Joint Final Report of EORTC 26951 and RTOG 9402
Phase III Trials With Procarbazine, Lomustine, and Vincristine Chemotherapy for Anaplastic Oligodendroglial Tumors
Lassman AB, Hoang-Xuan K, Polley MYC, et al.
Journal of Clinical Oncology 2022;40:2539-2545
Presenter: [Your Name]
Journal Club - [Date]
Background
- Anaplastic oligodendroglial tumors (AOTs) are chemotherapy-sensitive brain tumors
- European Organization for Research and Treatment of Cancer (EORTC) 26951 and Radiation Therapy Oncology Group (RTOG) 9402 phase III trials were launched in the 1990s
- Initial reports showed improved progression-free survival (PFS) but not overall survival (OS) with the addition of PCV
- With longer follow-up, improved OS was observed, particularly in patients with 1p/19q codeleted tumors
- This is the final report with extremely long-term follow-up (18-19 years)
- When trials were launched, the importance of molecular markers was not yet established:
- 1p/19q codeletion
- IDH1 and IDH2 mutations
- MGMT promoter methylation
Methods
Study Design
- Two phase III trials testing the addition of procarbazine, lomustine (CCNU), and vincristine (PCV) to radiotherapy (RT)
- EORTC 26951: RT + adjuvant PCV (up to 6 cycles) vs. RT alone
- RTOG 9402: Neoadjuvant intensified PCV (up to 4 cycles) + RT vs. RT alone
- Both trials used the same RT dose: 59.4 Gy in 33 fractions
Patient Population
- EORTC 26951: 368 patients enrolled between 1996-2002
- RTOG 9402: 289 patients enrolled between 1994-2002
- Anaplastic oligodendroglioma or oligoastrocytoma (per WHO classification at that time)
