Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma
The Children's Oncology Group ACNS0121 Trial
Merchant et al.
Journal of Clinical Oncology, 2019
Merchant TE, et al. J Clin Oncol 37:974-983, 2019
Introduction
- Ependymoma accounts for 8-10% of pediatric brain tumors
- Survival historically disappointing: 5-year EFS 23-45%
- Typically treated with maximal surgical resection
- Controversy around radiation in young children (< 3 years)
- Previous approaches:
- Young children: surgery + chemotherapy, delay RT
- Older children: immediate post-operative RT
- Recurrences primarily local (80%), but 20% distant failures
Duffner PK, et al. Neuro-oncol 1:152-161, 1999
Strother DR, et al. Neuro-oncol 16:457-465, 2014
- Maximal safe surgical resection
- Multiagent chemotherapy regimens:
- POG 9233: Standard or dose-intensified chemotherapy
- Head Start: Intensive chemotherapy with autologous stem cell rescue
- Baby POG/COG: Cyclophosphamide, vincristine, cisplatin, etoposide
- Radiation therapy delayed by 1-2 years or until progression
- Poor outcomes with chemotherapy alone:
- POG 9233: 24.4% 5-year EFS
- High progression rates during chemotherapy
- Maximal safe surgical resection
- Immediate post-operative conventional irradiation
- Typically involved-field RT
- Large clinical margins (1.5-2.0 cm)
- Often craniospinal irradiation for infratentorial tumors
- Occasional use of adjuvant chemotherapy
- CCG 9942 (1995-1999) results:
- 5-year EFS: 57%
- 5-year OS: 71%
- Delay of RT in young children resulted in poor disease control
- Large RT fields increased risk of neurocognitive, endocrine, and auditory side effects
- Lack of consensus on optimal RT volumes and doses
- Incomplete understanding of molecular risk factors
- Limited data on observation-only approach for completely resected supratentorial tumors
Study Objectives
- Estimate local control and pattern of failure in completely resected, classic supratentorial ependymoma after surgery alone
