AHOD0431: PET-adapted therapy for early-stage, low-risk pediatric Hodgkin lymphoma
Parekh et al.
Blood 2022
Background
- Pediatric early-stage classic Hodgkin lymphoma (cHL) is highly curable
- Late toxicity from chemotherapy and radiation therapy is a concern
- Treatment strategies aim to maintain high cure rates while minimizing late effects
- Response-adapted protocols using PET scans can identify patients for whom radiotherapy may be omitted
- COG developed AHOD0431 to de-escalate treatment in early-stage, low-risk cHL
Study Objectives
- Primary: Evaluate if reducing up-front treatment using a response-based approach with minimal initial chemotherapy and omission of IFRT in complete responders could maintain overall survival and event-free survival while reducing late toxicity
- Secondary: Investigate the impact of PET response after 1 cycle (PET1) on outcomes and patterns of relapse
Eligibility Criteria
- Age: 0 to 21 years
- Diagnosis: Low-risk classic Hodgkin lymphoma
- Stage: Ann Arbor stage IA and IIA nonbulky disease
- Bulk definition:
- Mediastinal mass > one-third of longest thoracic dimension on upright PA chest x-ray
- Any contiguous nodal aggregate > 6 cm across longest transverse diameter on axial imaging
AHOD0431 Trial Schema
PET Response Assessment
PET negative defined as:
Activity below or at the level of the mediastinal blood pool (Below Deauville 3)
PR Definition:
defined as >50% but <80% decrease in perpendicular dimension on CT or positive PET or gallium scan
CR Definition:
≥80% reduction in the product of perpendicular dimensions and no residual extramediastinal lymph node mass >2.0 cm
Study Accrual
AHOD0431 was temporarily closed to accrual on 4 December2008 because of an increased risk of relapse among PET1 negative patients who did not receive IFRT because they had achieved aCR