- Molecular analyses performed centrally post-hoc
Trial comparison
RTOG 9402 Trial Design
Patients with newly diagnosed anaplastic oligodendroglial tumors
Enrollment: July 1994 - March 2002
↓
Randomization
N = 289
↓
RT Alone Arm
n = 143
↓
Radiotherapy
59.4 Gy in 33 fractions of 1.8 Gy
↓
Follow-up
PCV allowed at progression
PCV + RT Arm
n = 146
↓
Neoadjuvant Intensified PCV
Up to 4 cycles
↓
Radiotherapy
59.4 Gy in 33 fractions of 1.8 Gy
↓
Follow-up
Median 18.1 years
↓
Molecular Analysis (Post-hoc)
1p/19q Codeletion
48% (125 of 261 cases)
IDH Mutation
74% (156 of 210 cases)
- Both trials: Median follow-up duration of 18-19 years
- EORTC 26951: 17% of patients still alive at data lock
- RTOG 9402: 21% of patients still alive, 16% free of progression
EORTC 26951 Trial Design
Patients with newly diagnosed anaplastic oligodendroglial tumors
Enrollment: Aug 1996 - March 2002
↓
Randomization
N = 368
↓
RT Alone Arm
n = 183
↓
Radiotherapy
59.4 Gy in 33 fractions of 1.8 Gy
↓
Follow-up
PCV allowed at progression
RT + PCV Arm
n = 185
↓
Radiotherapy
59.4 Gy in 33 fractions of 1.8 Gy
↓
Adjuvant PCV
Up to 6 cycles
↓
Follow-up
Median 19 years
↓
Molecular Analysis (Post-hoc)
1p/19q Codeletion
25% (80 of 316 cases)
IDH Mutation
46% (83 of 182 cases)
Patient Population
| Characteristic | EORTC 26951 | RTOG 9402 |
|---|---|---|
| Total Enrollment | 368 patients | 289 patients |
| Enrollment Period | August 1996 - March 2002 | July 1994 - March 2002 |
| RT Alone Arm | 183 patients | 143 patients |
| RT+PCV Arm | 185 patients (adjuvant PCV) | 146 patients (neoadjuvant PCV) |
| Median Follow-up | 19 years | 18.1 years |
