Multiscale modeling of the heart
Multiscale models
- to provide insight into the mechanism of cardiac arrhythmia
- for patient specific modeling
- for in-silico drug testing
- to test different techniques (e.g. phase mapping)

Fig by Axel Loewe
are used for:
Atrial fibrillation
Atrial tachycardia
Ventricular tachycardia
Ventricular fibrillation
Torsade de Pointes
Atrial fibrillation
Atrial tachycardia
Ventricular tachycardia
Ventricular fibrillation
Torsade de Pointes
-
Atrial fibrillation (AF): most prevalent cardiac arrhythmia in the world: current prevalence of AF is ~2% of the general population worldwide and is projected to more than double in the following decades
-
6-fold increase of stroke, leads to anxiety, depression, and reduced quality of life.
-
Types: paroxysmal (< 1w), persistent (> 1w), permanent (> 1y)
-
Ablation treatment for persistent and permanent AF have long term success rates being <30% for single ablation procedures. These numbers vary depending on the hospital!
Atrial fibrillation
Persistent and permanent AF: Reason for poor success: Mechanism is heatedly debated.
Atrial fibrillation


Paroxysmal AF: consensus: triggers from the PV -> basis for PVI isolation

Haissaguerre, cric, 1996
Hypothesis 1: Persistant AF is maintained by rotors and focal impulses
Atrial fibrillation
Clinical evidence:
- CONFIRM study: FIRM = "Focal Impulse and Rotor Modulation" works with phase mapping

Narayan et al, JACC, 2012
Narayan et al, JACC, 2014
Stable Rotors and localized focal sources were present in almost all of their patients with AF (98%; mean, 2.3±1.1 concurrent rotors and focal sources)
INTERMEZZO: PHASE MAPPING
Atrial fibrillation
Assign a phase to a local signal

0
2Pi
Method 1: activation time
For a review, see Umapathy et al, Circ A&E, 2010
Gray et al, Nature, 1998

Method 2: phase space
INTERMEZZO: PHASE MAPPING
Atrial fibrillation
Assign a phase to a local signal
Method 4: concept of sinusoidal recomposition and Hilbert transform
Kuklik et al, TBME, 2014

Bray et al, IEEE, 2002
Shors et al, IEEE, 1996

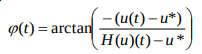

Method 3: Hilbert transform
INTERMEZZO: PHASE MAPPING
Atrial fibrillation


Hypothesis 1: Persistant AF is maintained by rotors and focal impulses
-
The technology was patented and works like a black box.
-
FIRM is not yet confirmed in other studies
Limitations
Atrial fibrillation
Gianni et al, Heart rhythm, 2016
Mohanty et al, JACC, 2016: study retracted
Buch et al, Heart rhythm, 2016
Hypothesis 1: Persistant AF is maintained by rotors
Atrial fibrillation
Clinical evidence:
2. Using a non-invasive ECG imaging (ECGI) approach
- AF is sustained by rotors: targeted ablation led to 85% of patients being freed from AF at 12 months post ablation.
- Reentries were not sustained, meandered substantially but recurred repetitively in the same region.
Haissaguerre et al., circ, 2014

Limitations
The torso works like a bandpass filter: limited sensitivity in the case of highly localized sources,
small signals and far-field signals, particularly in scar tissue
Atrial fibrillation
Hypothesis 1: Persistant AF is maintained by rotors
Computational studies
Multi-scale computer models of the human atria:
- Consistently demonstrated that AF is perpetuated by the re-entrant circuits persisting in the fibrotic boundary zones
Bayer et al., Front. Physiol., 2016
Morgan et al., Front. Physiol.,2016
Vigmond et al., Heart Rhythm, 2016
Zahid et al., Card Res, 2016
Zhao et al., Am. Heart Assoc, 2017
Atrial fibrillation
Hypothesis 1: Persistant AF is maintained by rotors
Computational studies
Zahid et al, Cardiovasular Research, 2016
"...throughout the re-entry, the RD-PS dynamic location was along a trajectory that followed a boundary between fibrotic and non-fibrotic tissue"

