STOCHASTIC THERMODYNAMICS OF COMPLEX SYSTEMS
Jan Korbel
CSH Friday Seminar "Analysis of complex systems" - 5.3.2021
Slides available at: https://slides.com/jankorbel
Outline
-
Review of equilibrium thermodynamics
-
Main results of stochastic thermodynamics
-
Thermodynamics of structure-forming systems
-
ST of non-linear systems
Introduction
Microscopic systems
Classical mechanics (QM,...)
Mesoscopic systems
Stochastic thermodynamics
Macroscopic systems
Thermodynamics
Statistical mechanics
Trajectory TD
Ensemble TD
Stochastic Thermodynamics is a thermodynamic theory
for mesoscopic, non-equilibrium physical systems
interacting with equilibrium thermal (and/or chemical)
reservoirs
History
Equilibrium thermodynamics (19 th century)
- Maxwell, Boltzman, Planck, Claussius, Gibbs...
- Macroscopic systems (\(N \rightarrow \infty\)) in equilibrium (no time dependence of measurable quantities - thermoSTATICS)
- General structure of thermodynamics
- Laws of thermodynamics (general)
- Response coefficients (system-specific)
- Applications: engines, refridgerators, air-condition,...


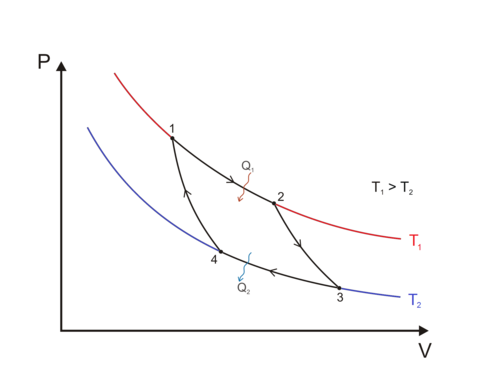
efficiency \(\leq 1-\frac{T_2}{T_1}\)
Heat engine: Carnot cycle
History
Laws of thermodynamics
Zeroth law:
Temperature can be measured. $$T_A = T_B \quad \mathrm{if} \quad A \ \mathrm{and} \ B \ \mathrm{are} \ \mathrm{in} \ \mathrm{equilibrium}.$$
First law (Claussius 1850, Helmholtz 1847):
Energy is conserved.
$${\color{aqua} d}U = {\color{orange} \delta} Q - {\color{orange} \delta} W$$ Second law (Carnot 1824, Claussius 1854, Kelvin):
Heat cannot be fully transformed into work. $${ \color{aqua} d} S \geq \frac{{\color{orange} \delta} Q}{T}$$ Third law: We cannot bring the system into the absolute zero
temperature in a finite number of steps. $$ \lim_{T \rightarrow 0} S(T) = 0$$
History
Local equilibrium thermodynamics (1st half of 20th cent.)
- Onsager, Rayleigh...
- Systems close to equilibrium - linear response theory
- Local equilibrium: subsystems a,b,c are each in equilibrium
Total entropy \(S \approx S^a + S^b + S^c + \dots\)
Entropy production \(\sigma^a = \frac{d S^a}{d t} = \sum_i Y_i^a J_i^a \)
\(Y_i^a\) - thermodynamic forces; \(J_i^a\) - thermodynamic currents
4th Law of thermodynamics (Onsager 1931): \( \sigma = \sum_{ij} L_{ij} \Gamma_i \Gamma_j\)
\(\Gamma_i = Y_i^a - Y_i^b \) - afinity, \(L_{ij}\) - symmetric
History and now
Stochastic thermodynamics (90s of 20th century - present)
- Evans, Jarzynski, Crooks, Seifert, van den Broek,....
- Mesoscopic systems far from equilibrium
- Combines stochastic calculus and non-equilibrium thermodynamics
- Main results: Trajectory thermodynamics, Fluctuation theorems, Thermodynamic uncertainty relations, Speed limit theorems,...