It was recommended that all patients with equivocal or positive PET1 receive 21-Gy IFRT, unless they were 12 months from completion of chemotherapy.
Radiation Therapy Details
- Dose: 21 Gy
- Fractionation: 1.5 Gy per fraction
- Total treatment time: 14 days
- Timing: 3-4 weeks after final cycle of chemotherapy
- Technique: Involved-field radiotherapy (IFRT)
- Volume: Sites initially involved by disease
- Delivery: Balanced anterior-posterior fields
Patient Characteristics
- Total patients: 222
- Median age: 15.3 years
- Male: 44.6%
- Stage II: 75.7%
- Rapid early responders (RERs): 54% (n=119)
- Slow early responders (SERs): 46% (n=103)
- SERs more likely to have:
- Stage II disease (p=0.0017)
- ESR >20 (p=0.0001)
- ≥3 sites of disease (p=0.0052)
Primary Outcome
- 10-year progression-free survival (PFS):
- RERs: 87.1%
- SERs: 73.5%
- p = 0.01
- RERs:
- With IFRT: 96.6%
- Without IFRT: 84.1%
- p = 0.10
- SERs:
- With IFRT: 80.9%
- Without IFRT: 64.0%
- p = 0.03
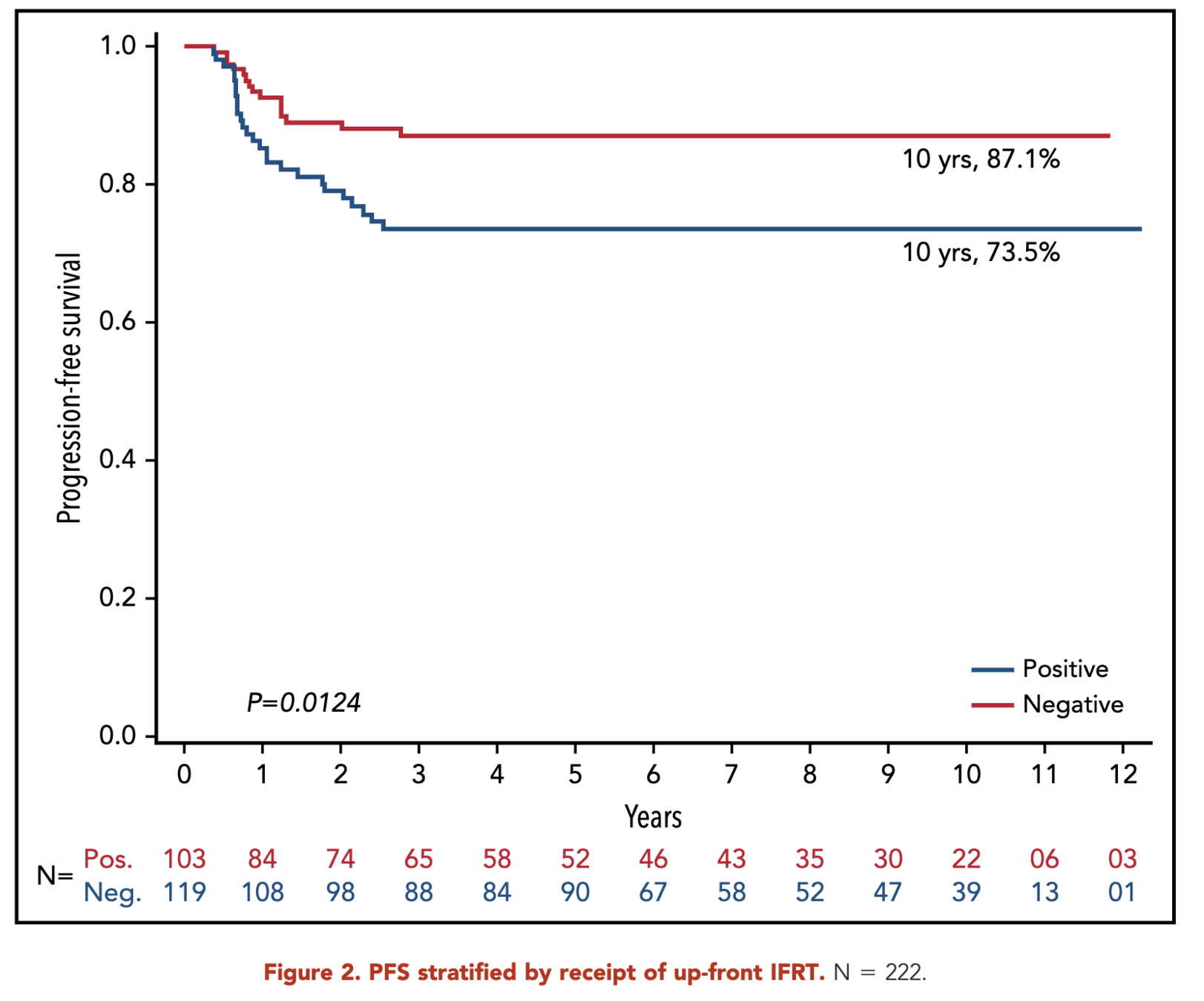
- 10-year progression-free survival (PFS):
- RERs: 87.1%
- SERs: 73.5%
- p = 0.01
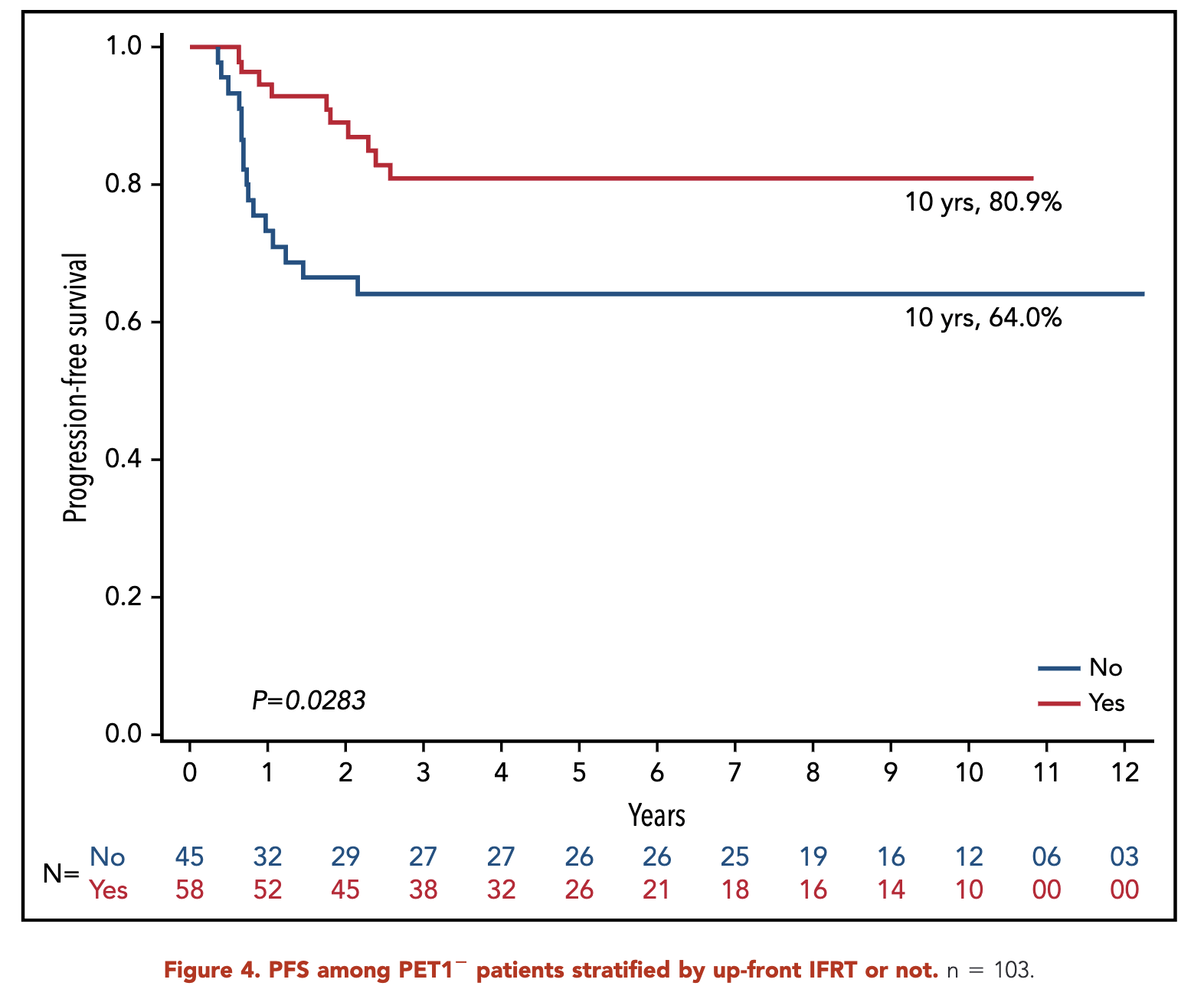
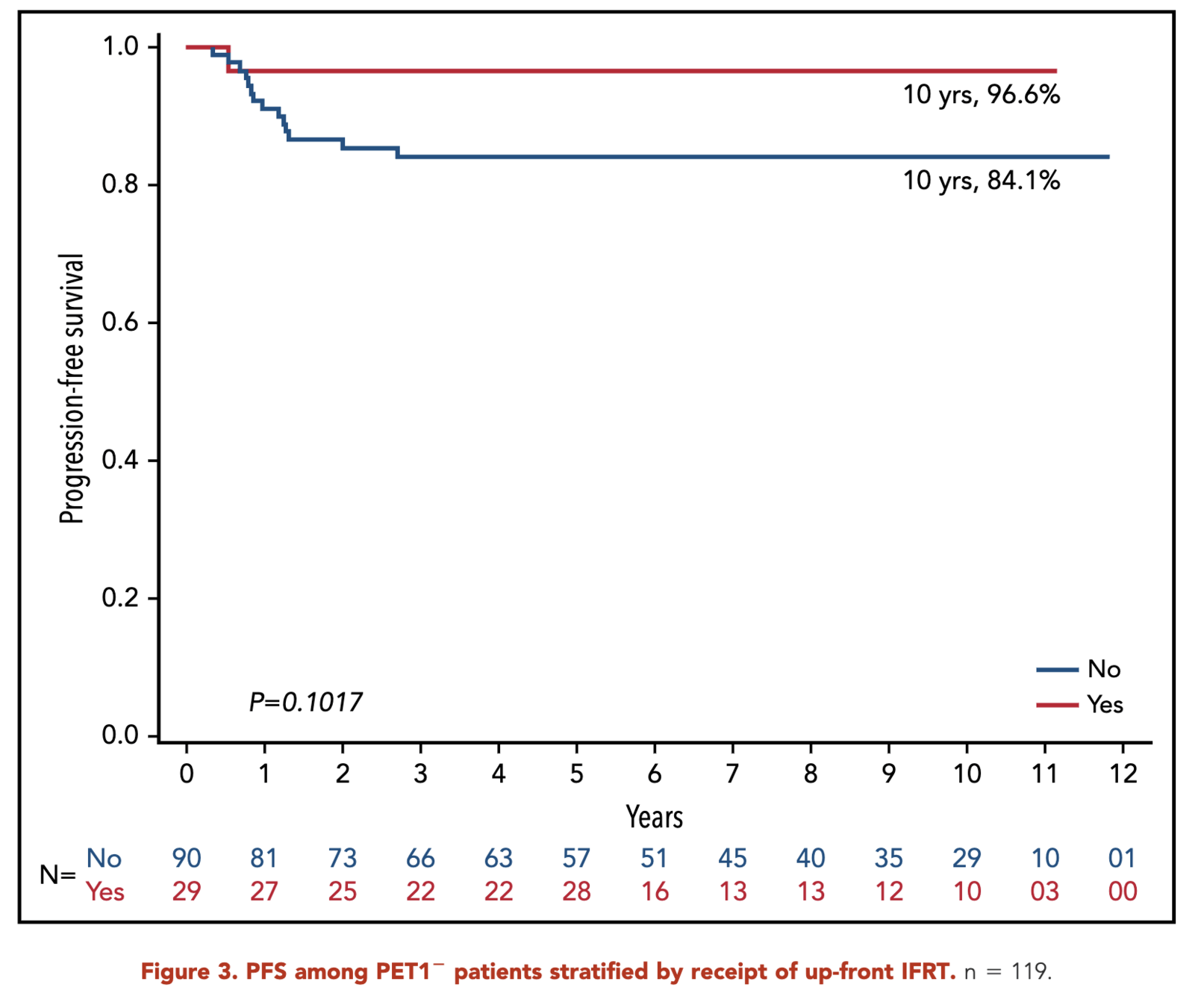

- RERs:
- With IFRT: 96.6%
- Without IFRT: 84.1%
- p = 0.10
- SERs:
- With IFRT: 80.9%
- Without IFRT: 64.0%
- p = 0.03
Primary Outcome (RERS)
- 10-year progression-free survival (PFS):
- RERs: 87.1%
- SERs: 73.5%
- p = 0.01
- RERs:
- With IFRT: 96.6%
- Without IFRT: 84.1%
- p = 0.10
- SERs:
- With IFRT: 80.9%
- Without IFRT: 64.0%
- p = 0.03

- 10-year progression-free survival (PFS):
- RERs: 87.1%
- SERs: 73.5%
- p = 0.01



- RERs:
- With IFRT: 96.6%
- Without IFRT: 84.1%
- p = 0.10
- SERs:
- With IFRT: 80.9%
- Without IFRT: 64.0%
- p = 0.03
Primary Outcome (sERs)
- 10-year progression-free survival (PFS):
- RERs: 87.1%
- SERs: 73.5%