- Estimate rate of complete resection with second surgery after chemotherapy
- Estimate local control and pattern of failure using 3D conformal RT with 1-cm CTV margin
- Determine influence of histologic grade on time to progression after CRT
- Determine effect of focal copy number gains/losses and genomic profiling on outcome
Study Design
- Phase 3 trial of Children's Oncology Group
- Enrolled 378 patients (356 eligible) from Oct 2003 to Sept 2007
- Age 1-21 years with newly diagnosed intracranial ependymoma
- Treatment stratified by extent of resection, tumor location, and histology
- First cooperative group trial to use immediate postoperative RT in children < 3 years old
- Median follow-up: 7.89 years (range 0.09-11.0 years)
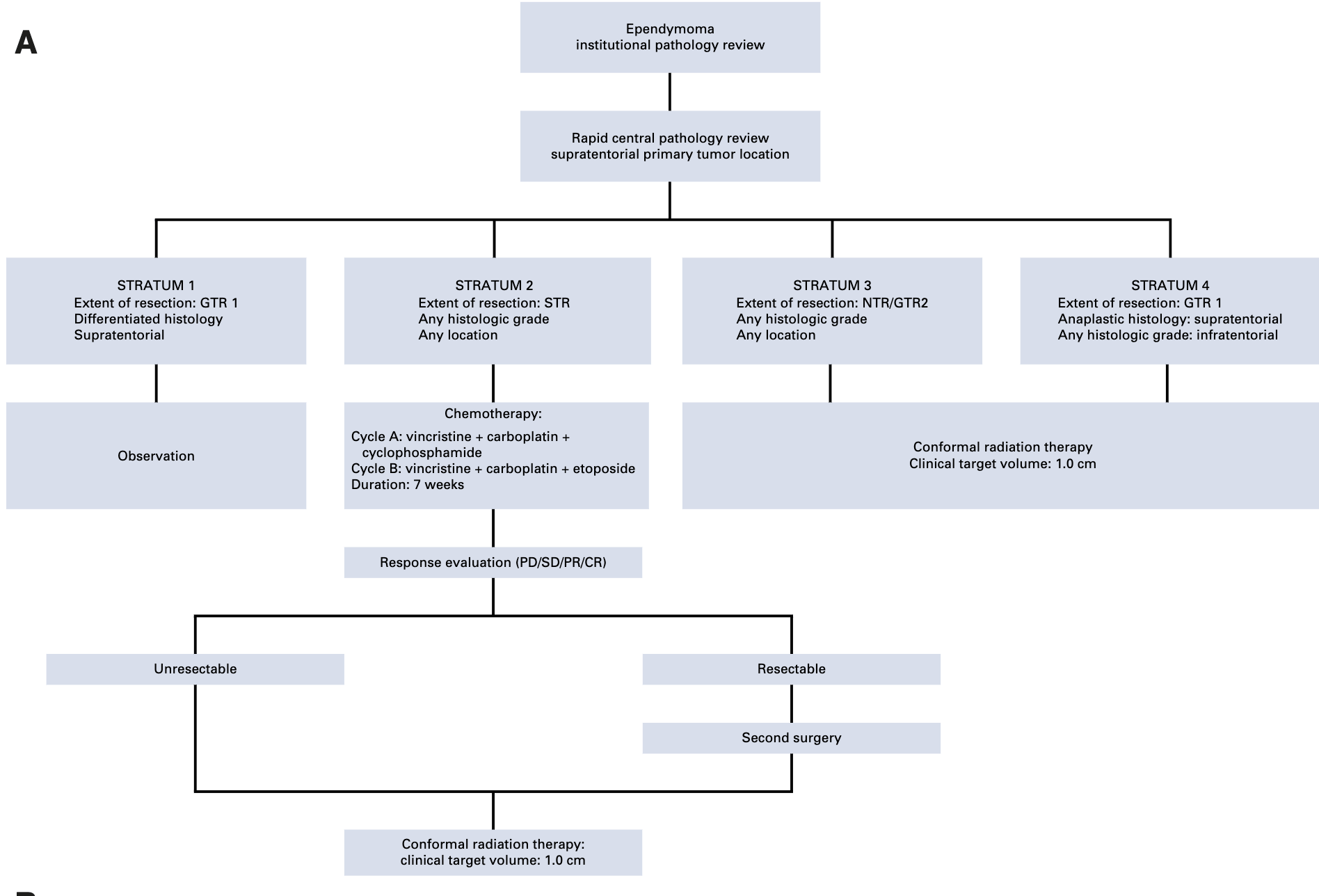
Trial Schema
GTR1: no visible residual tumor; GTR2: microscopically visible residual tumor; NTR: residual tumor ≤0.5cm; STR: residual tumor >0.5cm
Trial Schema
GTR1: no visible residual tumor; GTR2: microscopically visible residual tumor; NTR: residual tumor ≤0.5cm; STR: residual tumor >0.5cm
Trial Schema
GTR1: no visible residual tumor; GTR2: microscopically visible residual tumor; NTR: residual tumor ≤0.5cm; STR: residual tumor >0.5cm
Patient Characteristics
- Median age: 5.6 years (range 1.01-21.01)
- 28.6% were ≤ 3 years old
- 57.9% male, 42.1% female
- Classic ependymoma: 215 (60.4%)
- Anaplastic ependymoma: 141 (39.6%)
- Infratentorial: 258 (72.5%)
- Supratentorial: 96 (27.0%)
- Transtentorial: 2 (0.5%)
- Stratum 1 (observation): 11 patients
- Stratum 2 (STR → chemo): 64 patients
- Stratum 3 (NTR/GTR2 → RT): 118 patients
- Stratum 4 (GTR1 → RT): 163 patients
Treatment Methods
- CTV margin: 1.0 cm anatomically defined
- Dose: 59.4 Gy (1.8 Gy/fraction)
- Patients < 18 months with GTR: 54 Gy
- Photons or passive scattering protons allowed
- No elective nodal irradiation