- Applications: colloidal particles and soft matter, biochemistry, molecular motors
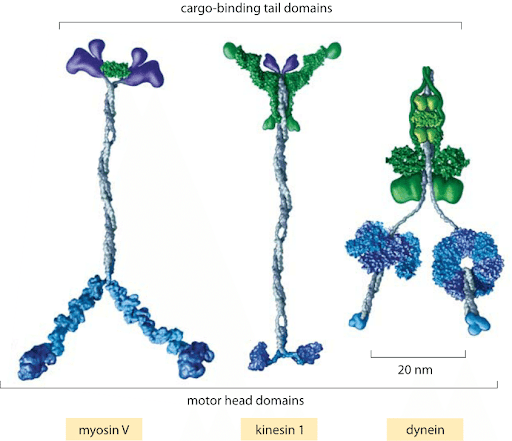
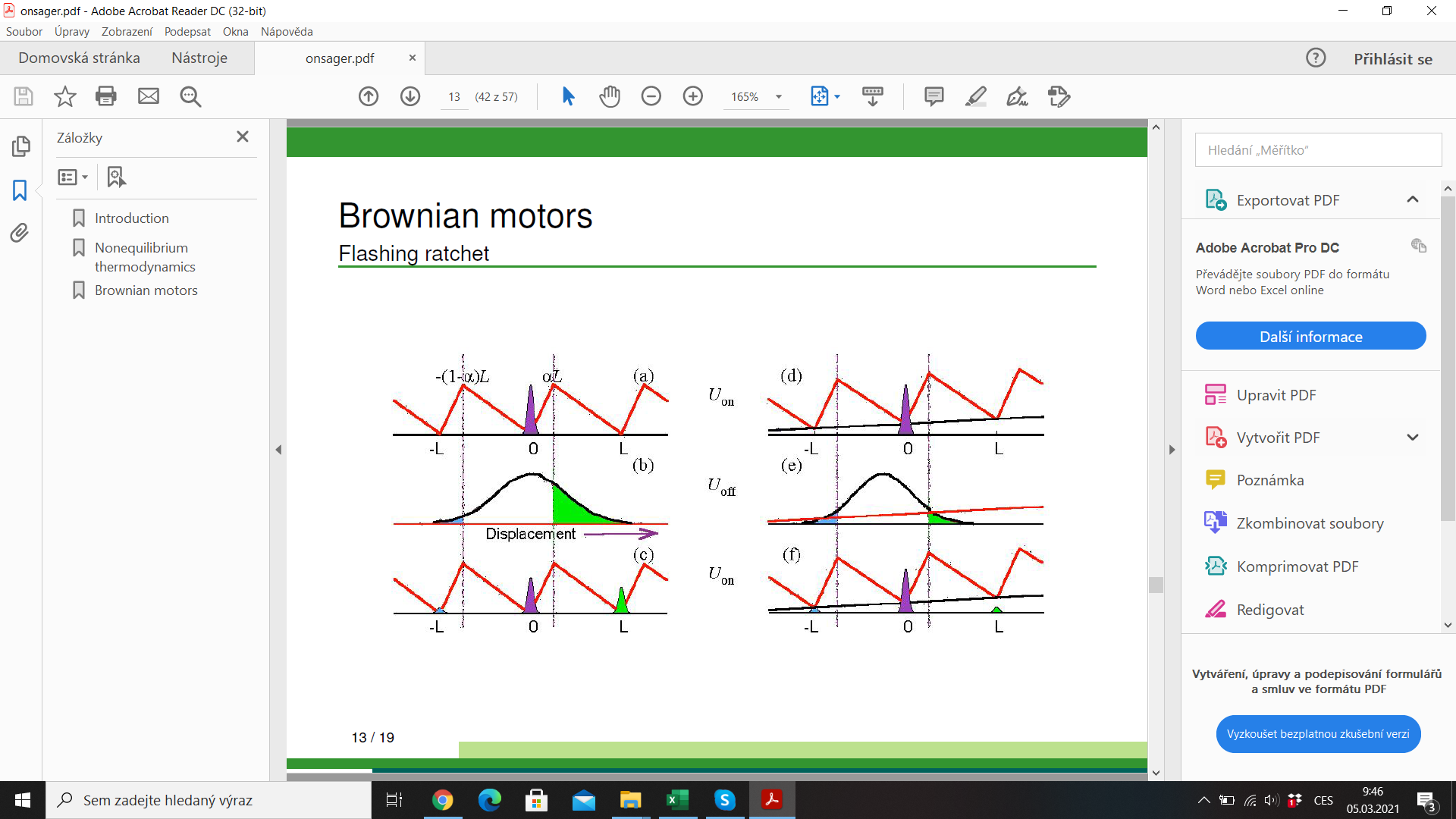
Molecular motor: kinesine walking on microtobulus
efficiency \( \leq 1\)
Stochastic thermodynamics
1.) Consider linear Markov (= memoryless) with distribution \(p_i(t)\).
Its evolution is described by master equation
$$ \dot{p}_i(t) = \sum_{j} [w_{ij} p_{j}(t) - w_{ji} p_i(t) ]$$
\(w_{ij}\) is transition rate.