Atrial fibrillation
Hypothesis 1: Persistant AF is maintained by rotors
Morgan et al, Front Physiol. 2016

(C) Shows a rotor pinned directly to a dense fibrotic region
(D) Shows a rotor with the core adjacent to a dense fibrotic region, but rotating within a border zone.
Atrial fibrillation
Hypothesis 1: Persistant AF is maintained by rotors
Zahid et al, Cardiovasular Research, 2016
Shows a rotor pinned directly to a dense fibrotic region
Why does fibrosis attract rotors?
Atrial fibrillation
This holds even for very large distances.
Vandersickel et al, PLOS COMP BIOLOGY, 2018
Atrial fibrillation
Why does fibrosis attract rotors?
Vandersickel et al, PLOS COMP BIOLOGY, 2018
Atrial fibrillation
Questions for modeling this first hypothesis
- How to model fibrosis?
A. change the overall electrical conductances of the tissue to remodel the fibrotic tissue
B. explicitly model fibroblasts and couple it to myocites
C. model fibroblasts as inexitable objects
How much do results depend on method A - B - C?
- How to model patient specific data of fibrosis?
LGE-MRI data is somehow matched to a model - How do you model anisotropy?
- How do you model heterogeneities?
Atrial fibrillation
Hypothesis 2: Multiple wavelet maintain AF
Clinical evidence:
- STARLIGHT study:
"PSs were distributed globally throughout both chambers with no clear anatomic predisposition."
"No sustained rotors or localized drivers were detected, and instead, the mechanism of arrhythmia maintenance was consistent with the multiple wavelet hypothesis, with passive activation of short-lived rotational activity."
Child, Clayton, Roney, et al, Circ A&E 2018

64 constellation catheter, bi-atrial
Atrial fibrillation

Atrial fibrillation
Hypothesis 2: Multiple wavelets maintain AF
Clinical evidence:
2. Allessie et al. recorded electrical activity of the atria of a canine heart in 192 points and showed that after acetylcholine application multiple wavelet AF can be observed?
4 - 6 wavelets
Allessie et al, 1985
Atrial fibrillation
Hypothesis 2: Multiple wavelets maintain AF

white arrows: wavelets
Atrial fibrillation
Hypothesis 2: Multiple wavelet maintain AF
Clinical evidence:
3. ECGI study "In the study phase, the most common patterns of AF were multiple wavelets (92), with pulmonary vein (69) and non-pulmonary vein (62) focal sites. Rotor activity was seen rarely (15)."

Cuculich et al, Circ, 2010
Atrial fibrillation
Hypothesis 2: Multiple wavelet maintain AF
Cuculich et al, Circ, 2010
2 to 3 simultaneous wavelets
Simulation studies
First simulation was done by Moe et al
Moe et al, 1964
AF is maintained by multiple independent reentrant wavelets which change position, shape, size and number with each successive excitation
Hypothesis 2: Multiple wavelets maintain AF
Atrial fibrillation
Simulation studies
Hypothesis 2: Multiple wavelets maintain AF
Atrial fibrillation

Reumann et al, Journal of electrophysiology, 2007
The cardiomyocytes are initialized to short refractive periods and the propagation velocity is reduced to about 40% like in Moe et al.
Simulation studies
Hypothesis 2: Multiple wavelets maintain AF
Atrial fibrillation

Reumann et al, Journal of electrophysiology, 2007
Fibrillation occurs as a result of the heterogeneity of cardiac tissue in the refractory period
Simulation studies
Hypothesis 2: Multiple wavelets maintain AF
Atrial fibrillation
Reumann et al, Journal of electrophysiology, 2007
The cardiomyocytes are initialized to short refractive periods and the propagation velocity is reduced to about 40% like in Moe et al.
Hypothesis 3: Double layer hypothesis
Atrial fibrillation
The fibrillation waves with a focal pattern of activation could result from endo-epicardial breakthrough
Allesie Circ:A&E, 2010
de Groot, Circ, 2010
de Groot, Circ A&E, 2016
Clinical evidence:
Hypothesis 3: Double layer hypothesis
Atrial fibrillation
Clinical evidence:
goats!
The longer AF is maintained, the bigger the dissociation
Eckstein et al, (schotten group), Cardiov res, 2010