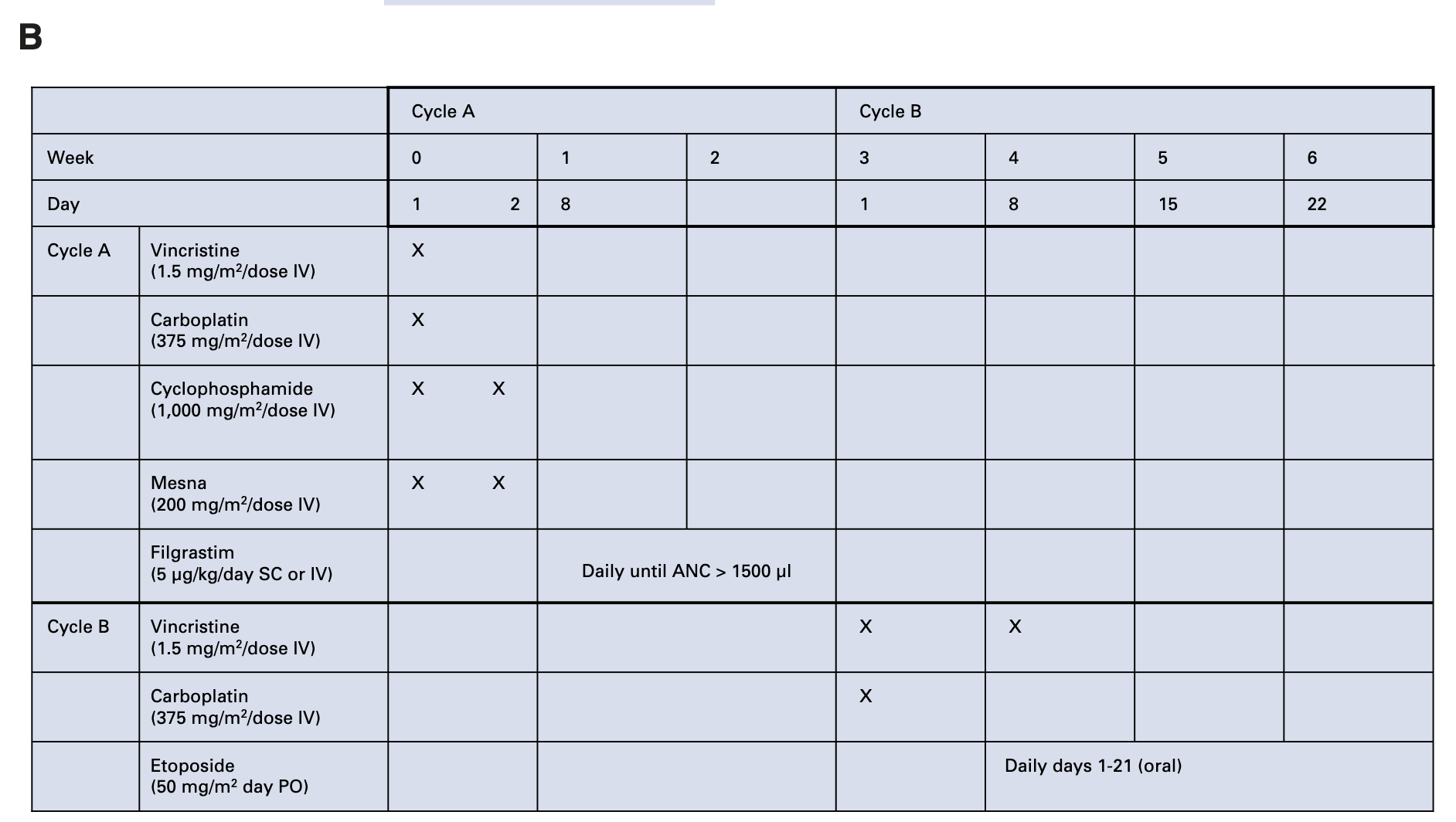
- Cycle A:
- Vincristine (1.5 mg/m²)
- Carboplatin (375 mg/m²)
- Cyclophosphamide (1,000 mg/m²)
- Cycle B:
- Vincristine (1.5 mg/m²)
- Carboplatin (375 mg/m²)
- Etoposide (50 mg/m² oral daily × 21 days)
- Total duration: 7 weeks
Merchant TE, et al. Int J Radiat Oncol Biol Phys 52:325-332, 2002
Treatment Methods
- Gross Tumor Volume (GTV):
- Tumor bed and residual tumor
- Based on pre-op & post-op imaging
- Clinical Target Volume (CTV):
- GTV + 1.0 cm anatomically confined margin
- Limited by bony calvarium, falx, tentorium
- Planning Target Volume (PTV):
- CTV + 0.3-0.5 cm geometric margin
- 3D conformal planning required (IMRT allowed)
- CT simulation ≤ 4 mm slice thickness
- MRI registration strongly encouraged
- Field shaping required (MLC allowed)
- Equipment: X-rays ≥ 4 MV or proton beams
- Minimize dose to critical structures (cochleae, hypothalamic-pituitary unit, temporal lobes)
- Protocol-defined 1.0 cm CTV margin was a key study objective
- Beam arrangements should minimize dose to auditory system, hypothalamic-pituitary unit, and supratentorial brain
Treatment Methods
| Patient Characteristics | Total Dose |
|---|---|
| Age < 18 months, GTR1/GTR2 | 54.0 Gy |
| Age ≥ 18 months, GTR1/GTR2 | 59.4 Gy |
| Any age, NTR/STR | 59.4 Gy |
- Fractionation: 1.8 Gy per day, 5 days/week
- Prescription: 100% isodose surface encompassing PTV (95% acceptable)
- Dose uniformity: ≤ 10% of PTV should receive > 110% of prescribed dose
- Spinal Cord:
- First 30 fractions: ≤ 1.85 Gy/fx (max 55.62 Gy)
- Last 3 fractions: ≤ 1.25 Gy/fx (max 7.50 Gy)
- Optic Chiasm:
- First 30 fractions: ≤ 1.85 Gy/fx (max 55.62 Gy)
- Last 3 fractions: ≤ 1.25 Gy/fx (max 7.50 Gy)
- Brainstem: No specific constraint
- Cochlea, pituitary, hypothalamus: Minimize dose without compromising target coverage
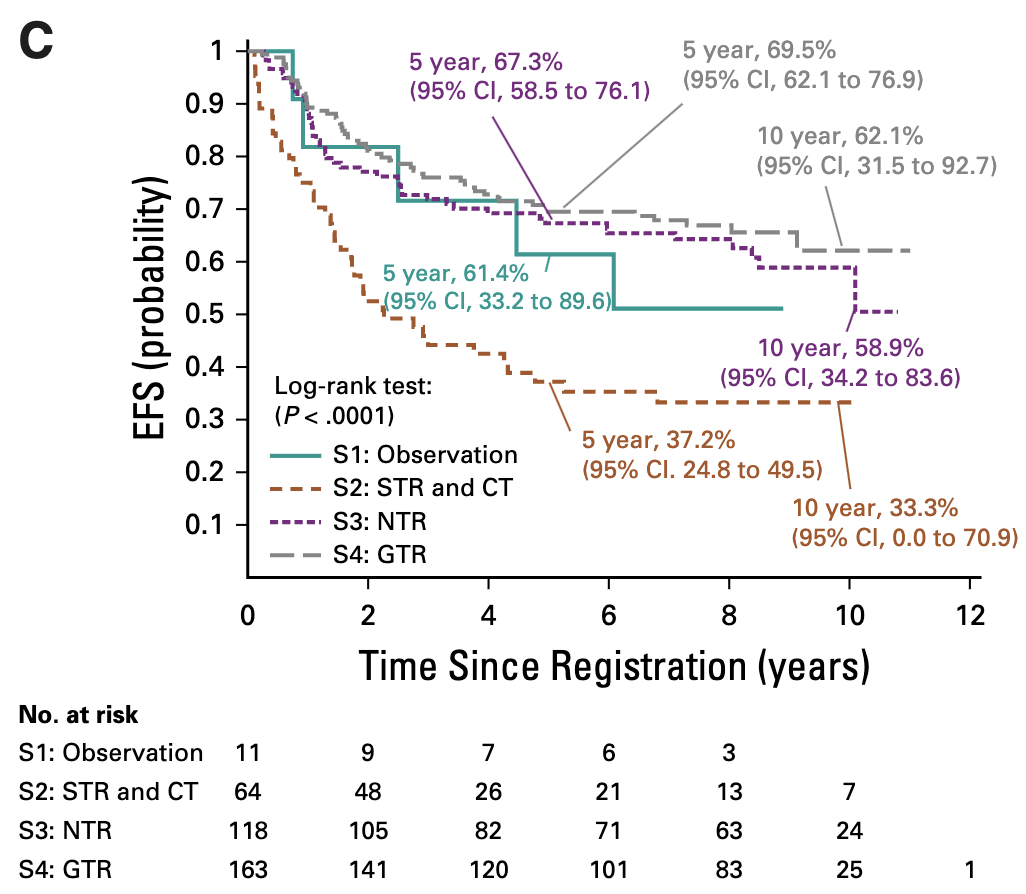
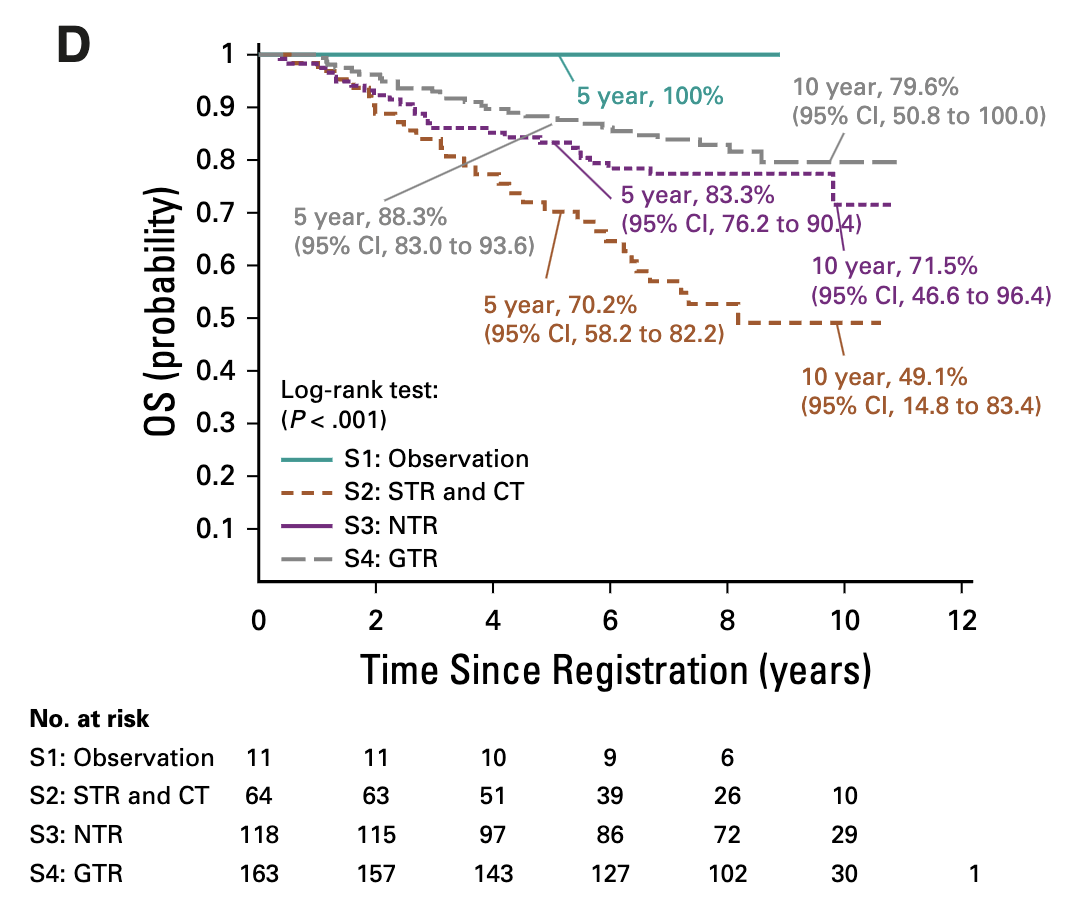
Primary Outcomes by Stratum