2.) Entropy of the system - Shannon entropy \(S(P) = - \sum_i p_i \log p_i\). Equilibrium distribution is obtained by maximization of \(S(P)\) under the constraint of average energy \( U(P) = \sum_i p_i \epsilon_i \)
$$ p_i^{eq} = \frac{1}{Z} \exp(- \beta \epsilon_i) \quad \mathrm{where} \ \beta=\frac{1}{k_B T}, Z = \sum_j \exp(-\beta \epsilon_j)$$
Stochastic thermodynamics
3.) Detailed balance - stationary state (\(\dot{p}_i = 0\) ) coincides with the equilibrium state (\(p_i^{eq}\)). We obtain
$$\frac{w_{ij}}{w_{ji}} = \frac{p_i^{eq}}{p_j^{eq}} = e^{\beta(\epsilon_j - \epsilon_i)}$$
4.) Second law of thermodynamics:
$$\dot{S} = - \sum_i \dot{p}_i \log p_i = \frac{1}{2} \sum_{ij} (w_{ij} p_j - w_{ji} p_i) \log \frac{p_j}{p_i}$$
$$ =\underbrace{\frac{1}{2} \sum_{ij} (w_{ij} p_j - w_{ji} p_i) \log \frac{w_{ij} p_j}{w_{ji} p_i}}_{\dot{S}_i} + \underbrace{\frac{1}{2} \sum_{ij} (w_{ij} p_j - w_{ji} p_i) \log \frac{w_{ji}}{w_{ij}}}_{\dot{S}_e}$$
\( \dot{S}_i \geq 0 \) - entropy production rate (2nd law of TD)
\(\dot{S}_e = \beta \dot{Q}\) entropy flow rate
Stochastic thermodynamics
5.) Trajectory thermodynamics - consider stochastic trajectory
\(x(t)= (x_0,t_0;x_1,t_1;\dots)\). Energy \(E_x = E_x(\lambda(t))\), \(\lambda(t)\) - control protocol
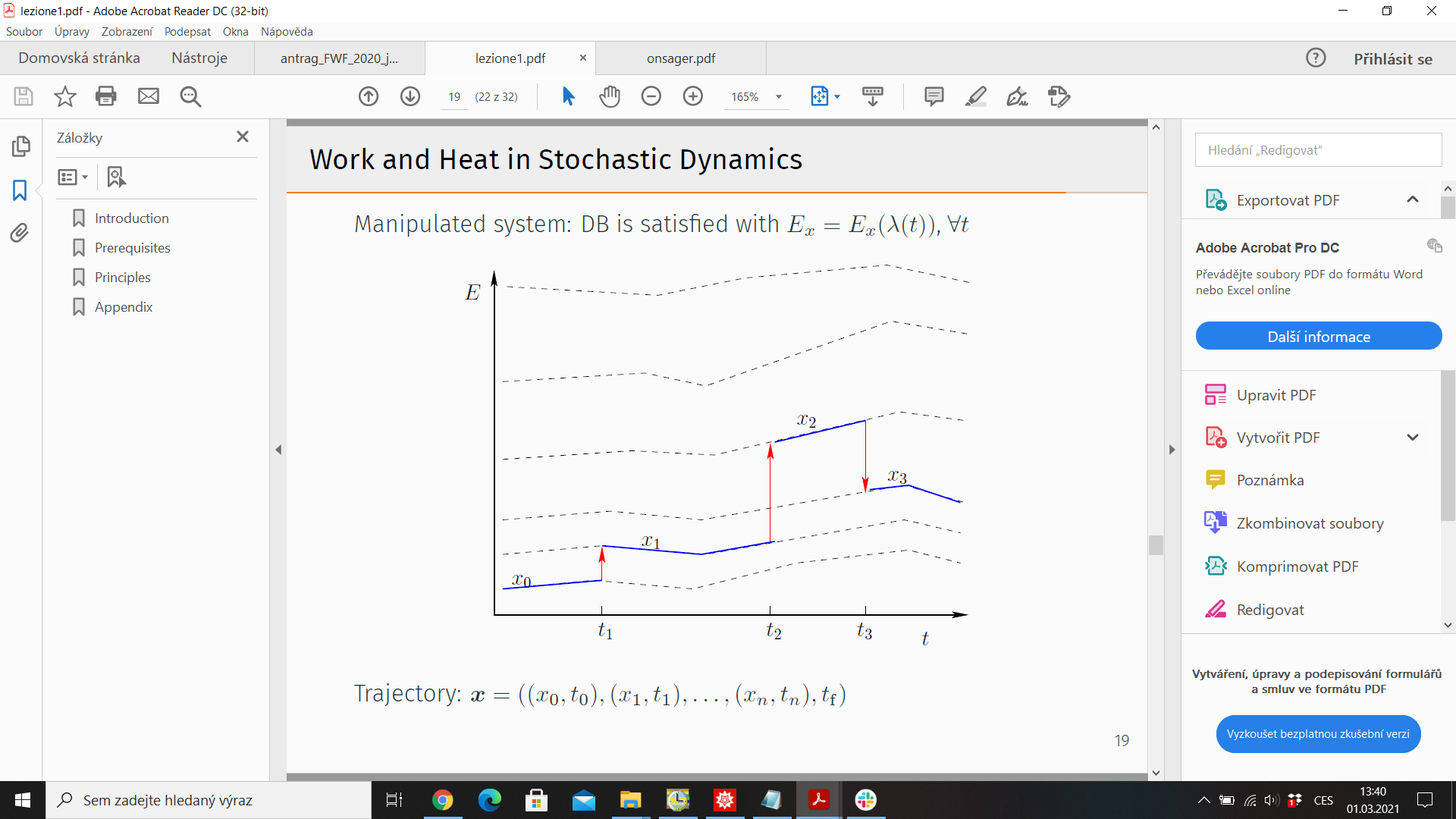
Probability of observing \( x(t)\): \(\mathcal{P}(x(t)\))
Time reversal \(\tilde{x}(t) = x(T-t)\)
Reversed protocol \(\tilde{\lambda}(t) = \lambda(T-t)\)
Probability of observing reversed trajectory under reversed protocol \(\tilde{\mathcal{P}}(\tilde{x}(t))\)
Stochastic thermodynamics
6.) Fluctuation theorems