| Patients Still Alive at Data Lock | 61 (17%) | 61 (21%) |
| Progression-free Patients | Not specifically reported | 47 (16%) |
| Median Follow-up of Survivors | 17.8 years (range > 0-21.7 years) | 18.1 years (range > 0-23.1 years) |
| PCV Regimen | Adjuvant: up to 6 cycles after RT | Neoadjuvant: up to 4 cycles of intensified PCV before RT |
| Radiotherapy Dose | 59.4 Gy in 33 fractions of 1.8 Gy | 59.4 Gy in 33 fractions of 1.8 Gy |
| Disease Assessment | Progression defined locally using Macdonald criteria | Progression defined locally using Macdonald criteria |
Molecular markers
EORTC 26951
- 1p/19q codeletion by FISH: 25% (80 of 316 cases)
- IDH1/IDH2 mutation: 46% (83 of 182 informative cases)
- 87% (39 of 45) of codeleted tumors were IDH-mutant
- 115 tumors analyzed with genome-wide methylation arrays
- 139 tumors analyzed by next-generation sequencing
RTOG 9402
- 1p/19q codeletion by FISH: 48% (125 of 261 cases)
- IDH mutation: 74% (156 of 210 informative cases)
- 90% of codeleted tumors were IDH-mutant
Molecular markers
| Molecular Marker | EORTC 26951 | RTOG 9402 | Key Findings |
|---|---|---|---|
| 1p/19q Codeletion (FISH) | 25% (80 of 316 informative cases) | 48% (125 of 261 informative cases) | Strong predictor of benefit from PCV in both trials |
| IDH1/IDH2 Mutation | 46% (83 of 182 informative cases) | 74% (156 of 210 informative cases) | Associated with better outcomes |
| Codeleted Tumors with IDH Mutation | 87% (39 of 45 codeleted tumors were IDH-mutant) | 90% of codeleted tumors were IDH-mutant | Strong correlation between codeletion and IDH mutation |