| Stratum | 5-year EFS (95% CI) | 5-year OS (95% CI) | Local Control | Local Failure | Local + Distant | Distant Failure |
|---|---|---|---|---|---|---|
| S1: GTR1, classic, supratentorial (observation) | 61.4% (33.2-89.6%) | 100% | 54.55% | 36.36% | 9.09% | 0% |
| S2: STR (chemo → surgery → RT) | 37.2% (24.8-49.5%) | 70.2% (58.2-82.2%) | 47.46% | 45.76% | 6.78% | 8.47% |
| S3: NTR/GTR2 (immediate RT) | 67.3% (58.5-76.1%) | 83.3% (76.2-90.4%) | 74.58% | 22.88% | 2.54% | 11.02% |
| S4: GTR1 anaplastic or infratentorial (immediate RT) | 69.5% (62.1-76.9%) | 88.3% (83.0-93.6%) | 79.14% | 18.40% | 2.45% | 7.98% |
| Combined strata 3+4 (all immediate RT) | 68.5% (62.8-74.2%) | 86.2% (81.9-90.6%) | 77.23% | 20.29% | 2.49% | 9.25% |
Primary Outcomes by Stratum
| Stratum | 5-year EFS (95% CI) | 5-year OS (95% CI) | Local Control | Local Failure | Local + Distant | Distant Failure |
|---|---|---|---|---|---|---|
| S1: GTR1, classic, supratentorial (observation) | 61.4% (33.2-89.6%) | 100% | 54.55% | 36.36% | 9.09% | 0% |
| S2: STR (chemo → surgery → RT) | 37.2% (24.8-49.5%) | 70.2% (58.2-82.2%) | 47.46% | 45.76% | 6.78% | 8.47% |
| S3: NTR/GTR2 (immediate RT) | 67.3% (58.5-76.1%) | 83.3% (76.2-90.4%) | 74.58% | 22.88% | 2.54% | 11.02% |
| S4: GTR1 anaplastic or infratentorial (immediate RT) | 69.5% (62.1-76.9%) | 88.3% (83.0-93.6%) | 79.14% | 18.40% | 2.45% | 7.98% |
| Combined strata 3+4 (all immediate RT) | 68.5% (62.8-74.2%) | 86.2% (81.9-90.6%) | 77.23% | 20.29% | 2.49% | 9.25% |
Outcomes by Tumor Grade
- Classic ependymoma:
- 5-year EFS 74.6% (67.5-81.7%)
- 5-year OS 89.4% (84.3-94.5%)
- Anaplastic ependymoma:
- 5-year EFS 60.7% (51.5-69.9%)
- 5-year OS 82.0% (74.7-89.3%)
- Significant difference (p=.0044)
- Children < 3 years had similar outcomes to older children with immediate RT
- Histology is a significant prognostic factor, independent of age and tumor location
- Immediate RT was well-tolerated across age groups