- p = 0.01
- RERs:
- With IFRT: 96.6%
- Without IFRT: 84.1%
- p = 0.10
- SERs:
- With IFRT: 80.9%
- Without IFRT: 64.0%
- p = 0.03

Patterns of Relapse
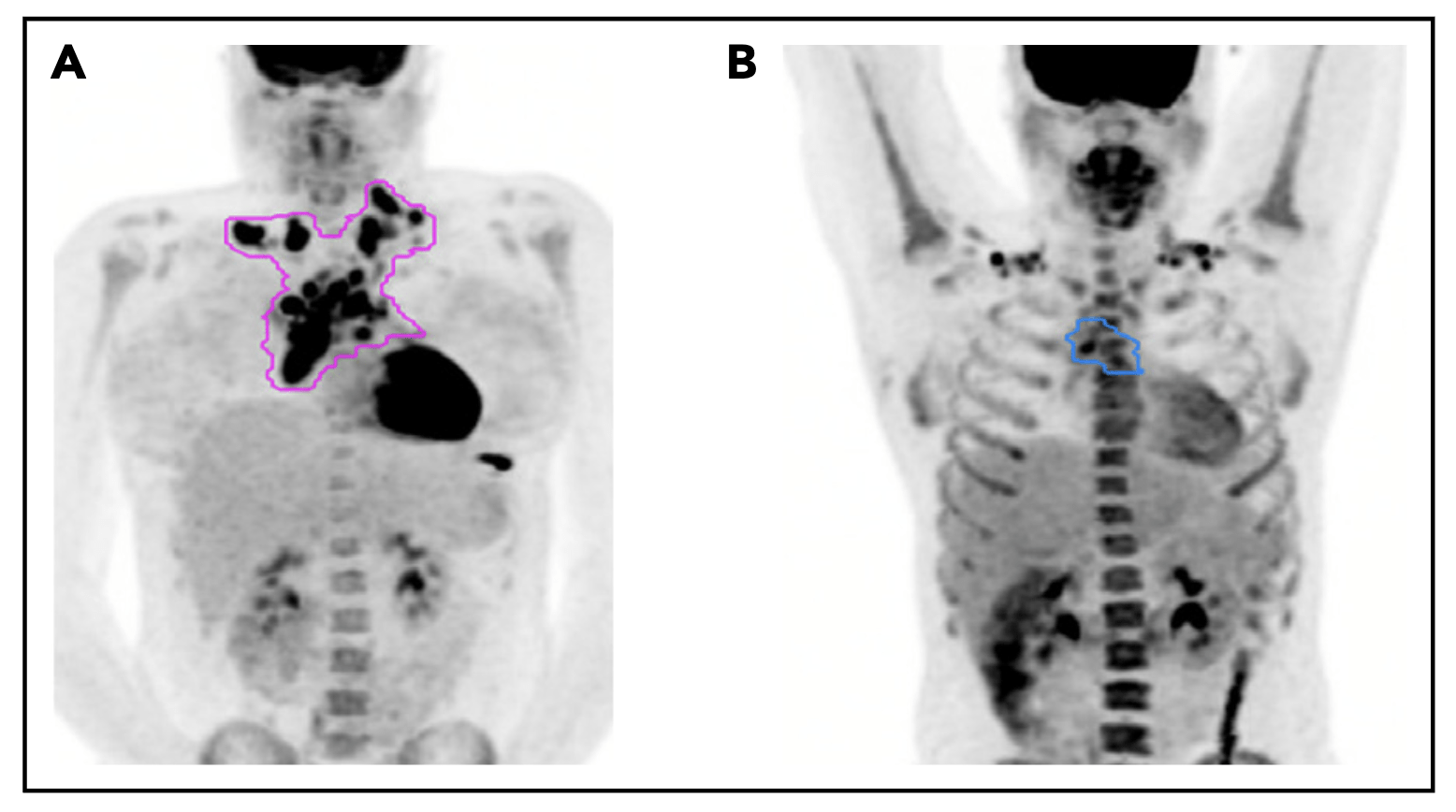
Pattern of relapse definitions. (A) Theoretical outline (pink) of initial site of disease based on the prechemotherapy PET/CT scan. A relapse within the out-lined region would have been categorized as a relapse in the “initial site” of disease. A relapse outside of the outlined region would be considered a “new site” ofrelapse. (B) Theoretical outline (blue) of fluorodeoxyglucose-avid residual disease greater than the mediastinal blood pool on PET/CT after 1 cycle of AVPC. For PET11patients, a relapse within the blue region would be considered a relapse at the “PET11 site,” whereas a relapse outside of the blue region but within the pink regionfrom panel A would be considered “initial site beyond the PET11 site.”