Hypothesis 3: Double layer hypothesis
Atrial fibrillation
Clinical evidence:

de Groot et al, circ A&E, 2016
humans!
Modeling studies:
Atrial fibrillation
Hypothesis 3: Double layer hypothesis

Gharaviri et al, EP Europace, 2012
Modeling studies:
Atrial fibrillation
Hypothesis 3: Double layer hypothesis
Now more complex models are simulated with multiple layers, based on MRI data including fibre orientation
Ghavarivi et al, 2020, Frontiers in physiology

Hypothesis 4: Mother rotor fibrillation
Atrial fibrillation
Clinical evidence
Jalife et al, cardiov res, 2002
- Faster reentrant circuits act as dominant frequency sources that maintain the overall activity.
- The rapidly succeeding wavefronts emanating from these sources propagate through both atria and interact with anatomic and/or functional obstacles, leading to wavelet formation.
Modeling studies
Atrial fibrillation
Hypothesis 4: Mother rotor fibrillation
Keldermann et al, Am J Physiol Heart Circ Physiol, 2008
Atrial fibrillation
Hypothesis 5: Atrial fibrillation driven by micro-anatomic intramural re-entry

Hansen, Fedorov et al, EHJ, 2015
- Micro-reentry via the pectinate muscle. Anchored to regions of the highly fibrosis-insulated and twisted intramural atrial bundles.
- Optically mapped simultaneously by three high-resolution CMOS cameras
Clinical
Atrial fibrillation
Hypothesis 5: Atrial fibrillation driven by micro-anatomic intramural re-entry
modeling