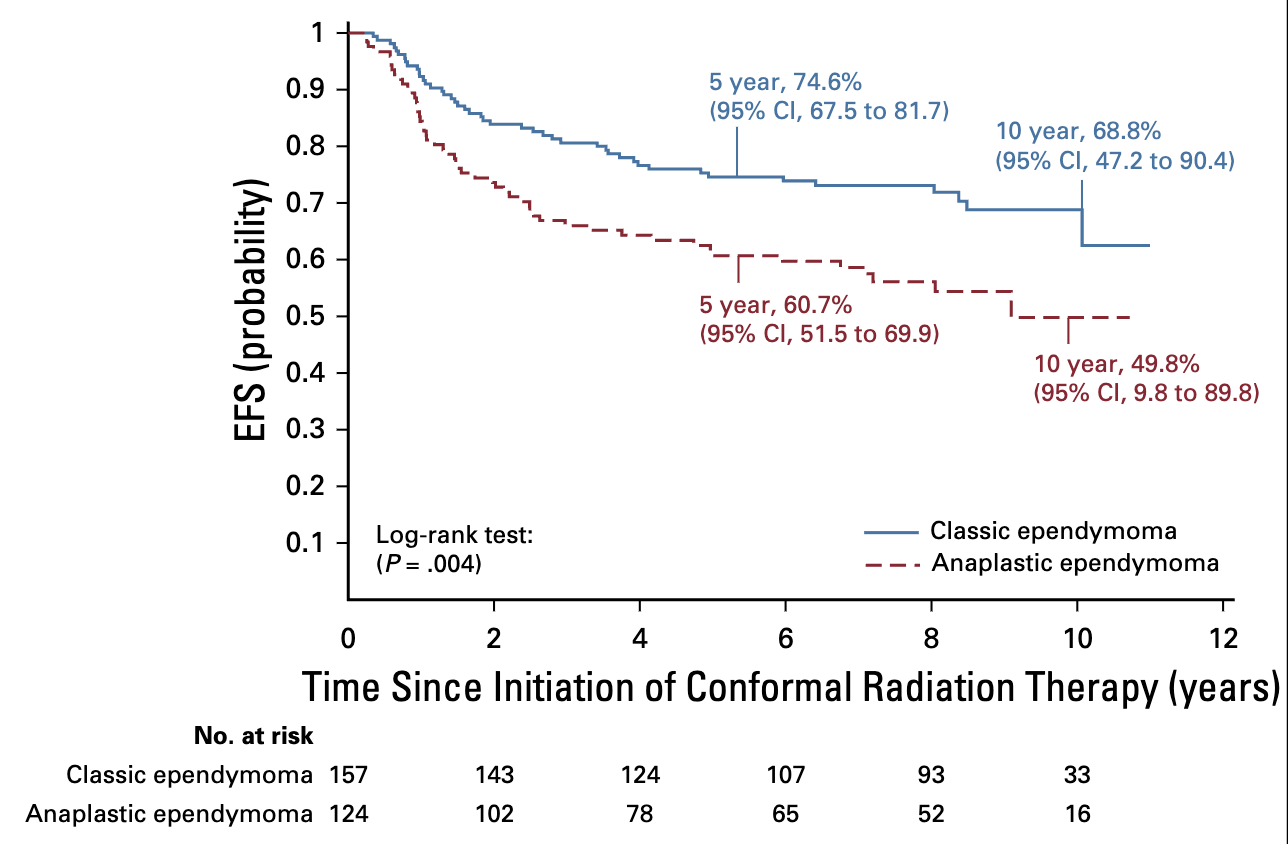
Outcomes by Age
- Age < 3 years:
- 5-year EFS 62.9% (51.9-73.9%)
- 5-year OS 87.4% (79.6-95.2%)
- Age ≥ 3 years:
- 5-year EFS 70.5% (63.8-77.2%)
- 5-year OS 85.8% (80.7-90.9%)
- Not significantly different (p=.2295)
- Children < 3 years had similar outcomes to older children with immediate RT
- Immediate RT was well-tolerated across age groups
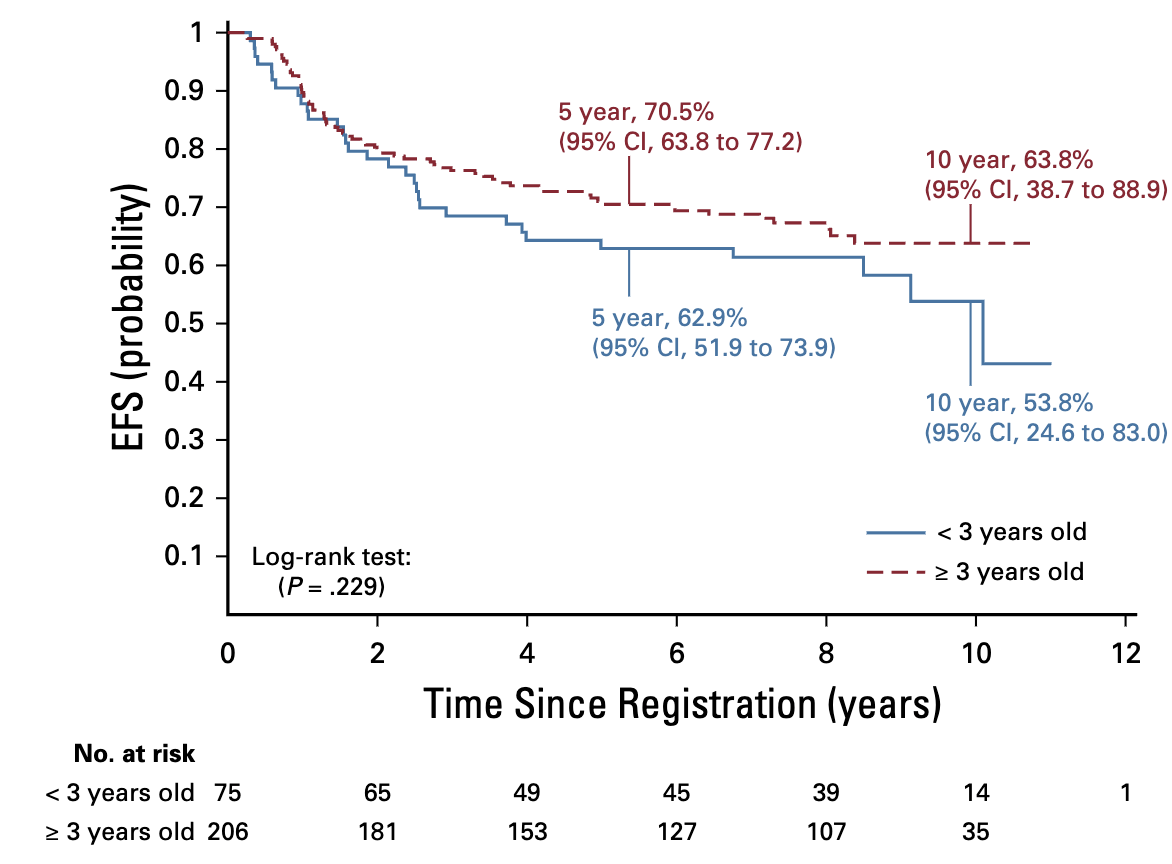
Molecular Markers and Outcomes
- Present in 30/39 (77%) of supratentorial tumors
- No significant difference in EFS, OS, or pattern of failure by RELA status
- Patients with RELA fusion responded well to RT
- No significant differences in outcome by PFA/PFB alone
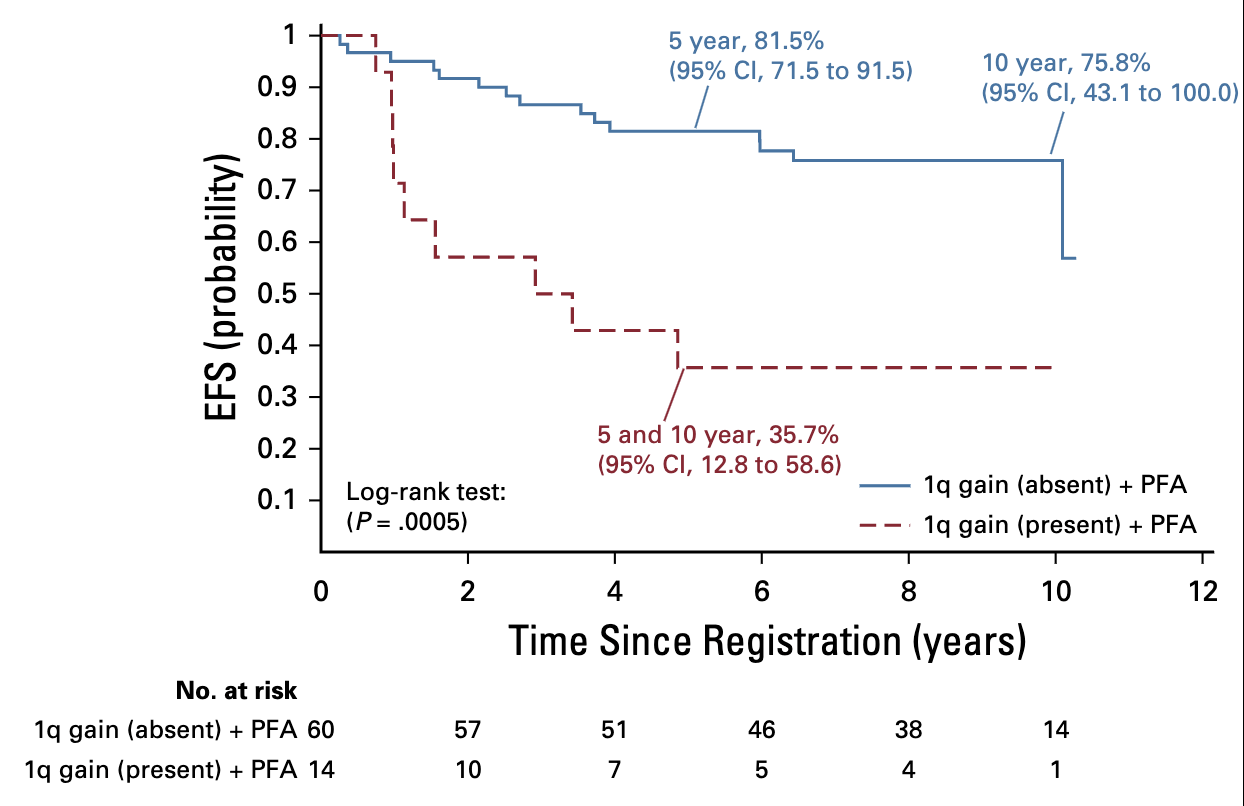
- Combined effect of PFA + 1q gain was significant:
- PFA without 1q gain: 5-year EFS 81.5%
- PFA with 1q gain: 5-year EFS 35.7%
- Significant difference (p=.0005)
- Present in 24/121 (20%) of infratentorial tumors
- For patients with immediate RT (strata 3+4):
- Without 1q gain: 5-year EFS 82.8%
- With 1q gain: 5-year EFS 47.4%
- Significant difference (p=.0013)
- Significant association with both local and distant failure patterns
- Local failure:
- With 1q gain: 31.6%
- Without 1q gain: 12.3%
- (p=.054)
- Distant failure:
- With 1q gain: 21.1%
- Without 1q gain: 6.1%
- (p=.040)
PFA: posterior fossa group A; PFB: posterior fossa group B
Molecular Markers and Outcomes
- Present in 24/121 (20%) of infratentorial tumors
- For patients with immediate RT (strata 3+4):
- Without 1q gain: 5-year EFS 82.8%
- With 1q gain: 5-year EFS 47.4%
- Significant difference (p=.0013)
- Significant association with both local and distant failure patterns
- Local failure:
- With 1q gain: 31.6%
- Without 1q gain: 12.3%
- (p=.054)
- Distant failure:
- With 1q gain: 21.1%
- Without 1q gain: 6.1%
- (p=.040)
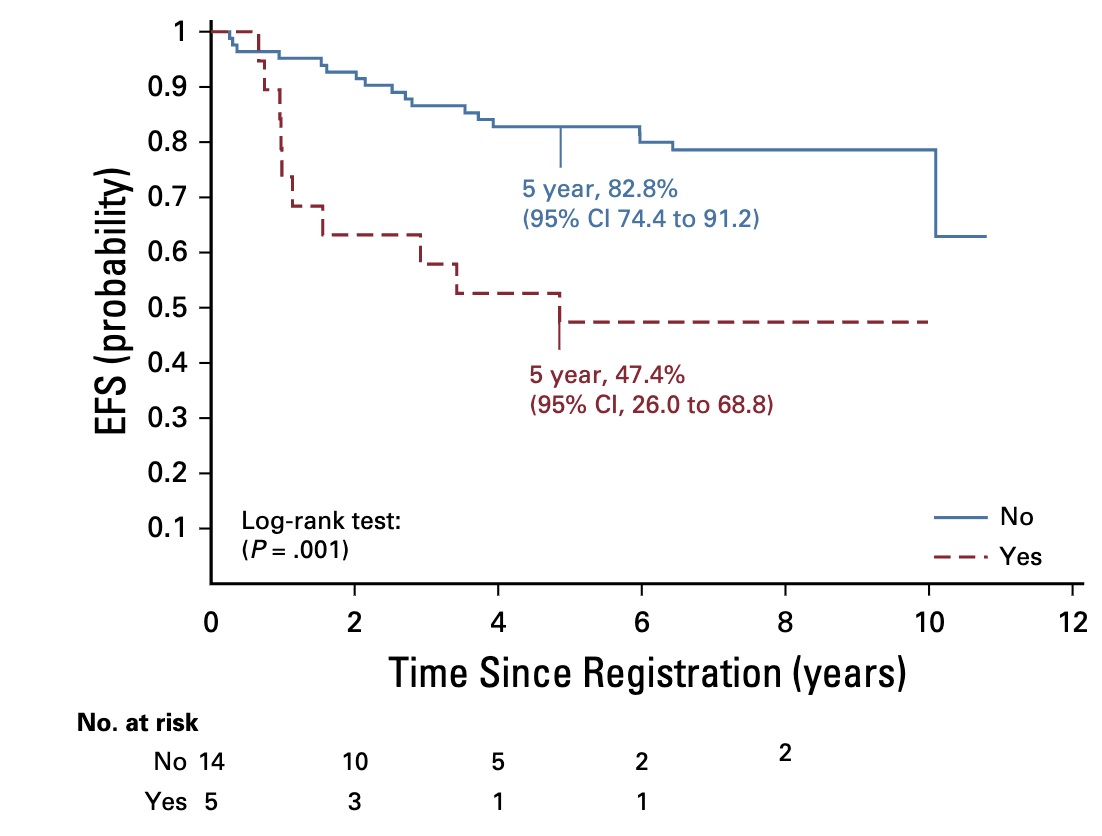
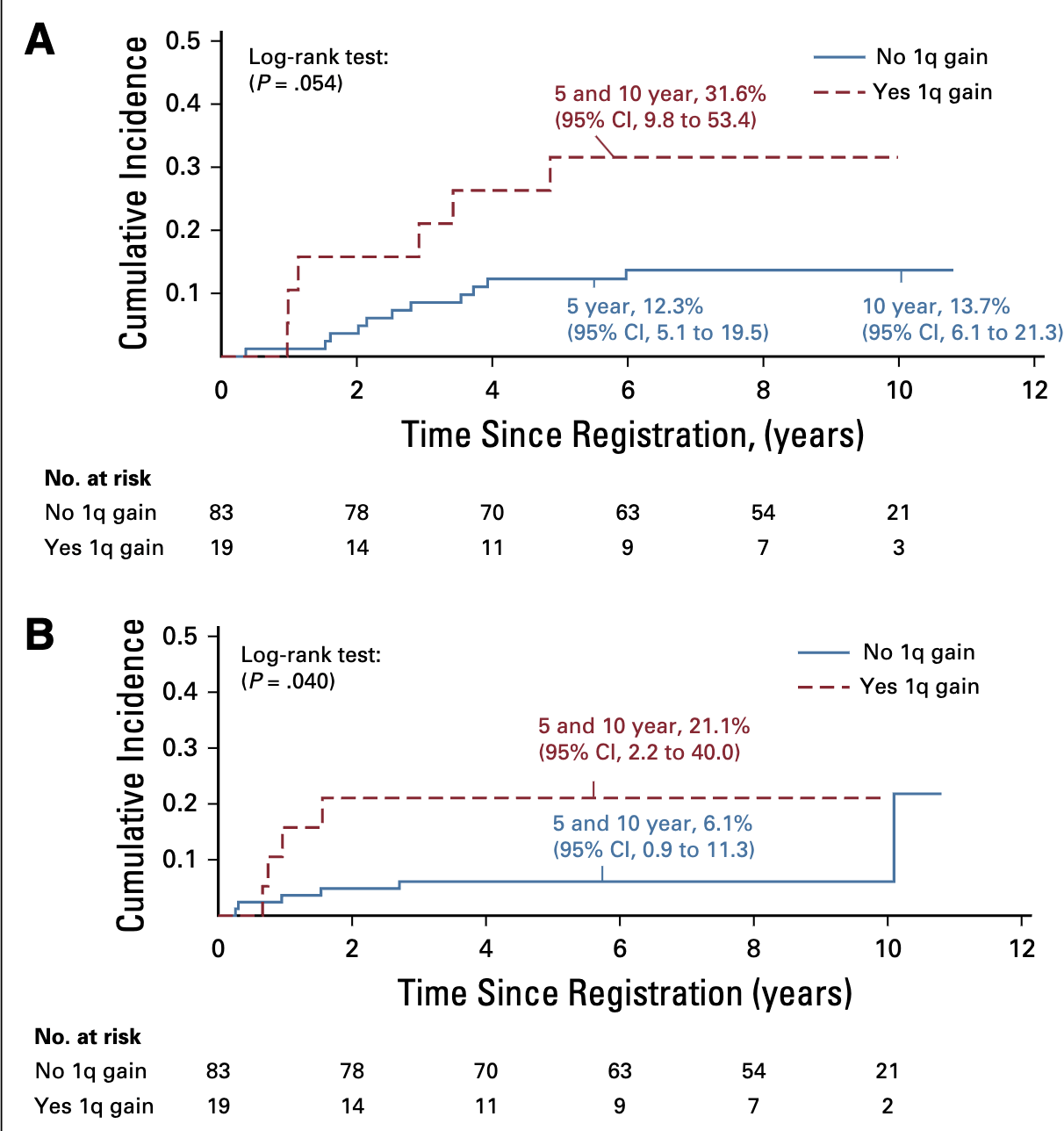