Patterns of Relapse - RERs
- Total relapses: 15
- All relapses involved an initial site of disease
- Without IFRT (14 relapses):
- 11 (78%): Initial site only
- 3 (21%): Initial plus new site
- With IFRT (1 relapse):
- 1 (100%): Initial irradiated site only
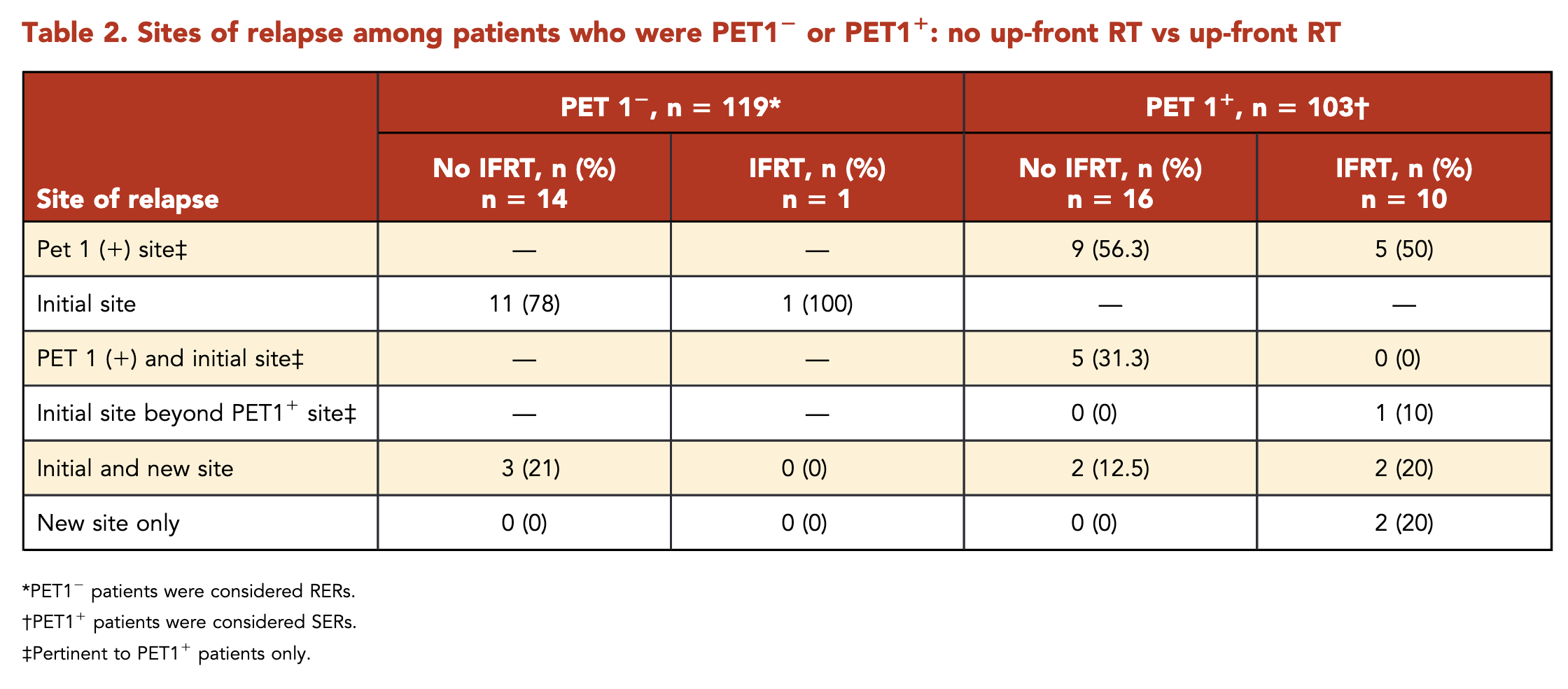
Patterns of Relapse - SERs

- Total relapses: 26
- 24 (92%) had relapse at an initial site
- 14 (54%) solely at the PET1+ site
- Without IFRT (16 relapses):
- 9 (56%): PET1+ site only
- 5 (31%): PET1+ site and outside PET1+ but within initial site
- 2 (12%): Initial and new site
- With IFRT (10 relapses):
- 5 (50%): PET1+ site only
- 1 (10%): Initial site beyond PET1+ site
- 2 (20%): Initial and new site
- 2 (20%): New site only
Comparison with Other Studies
| Study | PET Timing | Treatment | RT Criteria | RT Dose | Outcome |
|---|---|---|---|---|---|
| AHOD0431 (Current) | PET1 & PET3 | AVPC x3 | PR after 3 cycles (PET3+) | 21 Gy IFRT | 10-year PFS: RER: 96.6% (CMT) vs 84.1% (chemo alone) SER: 80.9% (IFRT) vs 64.0% (no IFRT) |
| EORTC H10 | PET2 | Standard: ABVD x3 + IFRT Experimental: ABVD x4 |
All in standard arm PET2+ in experimental arm |
30 Gy IFRT | 5-year PFS: 99.0% vs 87.1% |
| UK RAPID | PET3 | ABVD x3 ± IFRT | PET3+ or randomized to RT arm if PET3- | 30 Gy IFRT | 3-year PFS: 97.1% vs 90.8% |
| GHSG HD16 | PET2 | Standard: ABVD x2 + IFRT Experimental: ABVD x2 |
All in standard arm PET2+ in experimental arm |
20 Gy IFRT | 5-year PFS: 93.4% vs 86.1% |
- AHOD0431 unique in using both PET1 and PET3 for response assessment and allowing RT omission for SERs (PET1+) with CR at PET3
- Other studies used either PET2 or PET3 for response-adapted approach
- EORTC H10 and GHSG HD16 gave RT to all patients in standard arm, regardless of PET response
- UK RAPID randomized PET3- patients to RT or no RT
- AHOD0431 used lower RT dose (21 Gy) compared to EORTC H10 and UK RAPID (30 Gy)
- Results across studies consistently show improved outcomes with inclusion of RT, even in PET-negative patients
Comparison with Other Studies
| Study | PET Timing | Treatment | Outcome |
|---|---|---|---|
| AHOD0431 (Current) | PET1 | CMT Chemo alone |
10-year PFS: 96.6% 10-year PFS: 84.1% |
| EORTC H10 | PET2 | ABVD x3 + 30Gy IFRT ABVD x4 alone |
5-year PFS: 99.0% 5-year PFS: 87.1% |
| UK RAPID | PET3 | ABVD x3 + 30Gy IFRT ABVD x3 alone |