| MGMT Promoter Methylation | Assessed with methylation arrays | Not specifically reported | Significantly predictive of benefit from PCV (HR 0.41; 95% CI, 0.25-0.67; P < .0001) |
| Methylation Arrays | 115 tumors analyzed | Not specifically reported | Used to assess MGMT status |
| Next-Generation Sequencing | 139 tumors analyzed (using Ion Torrent) | Not specifically reported | Used for comprehensive molecular classification |
| IDH Mutation Detection Method | Sanger sequencing | Immunohistochemistry or DNA sequencing | Different techniques but consistent results |
| Patients with IDH-mutant non-codeleted | 43 patients | 66 patients | Also showed benefit from PCV but less robust than codeleted cases |
| Survivors with Codeleted Tumors | 33% (26 of 80) still alive at follow-up | Not specifically reported | Demonstrates long-term survival in this subgroup |
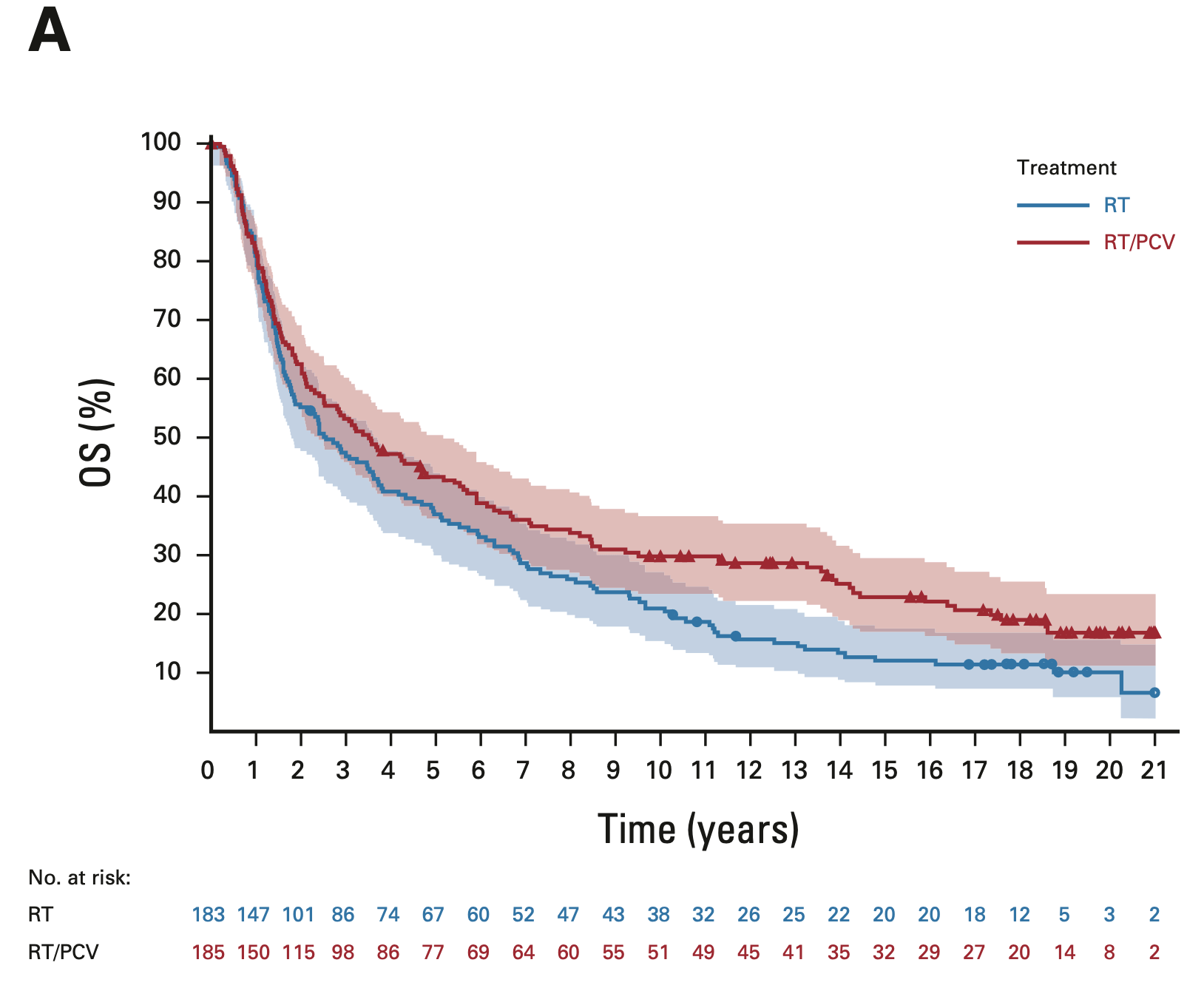
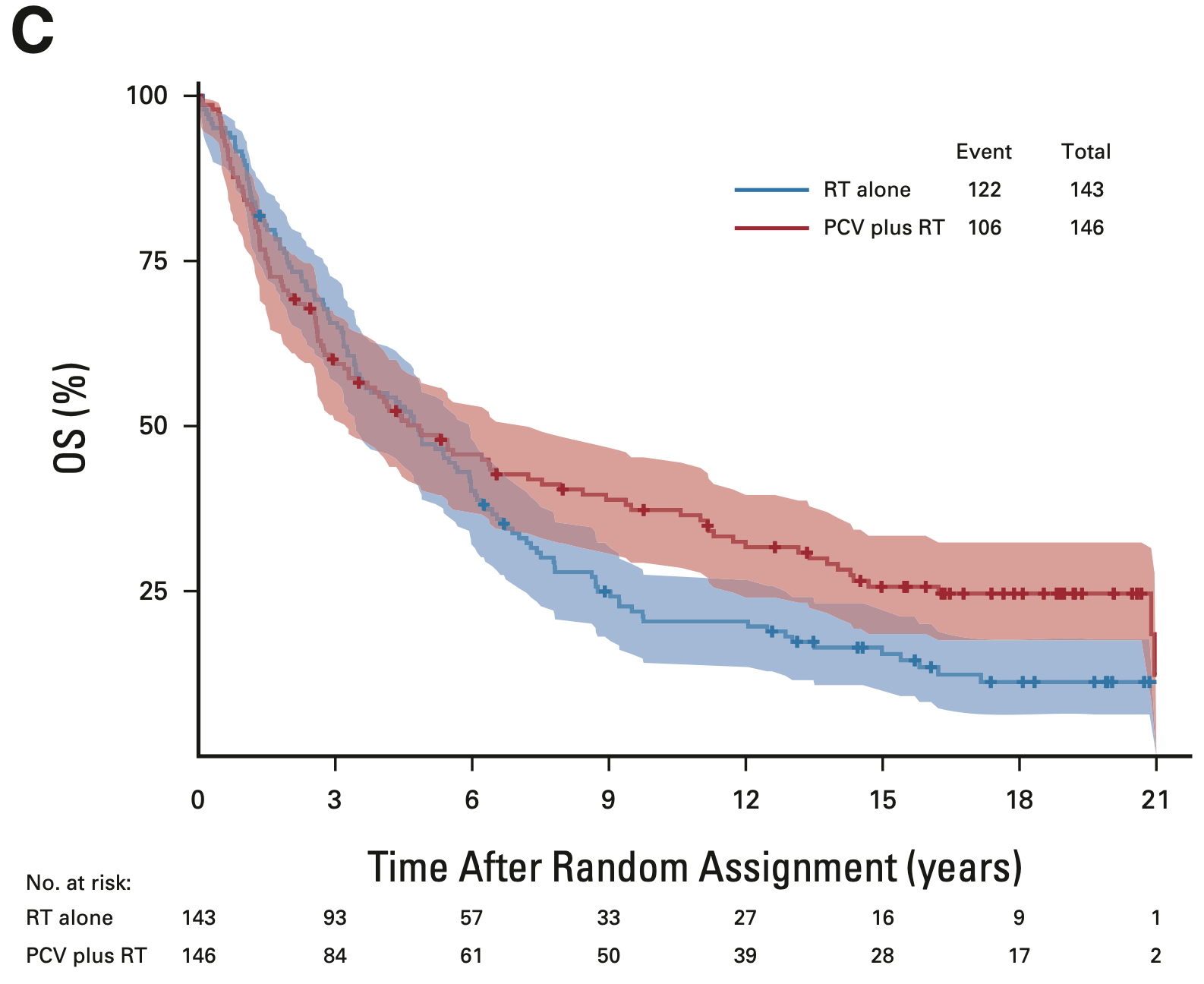
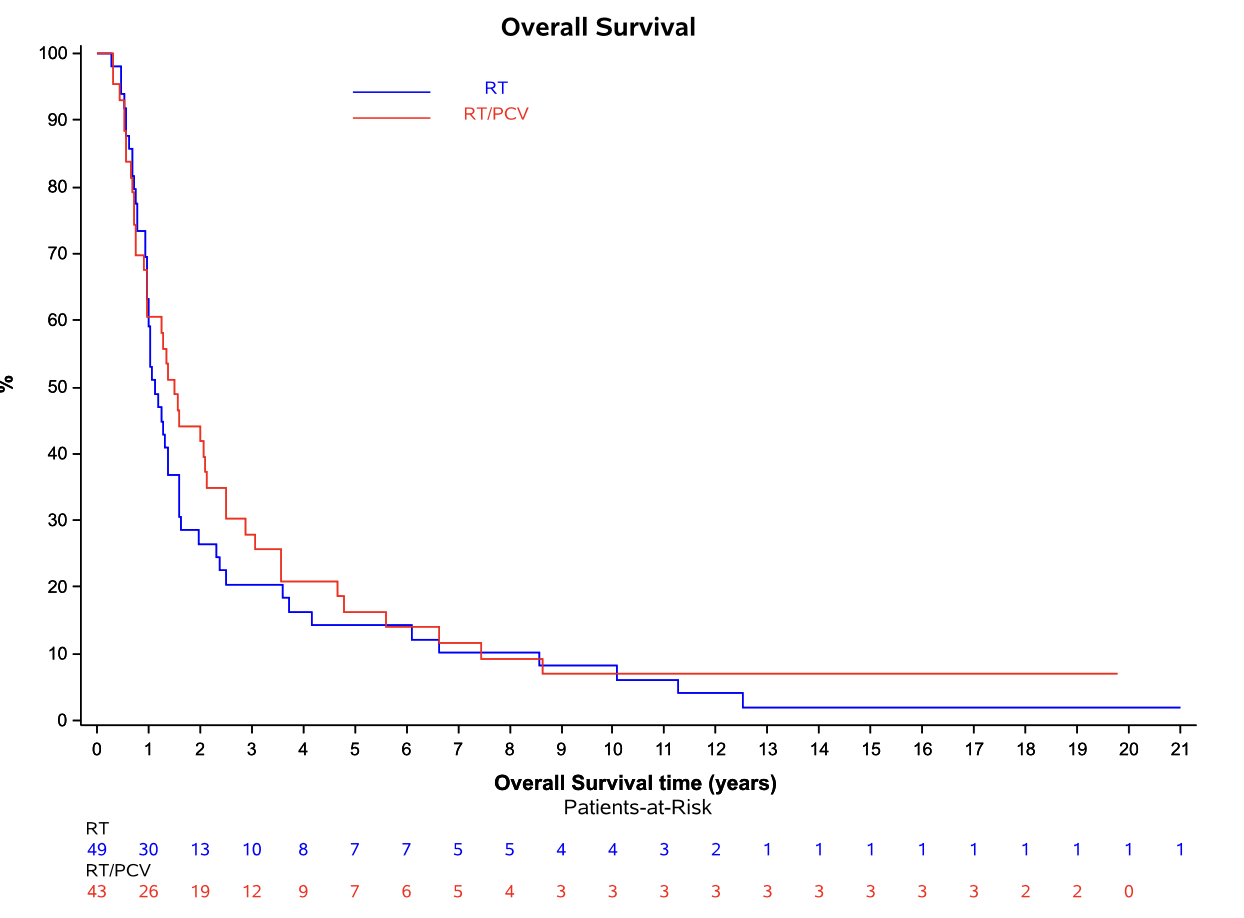
Overall survival: Intent-to-treat population
EORTC 26951
- Median OS: 3.5 vs. 2.6 years (HR 0.78; 95% CI, 0.63-0.98; P = 0.033)
- 14-year OS: 25.1% vs. 13.4%
- 20-year OS (est.): 16.8% vs. 10.1%
RTOG 9402
- Median OS: 4.8 vs. 4.8 years (HR 0.79; 95% CI, 0.61-1.03; P = 0.08)
- 14-year OS: 29.1% vs. 16.5%
- 20-year OS (est.): 24.6% vs. 11.2%
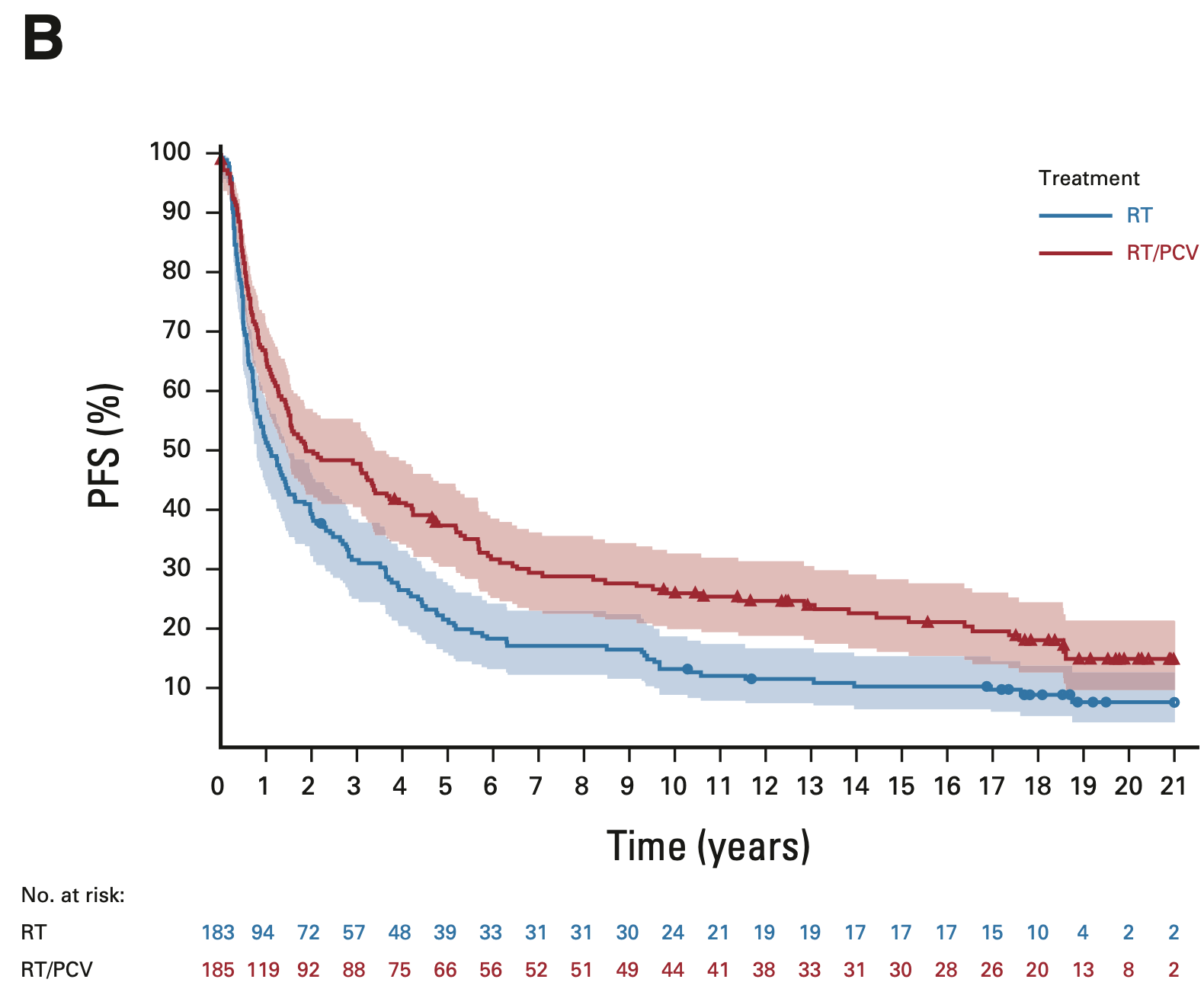
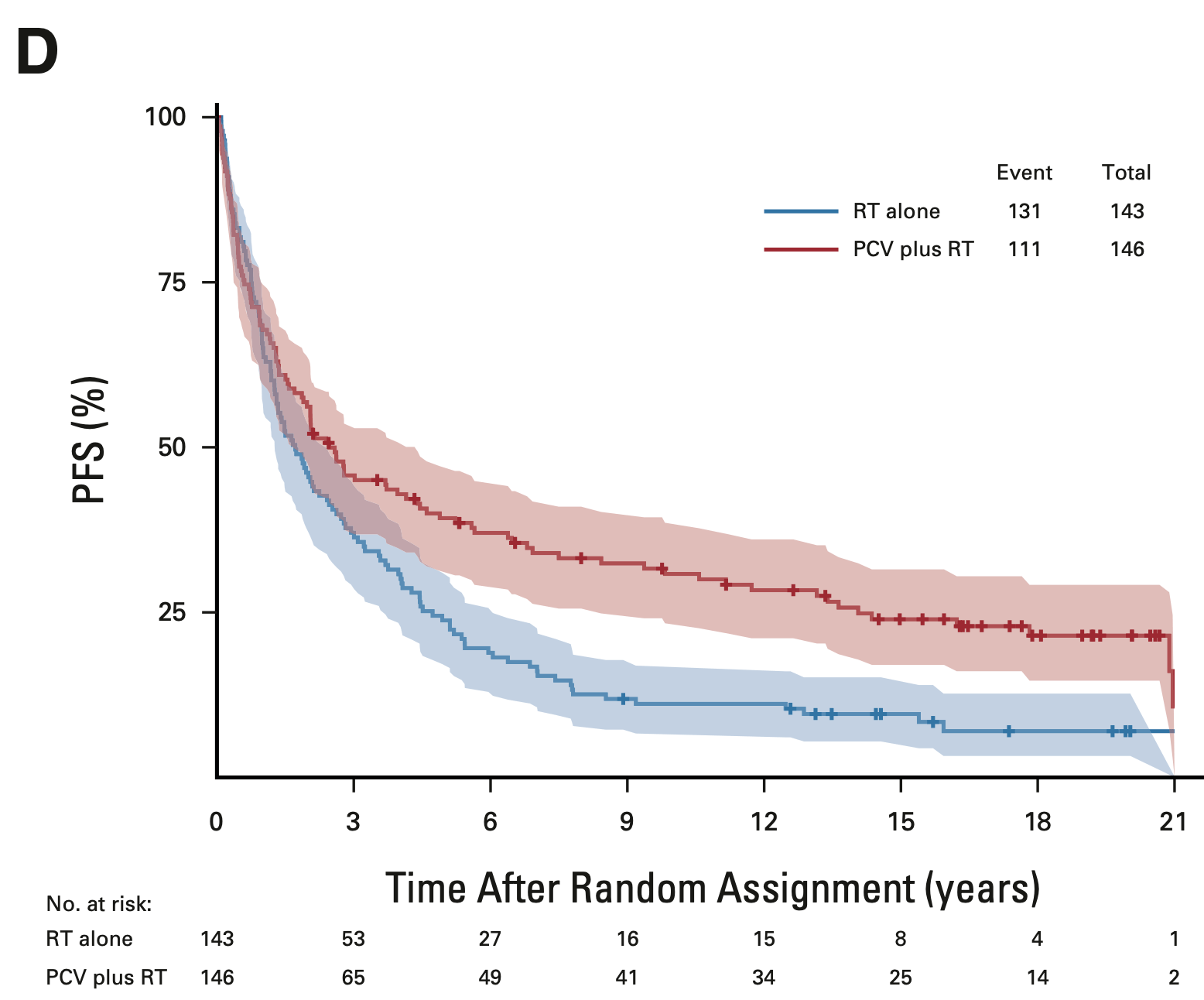
Progression-free survival: Intent-to-treat population
EORTC 26951
- Median PFS: 2.0 vs. 1.1 years (HR 0.69; 95% CI, 0.55-0.86; P = 0.001)
- 14-year PFS: 22.7% vs. 10.4%
- 20-year PFS (est.): 15.0% vs. 7.7%
RTOG 9402