Zhao et al, JAHA, 2017
Atrial fibrillation
Hypothesis 1: Persistant AF is maintained by rotors
Hypothesis 2: Multiple wavelets maintain AF
Hypothesis 3: Double layer hypothesis
Hypothesis 4: Mother rotor fibrillation
Many of the clinical methods use phase mapping to determine rotational activity
Which one is correct?
Maybe there are many different types of AF?
Hypothesis 5: Atrial fibrillation driven by micro-anatomic intramural re-entry
Kuklik et al, 2017, IEEE Trans Biomed Eng.:
"Great methodological care has to be taken before equating detected phase mapping with rotating waves and using phase mapping detection algorithms to guide catheter ablation of atrial fibrillation"
Atrial fibrillation
Problems with phase mapping
-
At least eight electrodes are required to avoid frequent false positive PS detection due to chance
- Phase mapping ignores fractionation and double potentials
- Ignores conduction block,
- Presents waves propagating around conduction block lines as rotors,
- Presents waves coming from opposite directions and (e.g. left part of system with wave going up->down and right part with wave gong down->up) as transient rotation.
Atrial fibrillation
Problems with phase mapping
Problems with phase mapping
Atrial fibrillation
2. Laura Martinez-Mateu, Jose Jalife, Javiev Saiz et al, PLOS COMP BIOLOGY
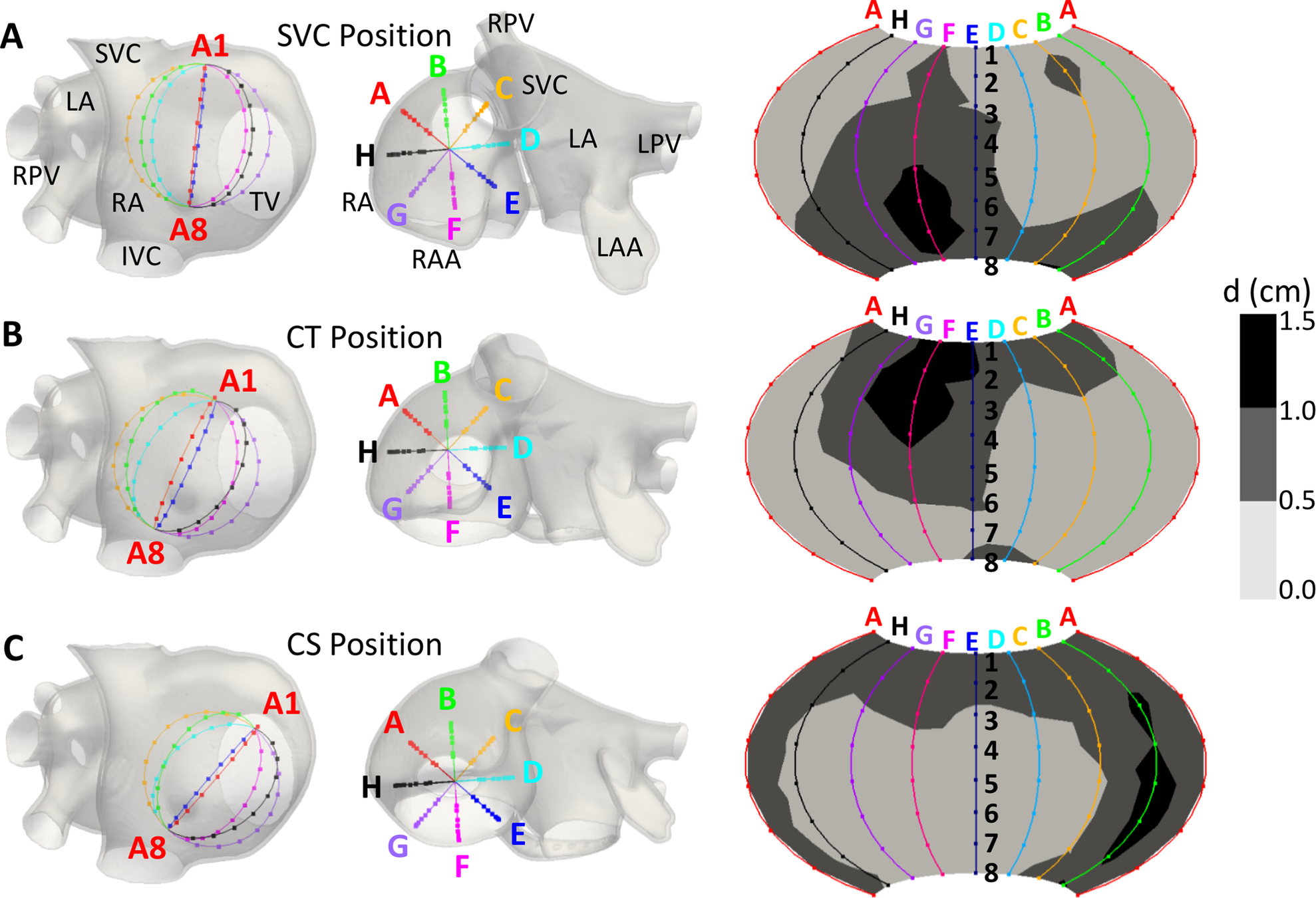
Atrial fibrillation
Martinez-Mateu, Jalife, Saiz et al, PLOS COMP BIOLOGY

- True Rotor detection between 35–94% of the simulation time, (depending on the basket’s position)
- Extra PS: due to the far-field sources
- Extra PS: due to interpolation between the electrodes;
Big Problem!!
Possible alternative to phase mapping:
consider the cardiac excitation as a directed network
Search algorithm
Brain
Network theory has many applications...