3-year PFS: 97.1% 3-year PFS: 90.8% |
| GHSG HD16 | PET2 | ABVD x2 + 20Gy IFRT ABVD x2 alone |
5-year PFS: 93.4% 5-year PFS: 86.1% |
- AHOD0431 used PET1, resulting in lower proportion of patients (54%) eligible for RT omission compared to PET2/PET3 studies (66-91%)
- In-field recurrences drove PFS differences in patients not receiving IFRT across studies
- AHOD0431 unique in allowing RT omission for SERs (PET1+)
- SERs showed improved outcomes with IFRT (10-year PFS: 80.9% vs 64%, p < .05)
- Results suggest potential benefit from dose intensification above 21 Gy to PET1+ regions in SERs
Conclusions
- PET1 response is a significant predictor of treatment outcome
- RERs showed favorable results with AVPC alone
- SERs had unfavorable outcomes with AVPC alone, but improved with 21 Gy IFRT
- RT remains an important component of treatment for SERs
- Most relapses occurred within the initial site of disease, often within the PET1+ site
- SERs may benefit from alternative systemic therapy and/or targeted RT intensification to PET1+ sites
Strengths and Limitations
- Strengths:
- Long-term follow-up (median 118 months)
- Centralized PET review
- Detailed analysis of relapse patterns
- Limitations:
- Exploratory analysis, not initially planned
- Small sample size in some subgroups
- Comparison with other response-adapted trials challenging due to differences in inclusion criteria and treatment regimens
Discussion Points
- How does the use of PET1 in this study compare to other trials using PET2 or PET3 for response assessment?
- What are the implications of the relapse patterns observed in this study for future RT planning in pediatric Hodgkin lymphoma?
- Should the radiation dose be increased for SERs, particularly to PET1+ sites? What are the potential benefits and risks?
- How might the results of this study influence the design of future pediatric Hodgkin lymphoma trials?
- What alternative systemic therapies could be considered for SERs to improve outcomes?
AHOD0431: PET-adapted therapy for early-stage, low-risk pediatric Hodgkin lymphoma
By RadMedSkiier
AHOD0431: PET-adapted therapy for early-stage, low-risk pediatric Hodgkin lymphoma
AHOD0431 trial, exploring PET-adapted therapy for low-risk pediatric Hodgkin lymphoma, with insights into patient outcomes, relapse patterns, and comparisons to existing studies.
- 16



