pice of uranium with a mass above the critical mass is required

Nuclear Fission Bomb
In a fission bomb two subcritical pieces of uranium are put together to form a super critical piece for detonation
Nuclear Fission Reactors
A nuclear fission reaction can be controlled to produce generate usable energy

The reaction is controlled by using Uranium with low percentages of U-235, and control rods that can be moved in and out and control the number of neutrons
Transmutation of Elements

Radioactive elements transform to other elements as they climb down the transmutation latter in the search for stabitlity


Waste Products of Fission
A major drawback to fission power is the generation of radioactive waste products
Towards Cleaner Nuclear Energy

Nuclear Fusion

In nuclear fusion, energy is released when light nuclei fuse together
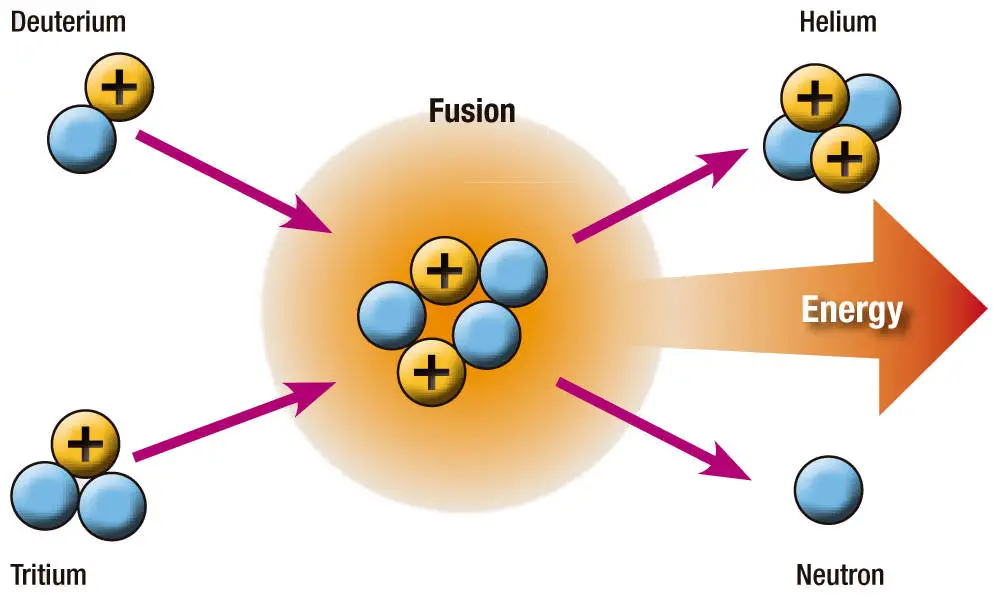
Thermonuclear Fusion
For fusion to occur, nuclei must collide at very high speeds to overcome electrical repulsion.
Fusion brought about by high temperatures is called thermonuclear fusion
In the central part of the sun, about 657 million tons of hydrogen are converted into 653 million tons of helium each second
The missing 4 million tons of mass is discharged as radiant energy
Natural Fusion Reactors
Fusion Reactors


The dream of clean energy production! No radioactive waste nor air pollution, and no risk of getting out of control

A real engineering challenge though! We haven't been able to produce sustainable fusion that produces more energy than what is input
The End
Good job for hanging in there, we made it!
1. Physics is the study of energy, forces, matter and the dynamics caused by their interactions
2. Energy and matter, waves and particles, they are all the same, just different representations of energy fields
3. Everything is vibrating, and vibrations produce waves of energy
Wish you all good vibes and positive energy!
If nothing else I hope you all take home these 3 things:
Nuclear Physics - Physics 2B
By Miguel Rocha
Nuclear Physics - Physics 2B
Physics 1 - Week 15 - Chapter 33-34
- 811