Trajectory entropy: \(s(t) = - \log p_x(t)\)
Trajectory 2nd law \(\Delta s = \Delta s_i + \Delta s_e\)
Relation to the trajectory probabilities
$$\log \frac{\mathcal{P}(x(t))}{\tilde{\mathcal{P}}(\tilde{x}(t))} = \Delta s_i$$
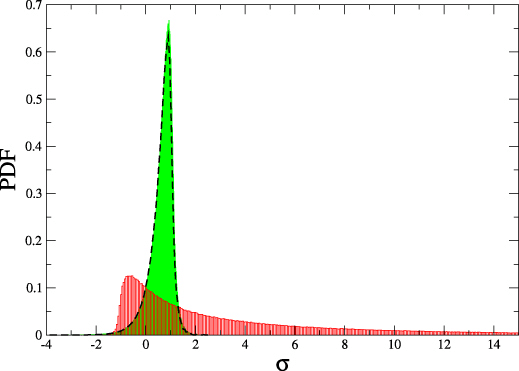
Detailed fluctuation theorem $$\frac{P(\Delta s_i)}{\tilde{P}(-\Delta s_i)} = e^{\Delta s_i}$$
Integrated fluctuation theorem $$ \langle e^{- \Delta s_i} \rangle = 1 \quad \Rightarrow \langle \Delta s_i \rangle = \Delta S_i \geq 0$$
Complex systems
- Complex systems are composed of many interacting subsystems
- Standard stochastic thermodynamics works with weakly-interacting subsystems that can be treated separately
- Shannon entropy and/or linear Markov dynamics do not have to be adequate description
We will discuss two examples of complex systems
-