- Median PFS: 2.5 vs. 1.7 years (HR 0.67; 95% CI, 0.52-0.86; P < 0.001)
- 14-year PFS: 25.7% vs. 9.6%
- 20-year PFS (est.): 21.5% vs. 7.0%
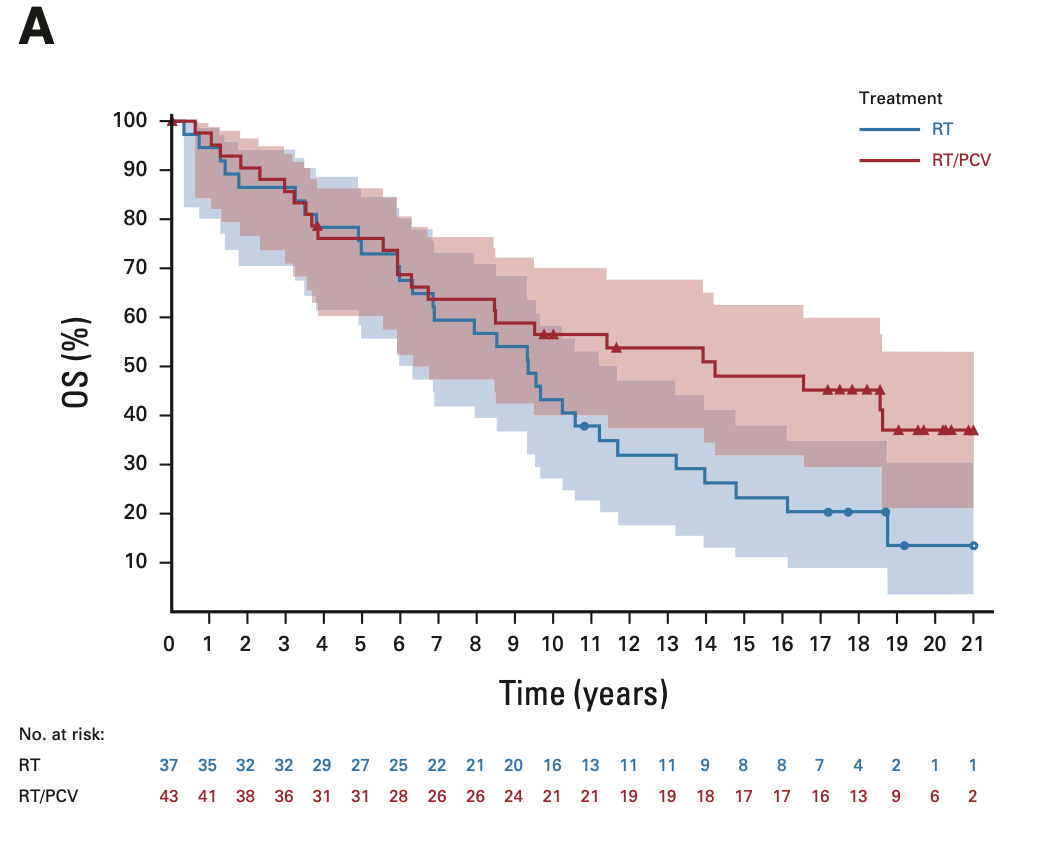
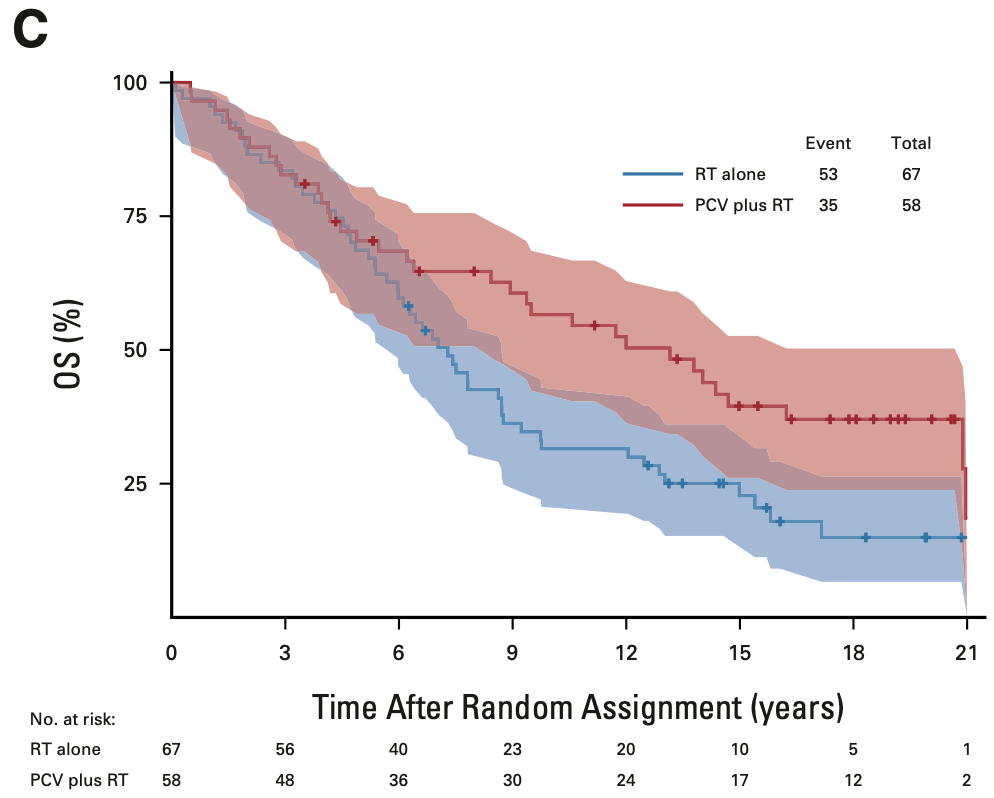
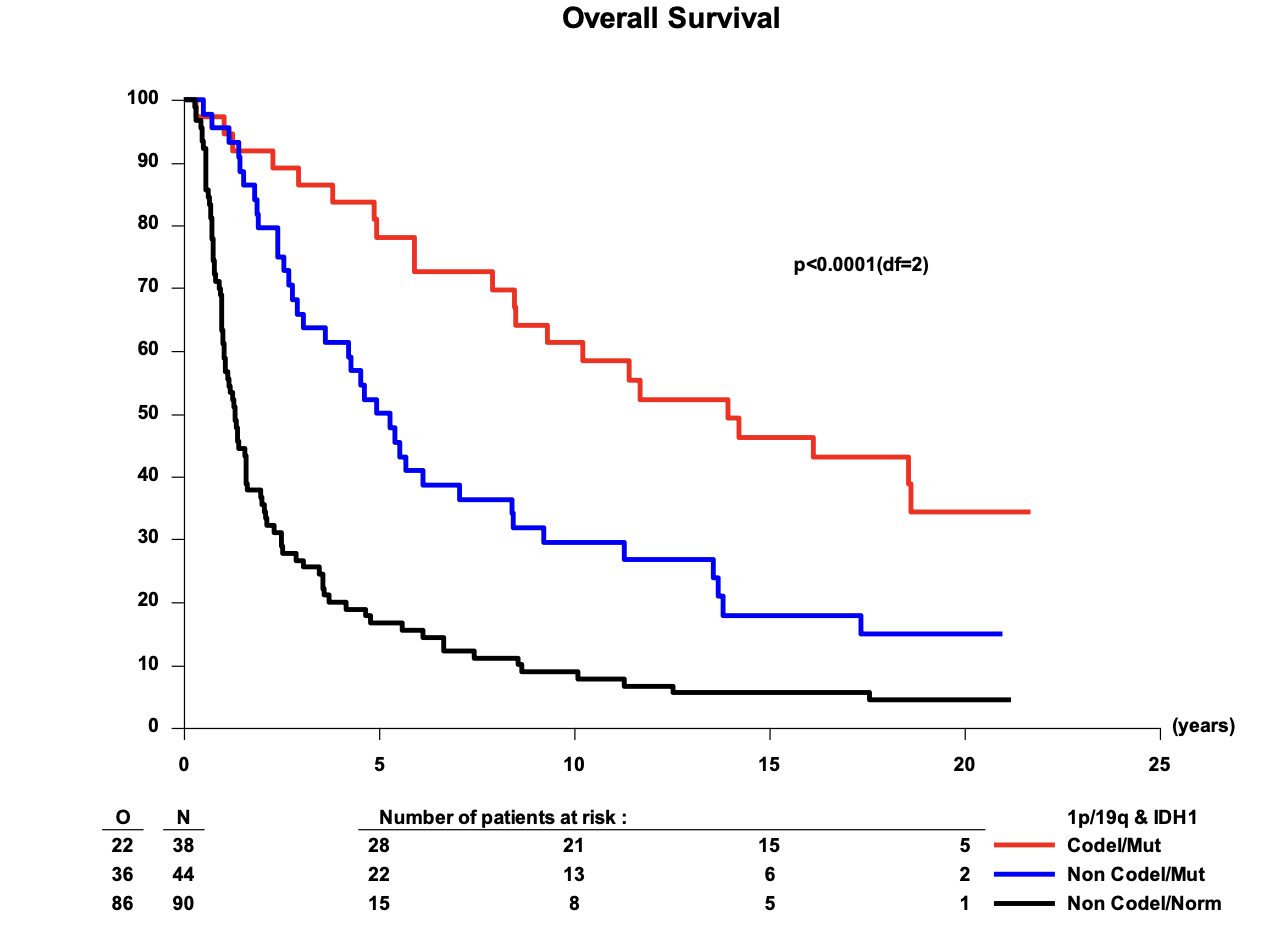
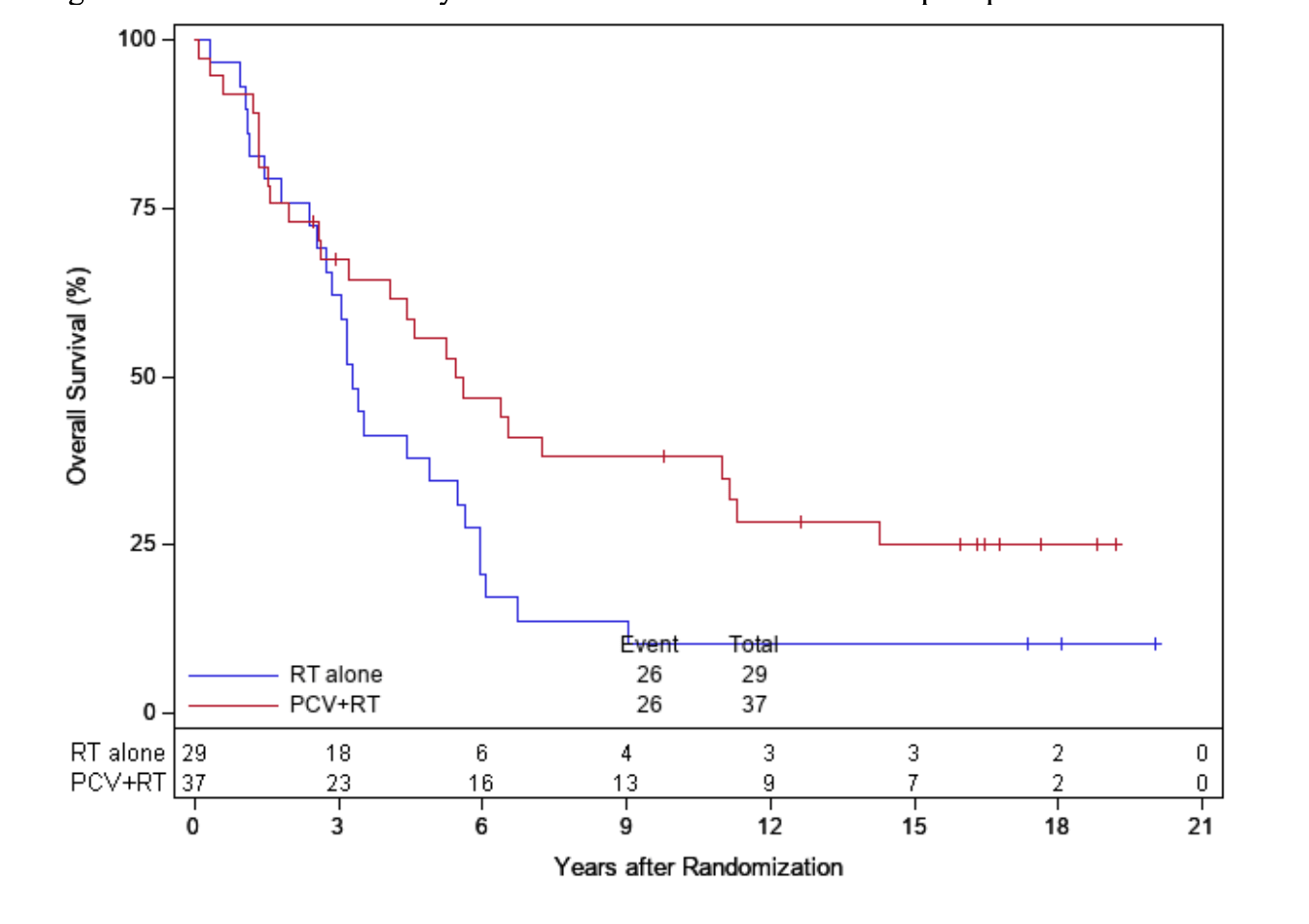
OS in 1p/19q codeleted tumors
EORTC 26951
- Median OS: 14.2 vs. 9.3 years (HR 0.60; 95% CI, 0.35-1.03; P = 0.063)
- 14-year OS: 51.0% vs. 26.2%
- 20-year OS (est.): 37.1% vs. 13.6%
RTOG 9402
- Median OS: 13.2 vs. 7.3 years (HR 0.61; 95% CI, 0.40-0.94; P = 0.02)
- 14-year OS: 46.1% vs. 25.0%
- 20-year OS (est.): 37% vs. 14.9%
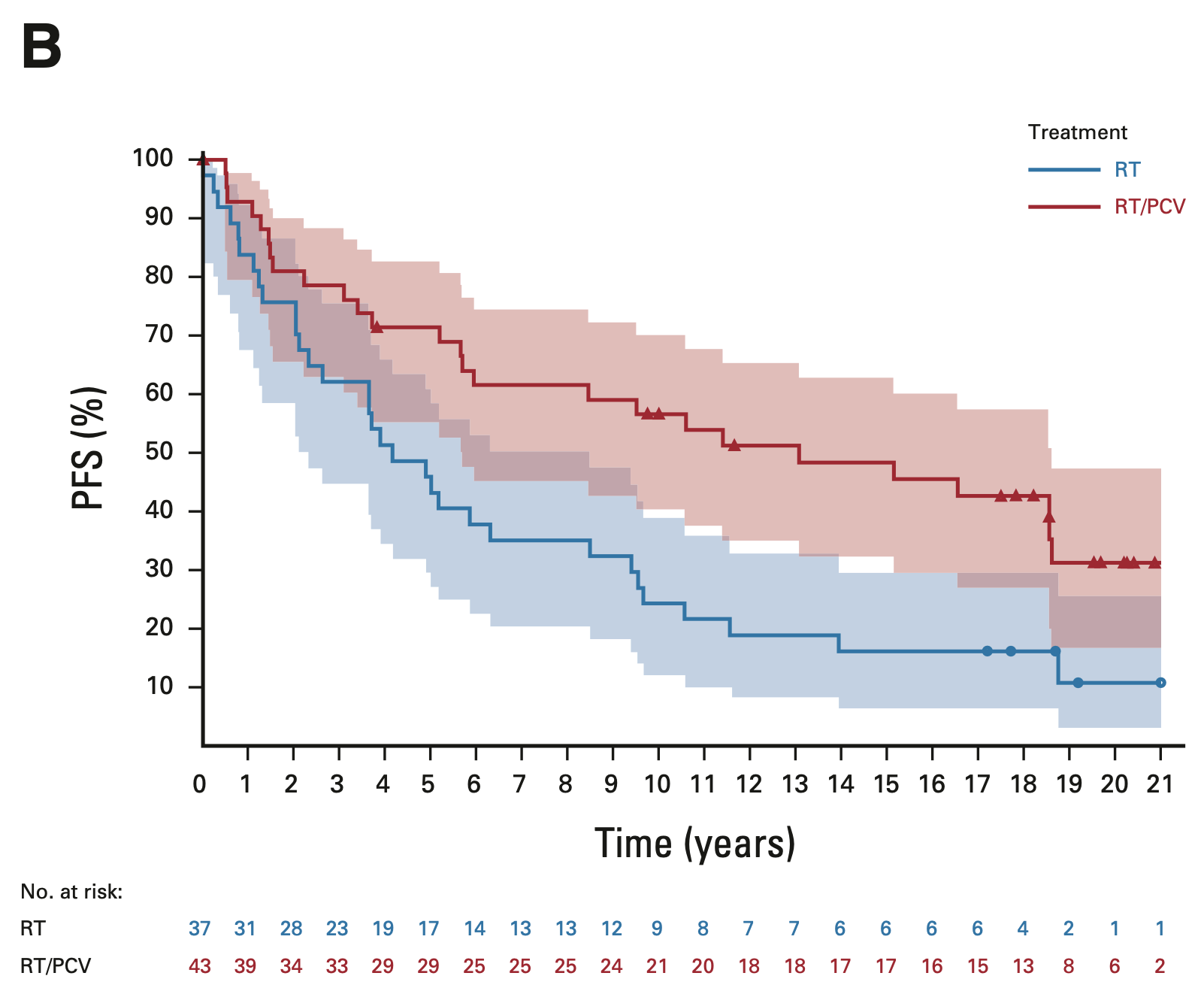
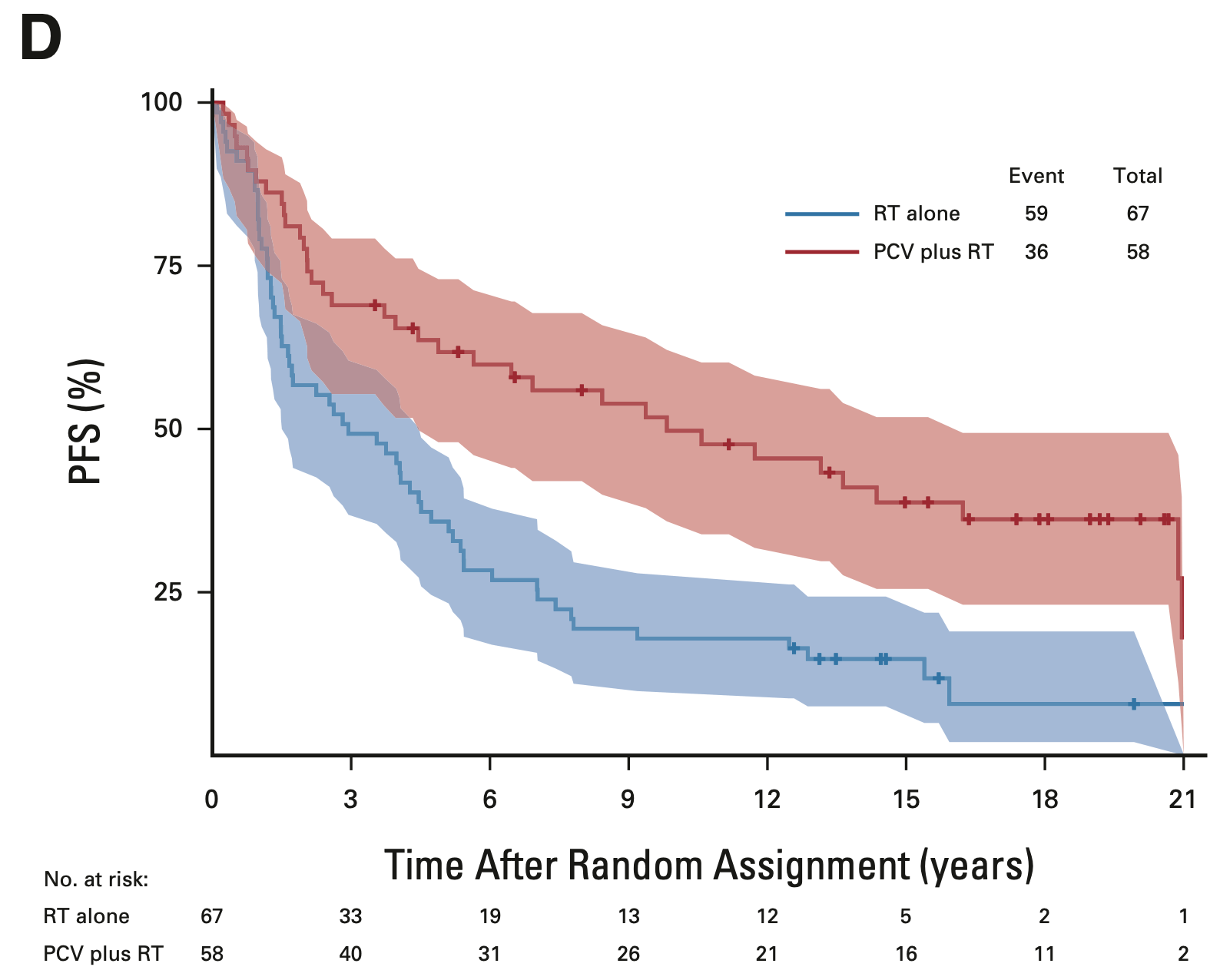
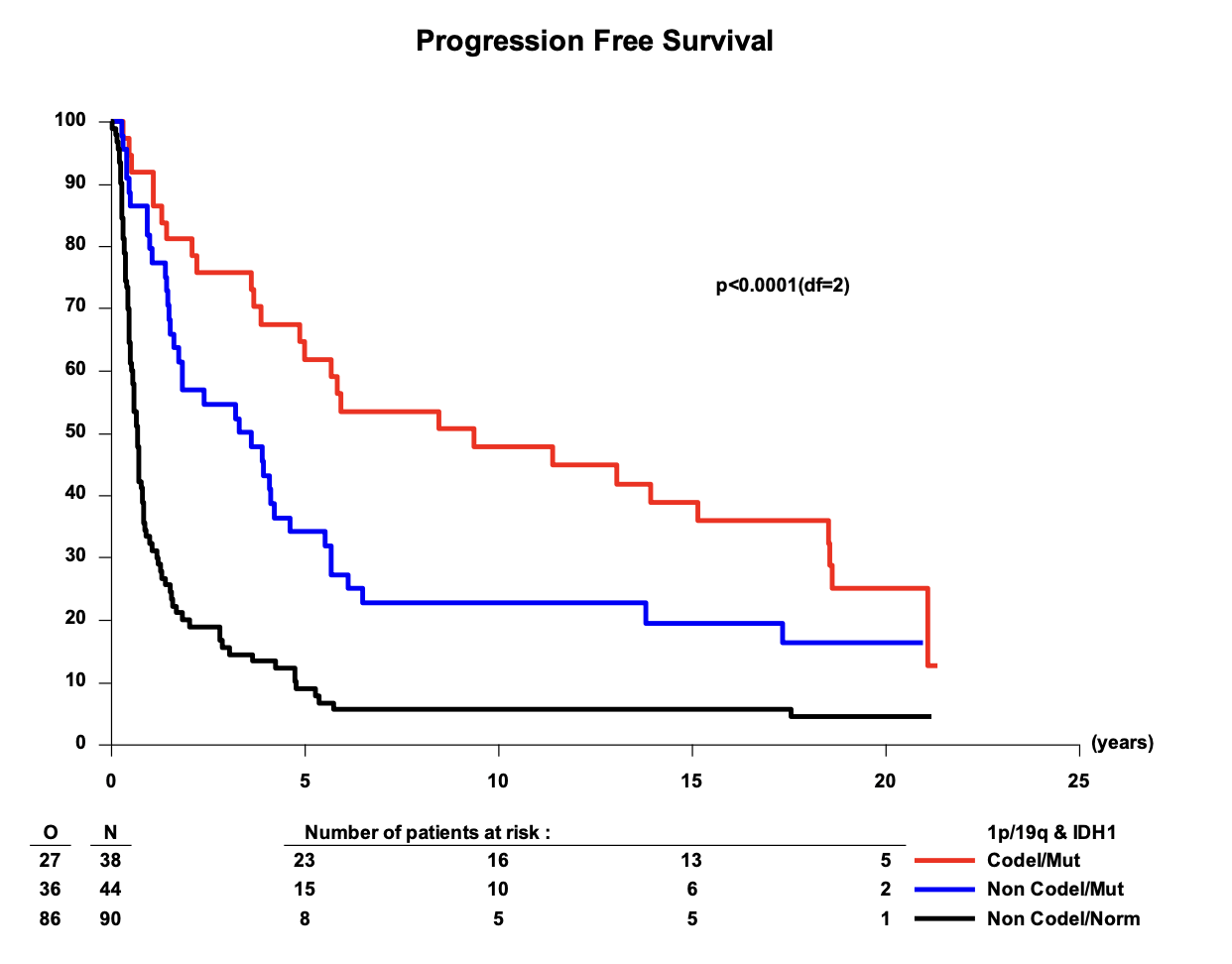
PFS in 1p/19q codeleted tumors
EORTC 26951
- Median PFS: 13.1 vs. 4.2 years (HR 0.49; 95% CI, 0.29-0.83; P = 0.007)
- 14-year PFS: 48.4% vs. 16.2%
- 20-year PFS (est.): 31.3% vs. 10.8%
RTOG 9402
- Median PFS: 9.8 vs. 2.9 years (HR 0.46; 95% CI, 0.30-0.70; P < 0.001)
- 14-year PFS: 41.0% vs. 14.8%
- 20-year PFS (est.): 36.2% vs. 7.9%
OS by molecular subgroups
IDH-mutant, non-codeleted tumors
EORTC 26951
- Median OS: 8.4 vs. 3.0 years (HR 0.60; 95% CI, 0.31-1.17; P = 0.131)
- 10-year OS: 43.5% vs. 15.0%
- 20-year OS (est.): 11.0% vs. NE
RTOG 9402
- Median OS: 5.5 vs. 3.3 years (HR 0.60; 95% CI, 0.34-1.03; P = 0.06)
- 10-year OS: 38.1% vs. 10.3%
- 20-year OS (est.): NE vs. 10.3%
NE = not evaluable
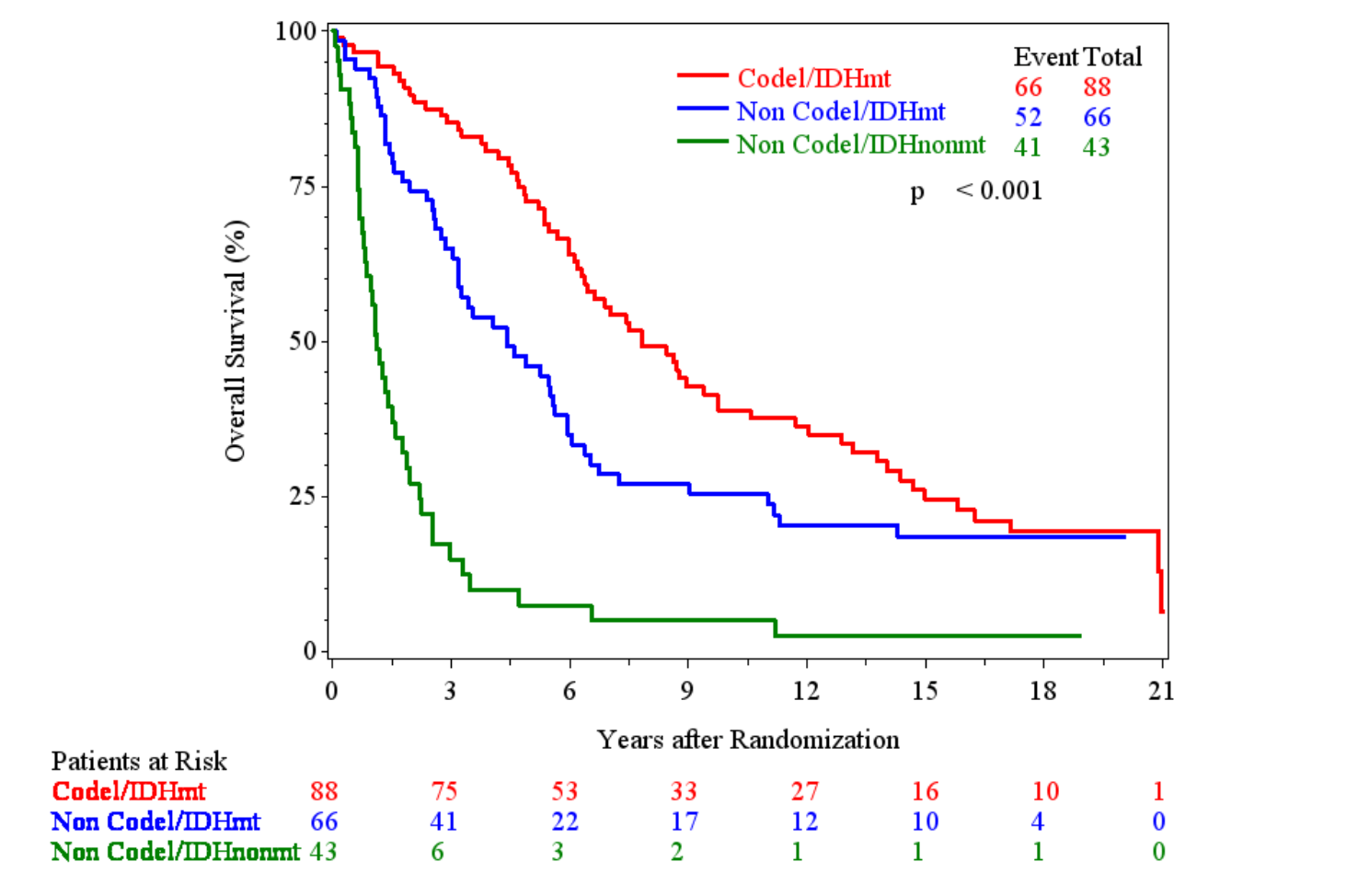
PFS by molecular subgroups
IDH-mutant, non-codeleted tumors
EORTC 26951
- Median OS: 8.4 vs. 3.0 years (HR 0.60; 95% CI, 0.31-1.17; P = 0.131)