Molecular Markers and Outcomes
- Present in 30/39 (77%) of supratentorial tumors
- No significant difference in EFS, OS, or pattern of failure by RELA status
- Patients with RELA fusion responded well to RT
- No significant differences in outcome by PFA/PFB alone
- Combined effect of PFA + 1q gain was significant:
- PFA without 1q gain: 5-year EFS 81.5%
- PFA with 1q gain: 5-year EFS 35.7%
- Significant difference (p=.0005)
Event-free survival (EFS) for patients with infratentorial tumors and posterior fossagroup A (PFA) classification who were treated with immediate postoperative radiationtherapy (strata 3 and 4) according to 1q gain status.;
PFA: posterior fossa group A; PFB: posterior fossa group B
Secondary Outcomes
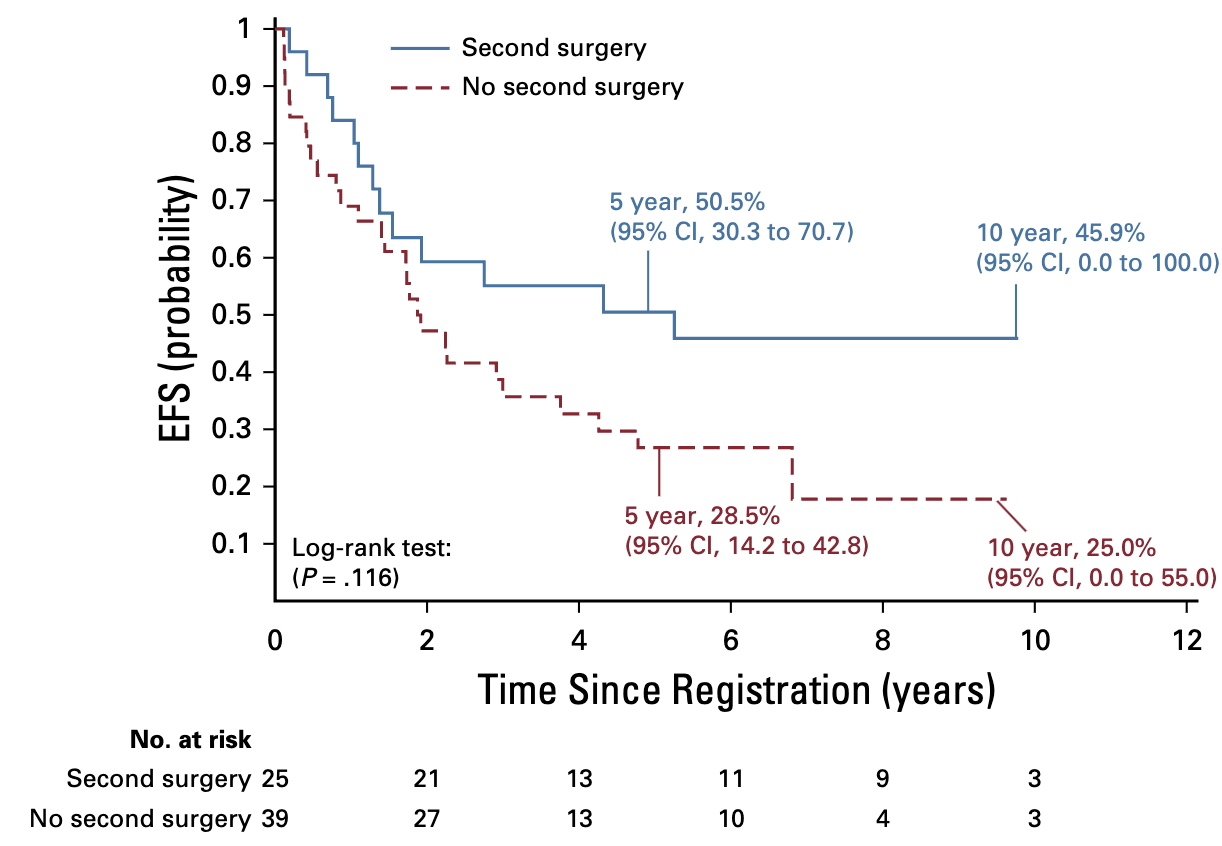
- 39% of patients underwent second surgery after chemo
- 56% achieved GTR at second surgery
- 5-year EFS with second surgery: 50.5%
- 5-year EFS without second surgery: 28.5%
- Trend toward benefit but not statistically significant (p=.1163)
- Patients with 1q gain:
- 2.5x higher risk of local failure
- 3.5x higher risk of distant failure
- 1q gain could identify patients who need intensified therapy
- 7 patients developed second malignancies
- 10-year cumulative incidence: 3.43% (0.4-6.5%)
- Distribution across strata:
- Stratum 2: n=2
- Stratum 3: n=1
- Stratum 4: n=4
- 11 eligible patients with classic supratentorial ependymoma after GTR1
- 5-year EFS: 61.4% (33.2-89.6%)
- 5-year OS: 100%
- Local failure occurred in 4 patients (36.36%)
- All failures salvageable with additional therapy
Secondary Outcomes
- 39% of patients underwent second surgery after chemo
- 56% achieved GTR at second surgery
- 5-year EFS with second surgery: 50.5%
- 5-year EFS without second surgery: 28.5%
- Trend toward benefit but not statistically significant (p=.1163)
Conclusions
