
Source: Stanford Research into the Impact of Tobacco Advertising
Direct to Consumer Advertising
Yina
Killian
Barrett
Nico
Qi
Stacey
Jones
Jan
Peter Conrad
-
American medical sociologist
-
medicalisation of deviance and the experience of illness
-
2007: “The Medicalisation of Society”
-
impact of medical professionals on medicalisation has diminished
-
pharmaceutical and biotechnical industries and patient as consumer have become major forces promoting medicalisation
-

Valerie Leiter
- Professor and Interim Chair of Sociology at Simmons College; Co-Director of the Program in Public Health
-
children and youth with disabilities, medicalisation, and gender and health.
-
Conrad’s grad student

From Lydia Pinkham to Queen Levitra: direct-to-consumer advertising and medicalisation
- role of direct-to-consumer advertising (DTCA) in the medicalisation of life problems
- compares patent medicine advertising with contemporary DTCA
- discusses the education of self-medicalization and how that’s led to the vast market of pharmaceutical drugs
Context of medicine in the 19th Century
- USA: many people saw hospitals as a place to go to die
- Two types of drugs existed that were used by the public:
-
"ethical drugs"
-
known composition
-
aligned with the medical profession
-
-
"patent drugs"
-
undeclared composition
-
advertised directly to the public
-
John Oliver on Marketing to Doctors
“Lydia E. Pinkham’s Vegetable Compound”



Text
Pinkham's Vegetable Compount was marketed towards all female sub-demographics
The Modern Era
-
1906: AMA sets standards for both advertising and prescribing medications with the publication of “New and Nonofficial Remedies”
-
drugs were not accepted if undisclosed ingredients, advertised to public, or had false claims
-
Inevitable: journalists were already publishing exposés 2-3 years before this exposing fraudulent patent medicines
-
1912: federal law amended to include claims of effectiveness (or lack thereof)
-
1920: included newspaper advertising
-
-
1915: Lydia Pinkham had to cease advertising specifically for women's disorders
-
"Recommended as a Vegetable Tonic in conditions for which the preparation has been adapted."
-
-
1906-1980: FDA consolidated regulatory authority over prescription drugs
1980s - DTCA of Prescription Drugs
- 1981: Rufen by Boots Pharmaceutical and Pneumovax by Merck Sharp & Dohme
- start of modern DTCA dilemma and regulation
- start of modern DTCA dilemma and regulation
- 1982: “In sum, my impression is that we may be on the brink of the exponential growth phase of direct-to-consumer promotion of prescription products.”
- boom of print advertising
- $12 billion in 1989
1997 - Pharma comes to TV
-
FDA issues draft guidelines for TV and radio
-
must include "major statement" of major risks
-
must include "major statement" of major risks
- "emphasised newer and more expensive medicines over cheaper existing ones, and regarding ‘the medicalising of normal human experience’ (Mintzes 2002, Frosch et al. 2007)"

-
broadcast DTCA $55M in 1991
-
$4.2B in 2005
-
$4.2B in 2005
-
most are for chronic conditions in relatively healthy people
-
these drugs are for allergy, anxiety, obesity, arthritis, erectile dysfunction, and high cholesterol
- about 20 drugs account for 60% of DTCA spending
Viagra
- 1998: approved by FDA
- started from advertising to older men to any man who wanted a boost in performance
- 2003: Levitra advertising on NFL with Mike Ditka as spokesman
- "Levitra Challenge"
- "Levitra Challenge"
- a week after Superbowl, prescriptions increased by 15%


From Lydia Pinkham to Queen Levitra: direct-to-consumer advertising and medicalisation
Conrad and Leiter
Peter Burke's Pentad
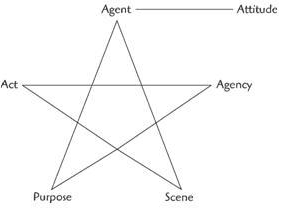
Act
- analysis of the advent of DTCA and FDA’s role in regulating DTCA in the United State
Scene
-
2008 in the United States
-
November, 2006: U.S. Government Accountability Office’s report: Improvements Needed in FDA’s Oversight of Direct-to-Consumer Advertising (1997-2005)
- 103.3% increase in R&D
- 296.4% increase in DTCA
-
November, 2006: U.S. Government Accountability Office’s report: Improvements Needed in FDA’s Oversight of Direct-to-Consumer Advertising (1997-2005)
Source: U.S. GAO
Agent
- Conrad and Leiter, sociologists of public health
- authoritative figures of medicine
Agency
-
historical accounts of FDA’s role in the regulation of DTCA
-
two examples of DTCA
-
one “extravagant” of Pinkham
-
one “contemporary” of Levitra
-
Purpose
-
ostensibly objective sociological analysis on DTCA
-
“grand promises printed on cheap, pulp paper” (833)
-
“In fact, direct access to consumers has increased the pharmaceutical industry’s incentive to medicalise human problems, encouraging consumers to self-diagnose and request drugs that they see on TV.” (834)
- “‘Every man is vulnerable to the power of suggestion and sought to make him sick so they could make him well’ (Young 1961: 184)”. (827)
-
“making physicians key gatekeepers to prescription drugs” (833)
-
Rhetoric of External Material
Advertising Standards Canada
DTCA Checklist
- "Is the advertising material directed to healthcare professionals; OR is the advertising material to be disseminated by healthcare professionals to patients?"
- "Does the advertisement directly or indirectly communicate a product characteristic or benefit"?
- "Can the product’s therapeutic indication possibly be identified by the way the product/package is depicted?"
Source: ASC Clearance Services
Food and Drug Administration
Guidance for Industry Direct-to-Consumer Television Advertisements
- "changes that are necessary to protect the consumer good and well-being, or that are consistent with prescribing information for the product under review"
- "statements for inclusion in the advertisement to address the specific efficacy of the drug as it relates to specific population groups, including elderly populations, children, and racial and ethnic minorities, if appropriate and if such information exists"
Source: FDA
Pharmaceutical Research and Manufacturers of America
Direct to Consumer Pharmaceutical Advertising
-
"America’s pharmaceutical research and biotechnology companies understand that accurate information about disease and treatment options makes patients and doctors better partners. And getting that information to patients and consumers is the goal of DTC pharmaceutical advertising about prescription medicines."
- "DTC advertising increases people’s awareness of disease and available treatments. Studies show DTC advertising brings patients into their doctors’ offices and starts important doctor-patient conversations about health that might otherwise not take place."
Source: PhRMA
Questions and Comments?
Have you been on the receiving end of DTCA? What were your impressions?
Is self-medicalization truly “educational” and “empowering”?
Would you prefer to be provided the choices and directed to your physician for advice or have your physician proactively recommend you products advertised to them as physicians?
The ‘call-to-action’ is a key part of sales strategy. With the current culture of self-diagnosis, how does the call-to-action relate?
You owe it to your family.
You owe it to yourself.
DTCA
By Nico Jan
DTCA
- 623