- 10-year OS: 43.5% vs. 15.0%
- 20-year OS (est.): 11.0% vs. NE
RTOG 9402
- Median OS: 5.5 vs. 3.3 years (HR 0.60; 95% CI, 0.34-1.03; P = 0.06)
- 10-year OS: 38.1% vs. 10.3%
- 20-year OS (est.): NE vs. 10.3%
NE = not evaluable
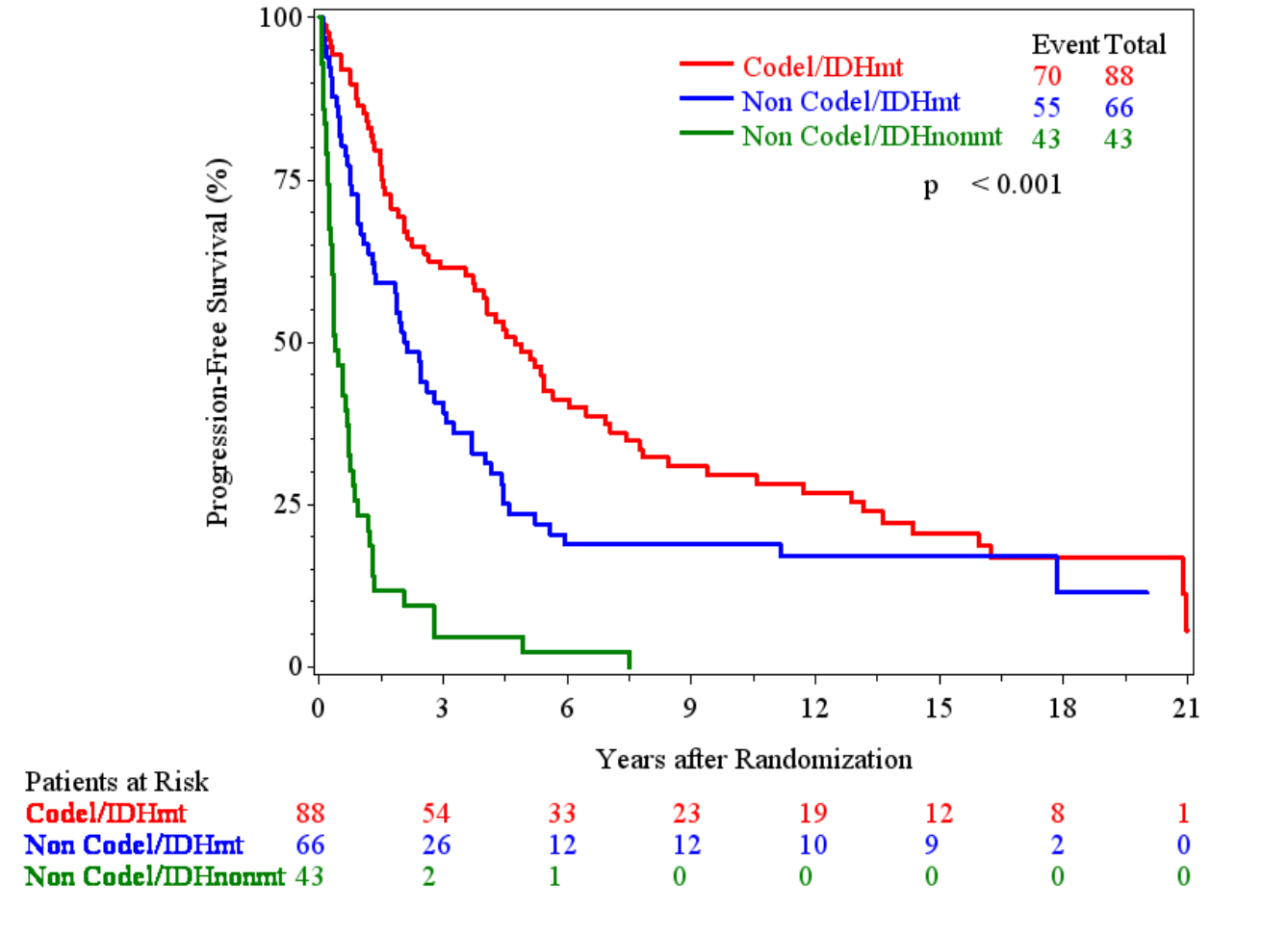
PFS by molecular subgroups
IDH-mutant, non-codeleted tumors
EORTC 26951
RTOG 9402
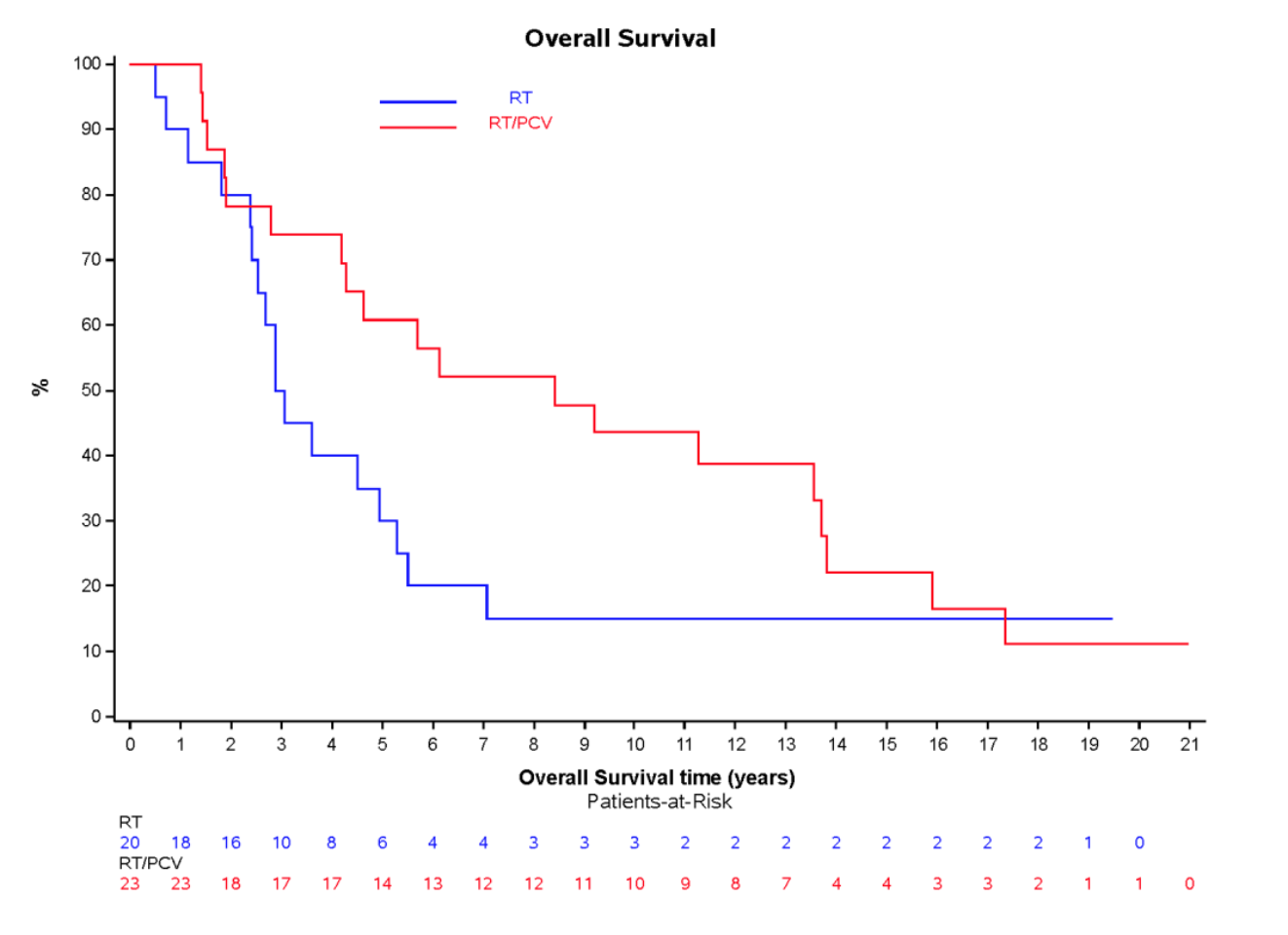
PFS by molecular subgroups
IDH-wt, non-codeleted tumors
EORTC 26951
RTOG 9402
NE = not evaluable
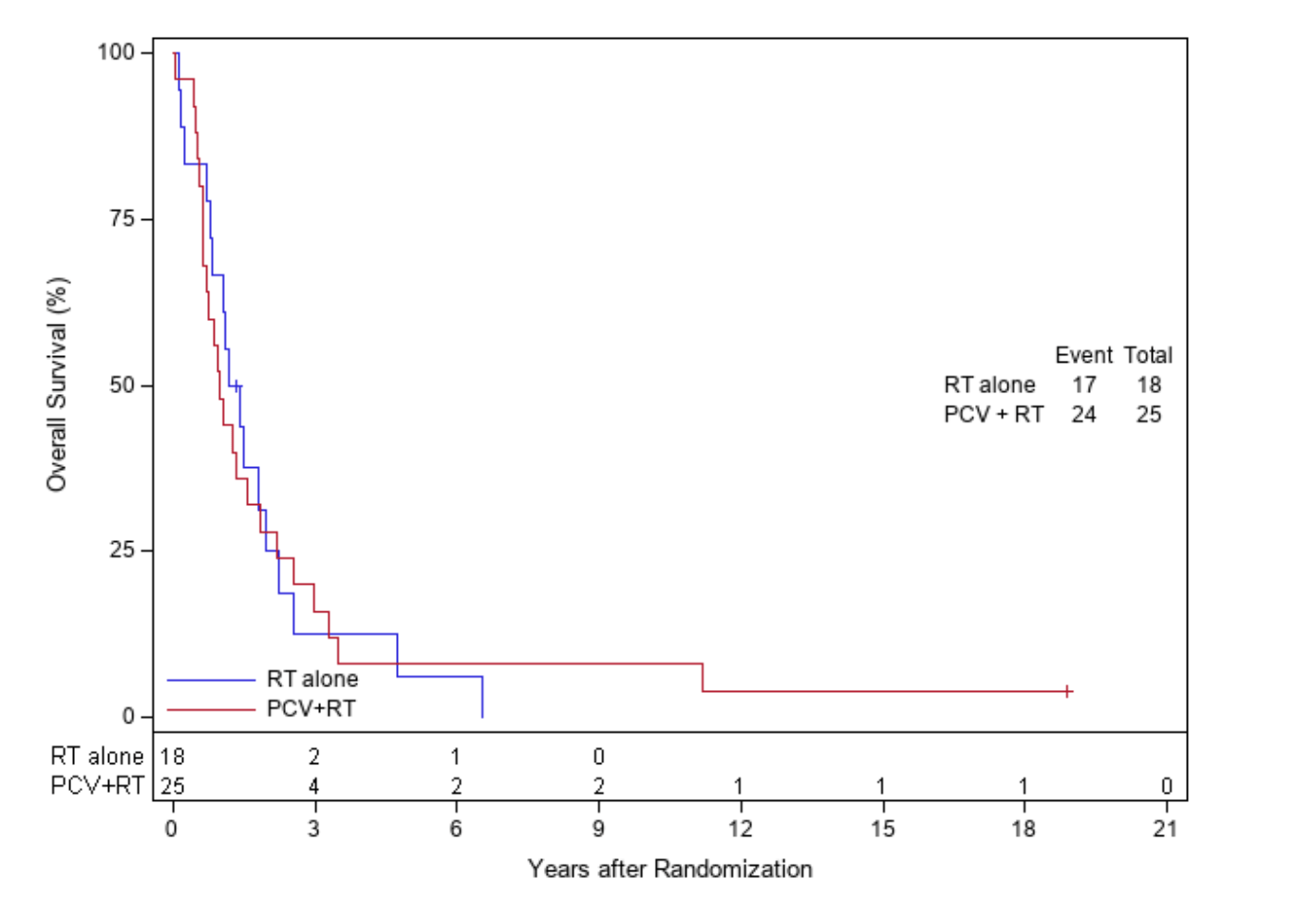
Additional molecular findings
Predictive biomarkers
- MGMT promoter methylation was significantly predictive of benefit from PCV (HR 0.41; 95% CI, 0.25-0.67; P < 0.0001)
- Strong association between IDH mutation and 1p/19q codeletion
- EORTC 26951: 87% of codeleted tumors were IDH-mutant
- RTOG 9402: 90% of codeleted tumors were IDH-mutant
Current WHO Classification (2021)
- Results align with 2021 WHO Classification of Tumors of the Central Nervous System
- Oligodendroglioma now requires both IDH mutation and 1p/19q codeletion
- Astrocytoma requires IDH mutation without 1p/19q codeletion
- The term 'oligoastrocytoma' is strongly discouraged since 2016
Summary of key findings
Both trials showed consistent long-term survival benefits from adding PCV to RT:
- 40% reduction in risk of death for 1p/19q codeleted tumors in both trials
- Substantial long-term survival (35-37% at 20 years) in codeleted tumors treated with PCV+RT
- IDH-mutant non-codeleted tumors also benefit from PCV, but to a lesser extent