Structure-forming systems
-
Non-linear systems
Thermodynamics of structure-forming systems
(with S. Lindner, R. Hanel, S. Thurner - Nat. Comm. 12 (2021) 1127)
- Many systems form structures: molecules of atoms, clusters of colloidal particles, (bio)polymers or micelles
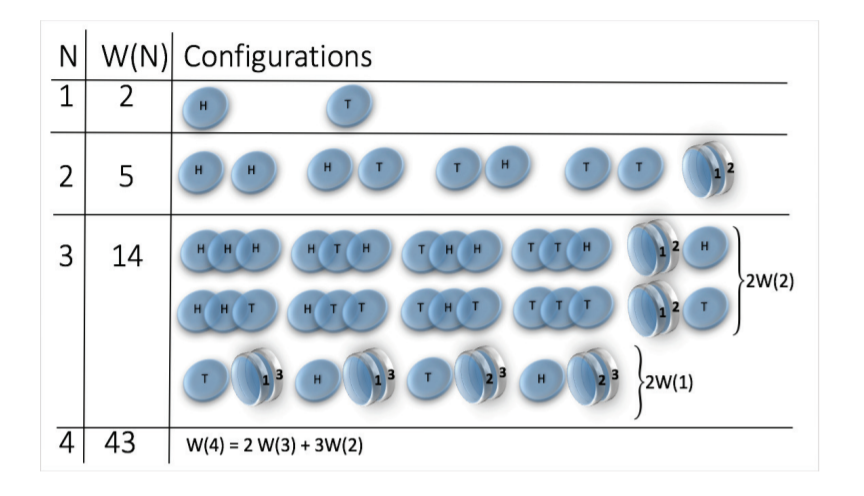
- Toy model: magnetic coin model
\(W(N) \sim e^{N \ln N}\)
"Simple systems" \(W(N) \sim a^N\)
Multiplicity of structure-forming systems
Boltzmann entropy formula: \(S = k_B \log W\)
\(W\) - is multiplicity, i.e., number of microstates corresponding to a mesostate
Microstate: state of each particle
if more particles are bound to a molecule, then state each molecule
Mesostate: how many particles and/or molecules are in given state
Example: magnetic coin model: 3 coins, magnetic
microstates mesostate multiplicity