- Immediate RT is effective across age groups: Children < 3 years treated with immediate postoperative CRT had similar outcomes to older children
- Maximal safe resection remains crucial: Extent of resection impacts outcome (68.5% 5-year EFS for NTR/GTR vs. 37.2% for STR)
- Tumor histology matters: Classic ependymoma had significantly better outcomes than anaplastic ependymoma
- 1q gain is a powerful prognostic marker: Specifically for infratentorial tumors, predicting both local and distant failure
- Observation may be reasonable for select patients: Completely resected classic supratentorial ependymoma had 100% OS with observation
Strengths and Limitations
- Large, multi-institutional prospective study
- Long follow-up period (median 7.89 years)
- First cooperative group trial to use immediate RT in children < 3 years
- Systematic evaluation of defined target volumes with 1-cm CTV margin
- Comprehensive molecular analysis
- Stratification by extent of resection, location, and histology
- Small sample size for some strata (especially stratum 1)
- Digital treatment planning data not uniformly collected
- Limited evaluation of late effects (neurocognitive, endocrine, etc.)
- Limited data on quality of life outcomes
- Single CTV margin size (1 cm) tested
Connection to ACNS0831
- ACNS0831 continues observation-only approach for completely resected classic supratentorial ependymoma
- Maintains immediate post-operative radiation in young children (building on ACNS0121 success)
- Key addition: Randomization to maintenance chemotherapy vs. observation after RT
- Main question: Will maintenance chemotherapy after RT improve outcomes?
- Continues comprehensive molecular analysis to further validate 1q and other biomarkers
Discussion Points
- Paradigm shift for young children: Does ACNS0121 definitively change our approach to RT in children < 3 years with ependymoma?
- Observation strategy: Is observation-only appropriate for completely resected classic supratentorial ependymoma given the 36% local failure rate?