- IDH wild-type tumors showed minimal benefit and poor outcomes regardless of treatment
| Trial | Subgroup | Treatment | Median OS | 20-yr OS | HR (95% CI) |
|---|---|---|---|---|---|
| EORTC 26951 |
All Patients |
RT alone | 2.6 yrs | 10.1% | 0.78 (0.63-0.98) p=0.033 |
| RT+PCV | 3.5 yrs | 16.8% | |||
| EORTC 26951 |
1p/19q Codeleted |
RT alone | 9.3 yrs | 13.6% | 0.60 (0.35-1.03) p=0.063 |
| RT+PCV | 14.2 yrs | 37.1% | |||
| EORTC 26951 |
IDHmt Non-codel |
RT alone | 3.0 yrs | NE | 0.60 (0.31-1.17) p=0.131 |
| RT+PCV | 8.4 yrs | 11.0% | |||
| RTOG 9402 |
All Patients |
RT alone | 4.8 yrs | 11.2% | 0.79 (0.61-1.03) p=0.08 |
| RT+PCV | 4.8 yrs | 24.6% | |||
| RTOG 9402 |
1p/19q Codeleted |
RT alone | 7.3 yrs | 14.9% | 0.61 (0.40-0.94) p=0.02 |
| RT+PCV | 13.2 yrs | 37.0% | |||
| RTOG 9402 |
IDHmt Non-codel |
RT alone | 3.3 yrs | 10.3% | 0.60 (0.34-1.03) p=0.06 |
| RT+PCV | 5.5 yrs | NE |
Strengths and limitations
Strengths
- Extremely long-term follow-up (18-19 years)
- Two independent studies with very similar results
- Comprehensive post-hoc molecular analyses
- Results aligned with current WHO classification system
- Demonstration of durable disease control in a significant proportion of patients
Limitations
- Molecular information not available for all patients
- Studies designed before current molecular classification era
- No comparison with temozolomide, which has largely replaced PCV in clinical practice due to better tolerability
- Limited data on long-term toxicities, including cognitive outcomes
- Limited information on treatments received at recurrence
Clinical implications