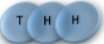
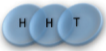
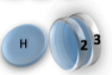
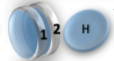

2 head, 1 tail
1 head, 1 sticked
3
3
Multiplicity
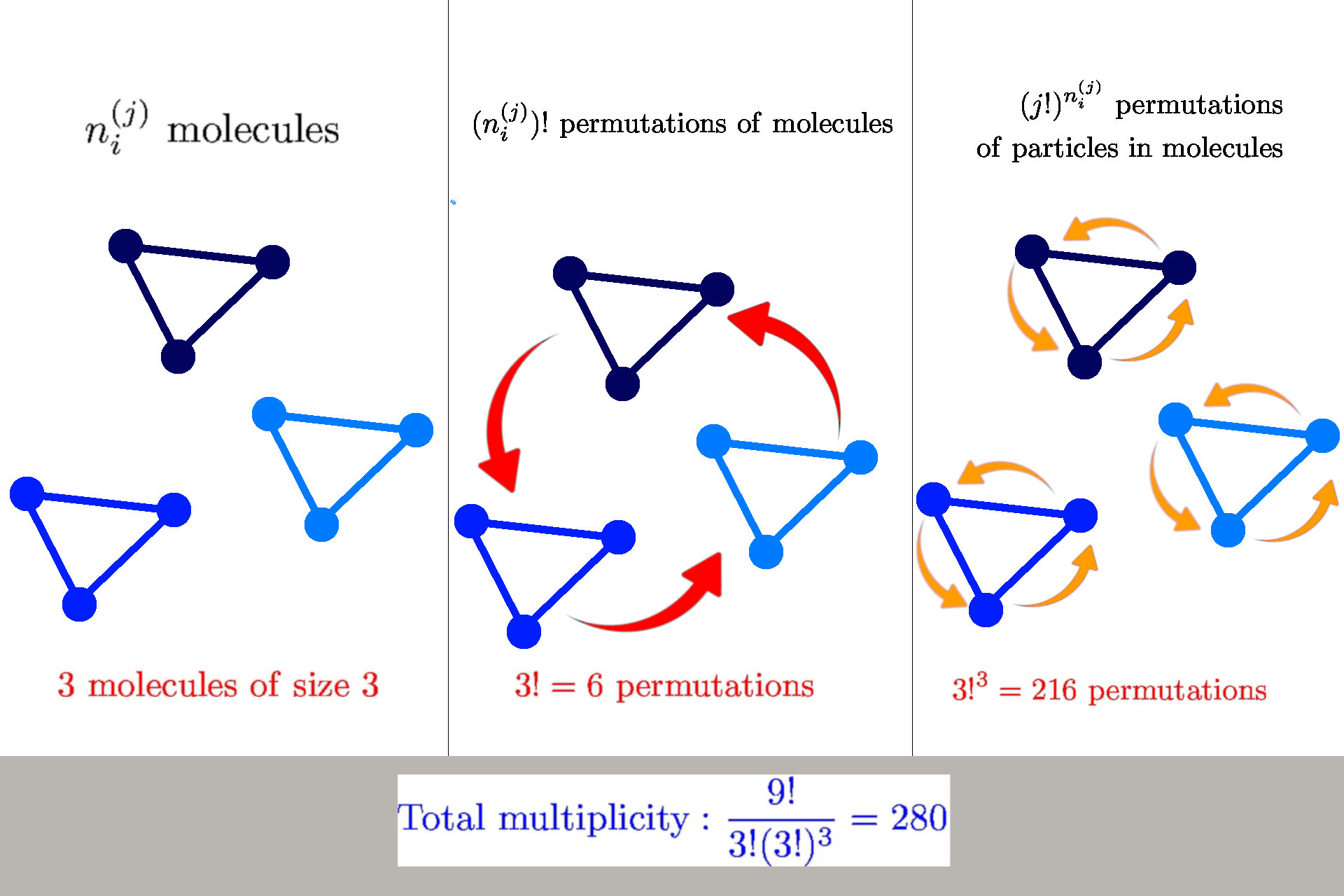
General formula: \(W = \frac{n!}{\prod_{ij} n_i^{(j)}! (j!)^{n_i^{(j)}}}\)

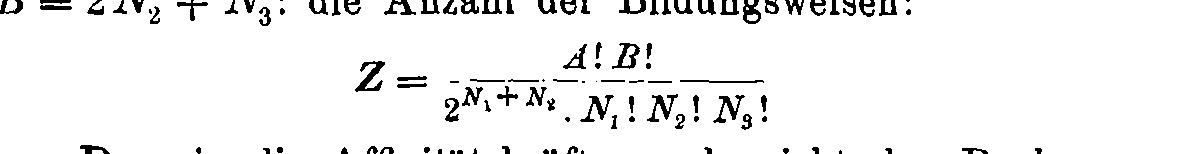
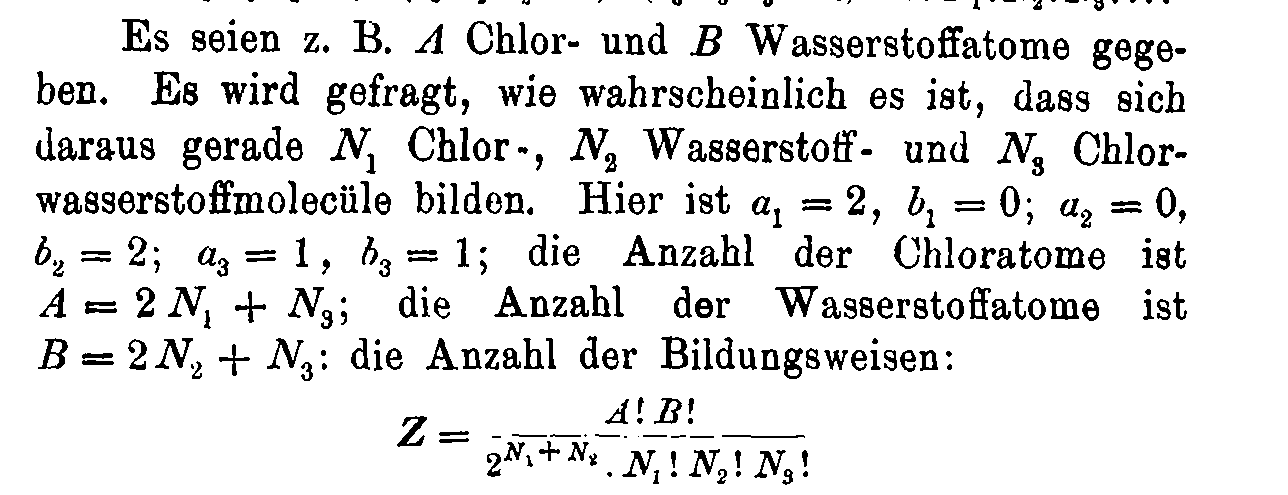
Boltzmann's 1884 paper
Entropy of structure-forming systems
$$ S = \log W \approx n \log n - \sum_{ij} \left(n_i^{(j)} \log n_i^{(j)} - n_i^{(j)} + {\color{aqua} n_i^{(j)} \log j!}\right)$$
Introduce "probabilities" \(\wp_i^{(j)} = n_i^{(j)}/n\)
$$\mathcal{S} = S/n = - \sum_{ij} \wp_i^{(j)} (\log \wp_i^{(j)} {\color{aqua}- 1}) {\color{aqua}- \sum_{ij} \wp_i^{(j)}\log \frac{j!}{n^{j-1}}}$$