- Molecular stratification: How should molecular markers (1q gain, PFA/PFB, RELA) influence our treatment decisions?
- Field reduction: Is a 1-cm CTV margin adequate for all patients? ACNS 0831 then shrank fields to 0.5 cm
- ACNS0831 design: Will maintenance chemotherapy after RT provide additional benefit? For which molecular subtypes?
References
- Merchant TE, Bendel AE, Sabin ND, et al: Conformal radiation therapy for pediatric ependymoma, chemotherapy for incompletely resected ependymoma, and observation for completely resected, supratentorial ependymoma. J Clin Oncol 37:974-983, 2019
- Merchant TE, Li C, Xiong X, et al: Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol 10:258-266, 2009
- Pajtler KW, Witt H, Sill M, et al: Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 27:728-743, 2015
- Ramaswamy V, Hielscher T, Mack SC, et al: Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: a retrospective multicohort analysis. J Clin Oncol 34:2468-2477, 2016
- Godfraind C, Kaczmarska JM, Kocak M, et al: Distinct disease-risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol 124:247-257, 2012
- Duffner PK, Horowitz ME, Krischer JP, et al: The treatment of malignant brain tumors in infants and very young children: an update of the Pediatric Oncology Group experience. Neuro-oncol 1:152-161, 1999
- Strother DR, Lafay-Cousin L, Boyett JM, et al: Benefit from prolonged dose-intensive chemotherapy for infants with malignant brain tumors is restricted to patients with ependymoma: a report of the Pediatric Oncology Group randomized controlled trial 9233/34. Neuro-oncol 16:457-465, 2014
Conformal Radiation Therapy for Pediatric Ependymoma: The ACNS0121 Trial
By RadMedSkiier
Conformal Radiation Therapy for Pediatric Ependymoma: The ACNS0121 Trial
Journal club presentation on the Children's Oncology Group ACNS0121 phase 3 trial for pediatric ependymoma
- 55



