Phase Changes
M. Rocha
Physics 4C
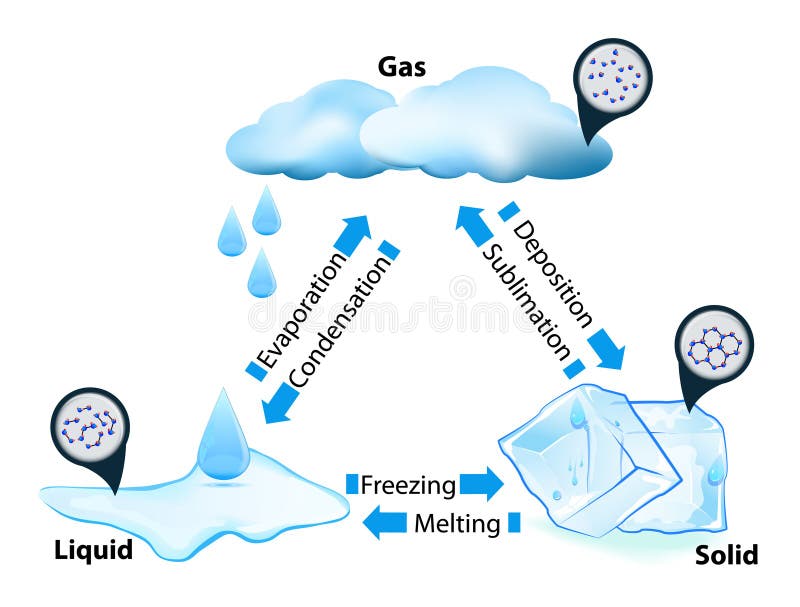
Phase Transitions
Inter Molecular Forces in Play
Van der Waals (Atraction)

Electron Overlap (Repulsion)


Inter Molecular Forces in Play
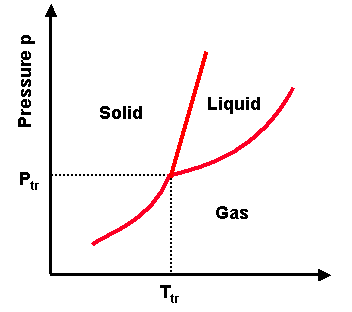
Temperature and pressure make molecules be either too far, at the optimal distance for attraction, or too close
Potential Energy
Phase transitions depend on the molecular properties of the material, temperature and pressure

Evaporation
Evaporation is the change of phase from liquid to gas
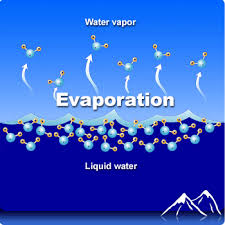
Evaporation
Evaporation is the change of phase from liquid to gas

Evaporation can happen below the surface when boiling
Evaporative Cooling
Because only the most energetic molecules can escape the surface, evaporation removes internal energy from the liquid, thus evaporation cools




Condesation
Condensation is the reverse of evaporation, a change of phase from gas to liquid



Condesation
Condensation is the reverse of evaporation, a change of phase from gas to liquid
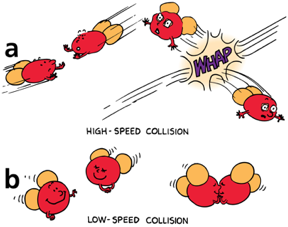
a) At high speeds, molecules of water vapor bounce apart and remain a gas.
b) At lower speeds, molecules of water vapor are more likely to stick together and form a liquid
Checkpoint
Why is it that a 90 degree day in a dry climate feels more comfortable than a 90 degree day in a humid place?
In a dry climate you’re cooled by evaporation, in a wet climate you’re heated by condensation
Melting

Melting is the change of phase from solid to liquid.
While melting, the solid absorbs heat from the environment


Freezing

Freezing is the change of phase from liquid to solid.
While freezing, the solid releases heat into the environment

Sublimation

Sublimation is the change of phase from solid to gas without passing through the liquid phase.

Solid carbon dioxide (dry ice) sublimates at -78 °C (-109 °F).

Energy and Changes of Phase

Energy and Change of Phase

1 Calorie = 4.2 Joules
Phase transitions require energy
Heating 1 gram of water

Water Heat Capacity in calories = 1 cal/g °C
Latent Heat
The energy / heat required for a phase transition is called Latent Heat. Latent heat is given per unit mass as the energy required for transitions is proportional to the number of molecules.
The energy required for (melting / freezing) is given by:

Where L_f is the Latent heat of melting / freezing
The energy required for (vaporizing / condensing) is given by:
Where L_v is the Latent heat of vaporization / condensation

Checkpoint
How much energy do you need to melt 1 gram of ice and end up with water at 20 °C ?
The latent heat of melting for water is
L_f = 80 Calories/gram,
and the specific heat capacity is
c = 1 Calorie/(g °C).
100 Calories
Checkpoint
Three ice cubes are used to chill a soda at 20°C with mass m_soda=0.25kg. The ice is at 0°C and each ice cube has a mass of 6.0 g. Assume that the soda is kept in a foam container so that heat loss can be ignored and that the soda has the same specific heat as water. Find the final temperature when all ice has melted.




The End
Phase Changes and Mechanisms of Heat Transfer (Physics 4C)
By Miguel Rocha
Phase Changes and Mechanisms of Heat Transfer (Physics 4C)
Physics 1 - Week 7 - Chapters 17-18
- 718