- PCV is an effective therapeutic regimen for gliomas with IDH mutation and especially 1p/19q codeletion
- Long-term survival observed emphasizes need to better understand long-term effects of treatment on cognitive function and quality of life
- Concern that initial treatment with chemotherapy alone may be detrimental for survival
- Results demonstrate critical importance of governmentally funded networks in conducting long-term clinical trials
PCV vs. Temozolomide
- Many clinicians have transitioned to temozolomide due to lower toxicity and perceived equivalence of efficacy
- However, direct comparisons between PCV and temozolomide are limited
- Ongoing trials (e.g., CODEL) may provide further insights
Discussion points
- How should these results influence our approach to newly diagnosed 1p/19q codeleted oligodendroglioma?
- Does the 40% reduction in risk of death with PCV justify its toxicity compared to temozolomide?
- How do we balance the risk of late neurocognitive effects of early radiotherapy against potentially compromised survival with chemotherapy alone?
- What is the optimal timing of radiotherapy and PCV (pre- vs. post-RT)?
- What are the key long-term survivorship issues for patients with oligodendrogliomas who may live 20+ years after diagnosis?
Joint Final Report of EORTC 26951 and RTOG 9402: PCV Chemotherapy for Anaplastic Oligodendroglial Tumors
By RadMedSkiier
Joint Final Report of EORTC 26951 and RTOG 9402: PCV Chemotherapy for Anaplastic Oligodendroglial Tumors
Journal club presentation on the final very long-term survival results of two phase III trials for anaplastic oligodendroglial tumors
- 347