Atrial fibrillation
We can find these rotating electrical waves very easily, they are just the cycles in our network

But was not very often applied to the heart (directed networks)
Atrial fibrillation
We call this method DG-mapping
Atrial fibrillation

Atrial fibrillation
Martinez-Mateu, Jalife, Saiz et al, PLOS COMP BIOLOGY

Van Nieuwenhuyse et al, to be submitted
Atrial tachycardia
- Much more simple than atrial fibrillation
- Possible mechanisms:
(1) focal
(2) macro-reentry
(3) localized reentry - Can still be very complex to find, especially after ablation of AF
DG-mapping on clinical AT cases

Optimization protocols

DG-mapping on clinical AT cases
DG-mapping on clinical AT - DG GUI
DG-mapping on tested on clinical AT cases
Collaboration with Sint-Jan Bruges (Belgium): Prof. Dr. Mattias Duytschaever using CARTO from Biosense Webster
- 31 cases tested post-ablation:
Ablation target 100% correct - 51 (complex) cases: mechanism analyzed in detail during ablation with ppi:
DGM 72% correct <-> expert with HDAM 64% correct
Collaboration with LIRYC Bordeaux (France): Dr. Nicolas Derval using RHYTHMIA from Boston Scientific
- 28 (complex) cases: mechanism analyzed in detail during ablation with ppi:
DGM ablation target close to 100%
DG-mapping can automatically find the mechanism of an AT without manual interpretation of the colormap of the atrium
-
Operator independent and in some cases better than the operator: DG-mapping removes intuition and multiple interpretations, is thus more robust
-
Faster: DG-mapping is almost instantaneous
-
Goal? no more entrainment mapping (PPI)
DG-mapping on AT
Atrial fibrillation
Hypothesis 1: Persistant AF is maintained by rotors
Hypothesis 2: Multiple wavelets maintain AF
Hypothesis 3: Double layer hypothesis
Hypothesis 4: Mother rotor fibrillation
Maybe we need to use different measurement methods?
Which one is correct?
Maybe there are many different types of AF?
Hypothesis 5: Atrial fibrillation driven by micro-anatomic intramural re-entry
Modeling tools
There also exist some packages:
- Chaste
- Beatbox
- OpenCMISS
- Continuity
Only Chaste has a significant number of users outside of the institution in which it has been developed but the code is hard to understand without programming experience
Most groups have their own costom-made software
Modeling tools
Latest novel tool on the market:

openCARP is an open cardiac electrophysiology simulator for in-silico experiments
The idea is to build a sustainable cardiac simulator
Builds on:
- The Cardiac Arrhythmia Research Package (CARP) developed by Ed Vigmond and Gernot Plank
- acCELLerate developed by Gunnar Seemann and Axel Loewe
Modeling tools
Latest novel tool on the market:

Aim:
- To increase the productivity in our research field.
- Research time should be put in actually solving a scientific problem
- You can import CellML-based EP models to avoid error-prone and time-demanding manual implementation.
- Possible to upload in silico experiments of a published studies to share it with your colleagues => a more transparent and better reproducible cardiac modeling research.
Modeling tools

Next user meeting: 13-15 May 2020 in Freiburg, Germany
first version will be released in March 2020
More info: https://opencarp.org/

Enid Van Nieuwenhuyse
modeling
modeling

modeling
Alexander Panfilov

Clinical expert
Mattias Duytschaever

Nele Vandersickel
Ghent University and AZ-Sint Jan Bruges
Clinical expert
Clinical expert

Dr. Sebastien Knecht

Teresa Strisciuglio

modeling
Lars Lowie

Network specialists


Clinical expert
Jan Goedgebeur
Nico Van Cleemput
Anthony de Molder
Valencia
Bordeaux
Other algorithms are also developed:
- Novel single-signal algorithms based on instantaneous amplitude modulation (iAM) and iFM to detect rotational-footprints
DG mapping and double loops
DG mapping and double loops





single loop
double loop equal dominance
single loop
double loop:
scar dominant
double loop:
MV dominant

DG mapping and double loops
DG mapping and double loops

A node belongs to the ROI of a loop if there is a path from a node from this loop to the certain node
To know by which loop a node is excited?
To know the dominant loop:
- largest ROI
- path going from dominant loop to other loop

DG mapping and double loops

DG mapping and double loops
Multiscale modeling of the heart
By Nele Vandersickel
Multiscale modeling of the heart
- 403