Finite interaction range: concentration \(c = n/b\)
$$\mathcal{S} = S/n = - \sum_{ij} \wp_i^{(j)} (\log \wp_i^{(j)} {\color{aqua}- 1}) {\color{aqua}- \sum_{ij} \wp_i^{(j)}\log \frac{j!}{{\color{orange}c^{j-1}}}}$$
Equilibrium distribution:
$$\hat{\wp}_i^{(j)} = \frac{c^{j-1}}{j!} \exp(-\alpha j - \beta \epsilon_i^{(j)})$$
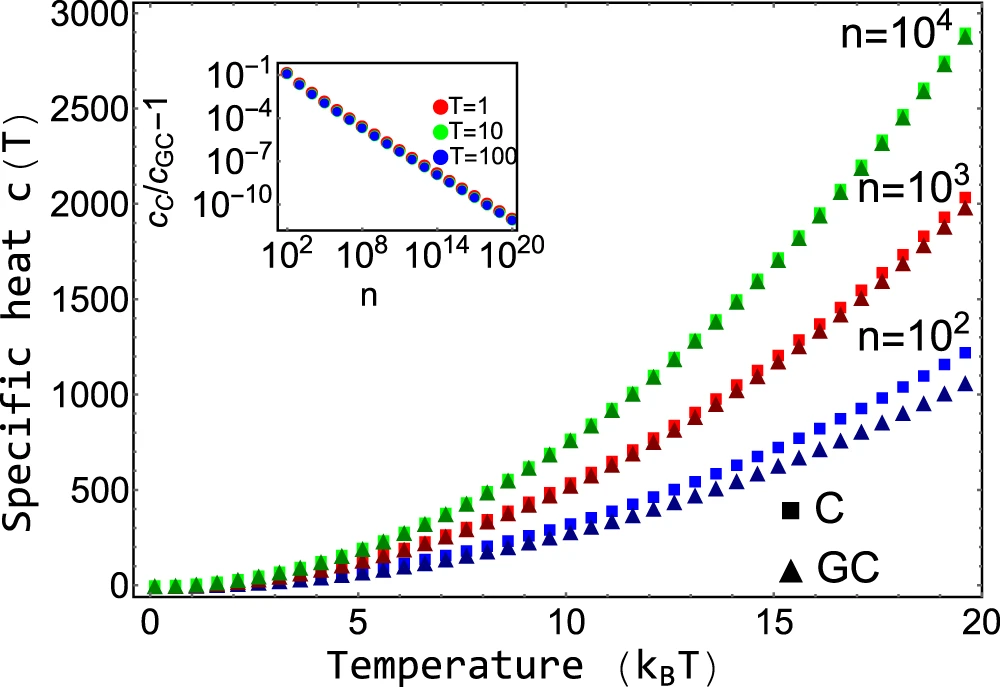
Comparison with Grand-canonical ensemble
Examples
Self-assembly of amphibolic particles
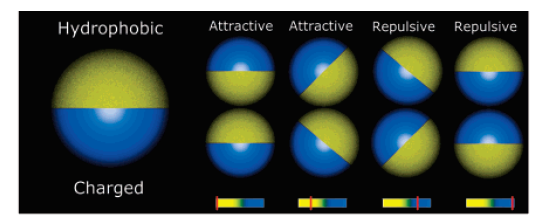
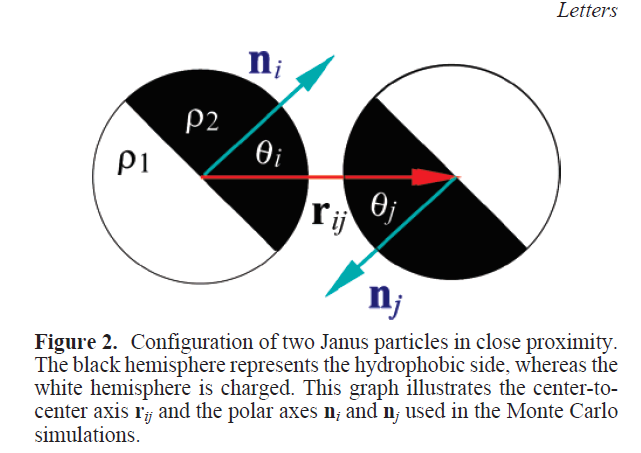
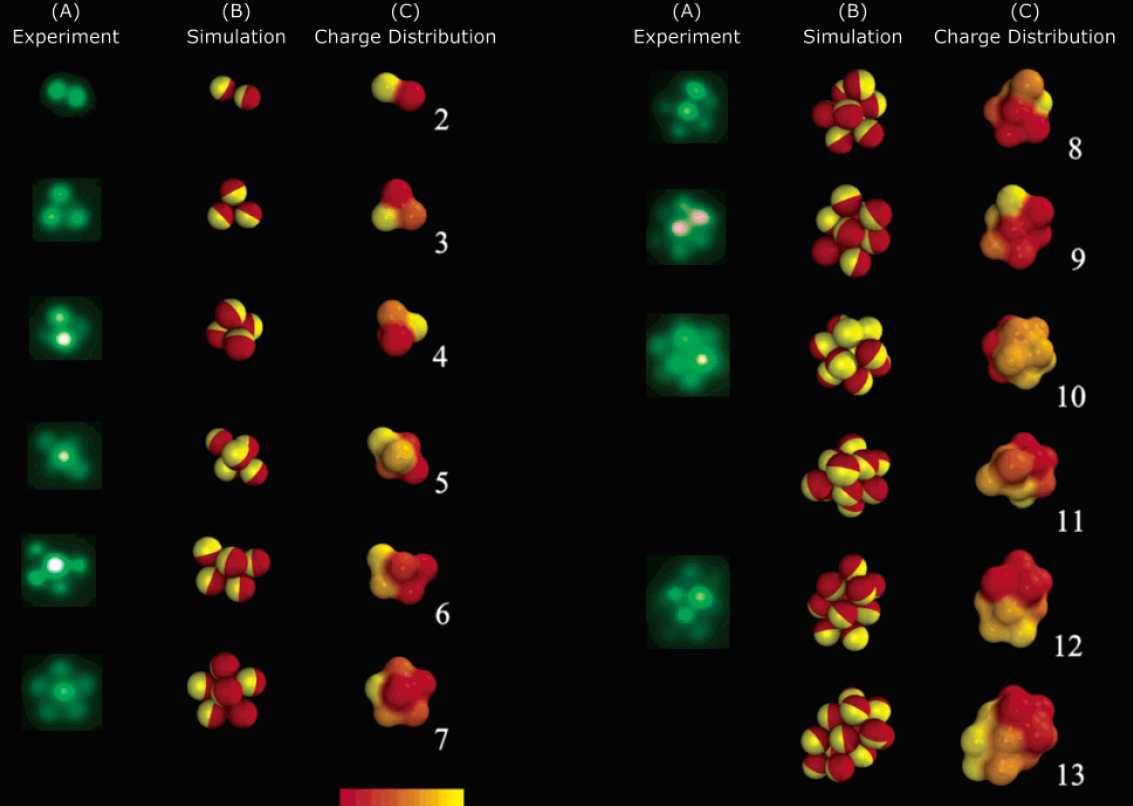
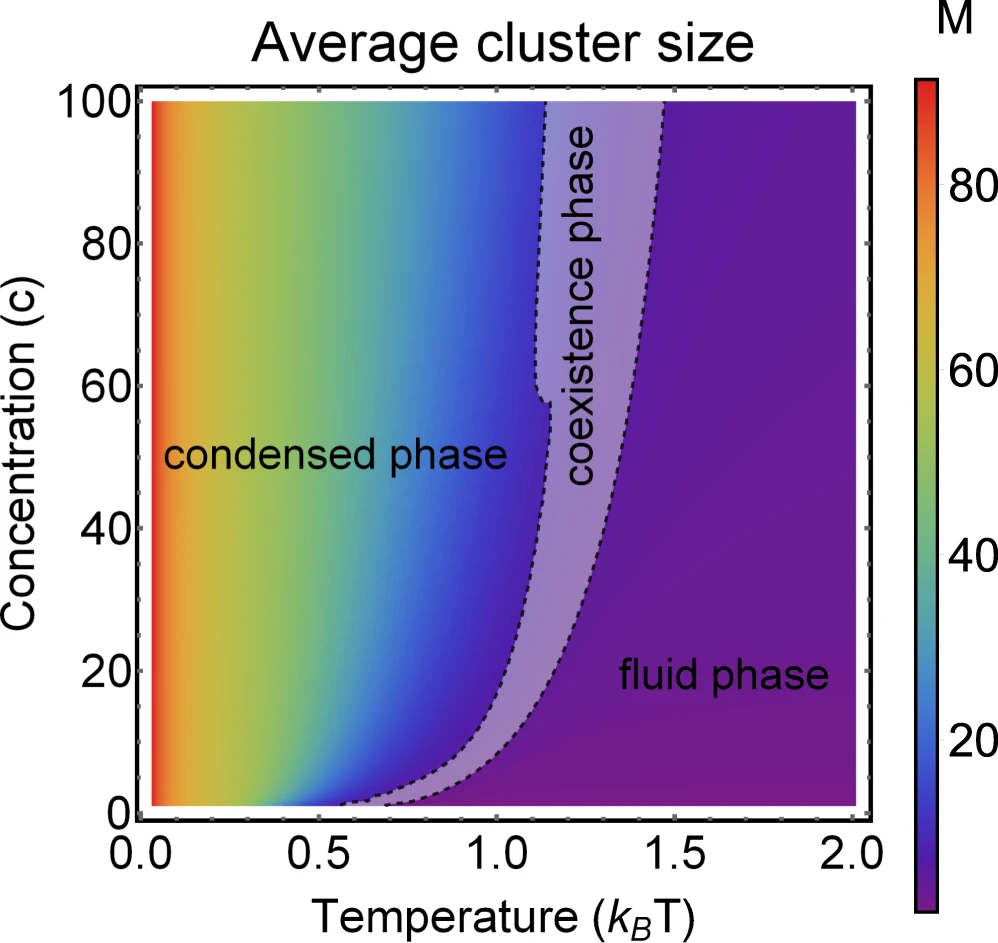
Phase diagram of amphibolic particle average cluster size
Currie-Weiss model with molecules
$$ H(\sigma_i) = - \frac{J}{n-1} \sum_{i \neq j, \ free} \sigma_i \sigma_j - h \sum_{j, \ free} \sigma_j $$
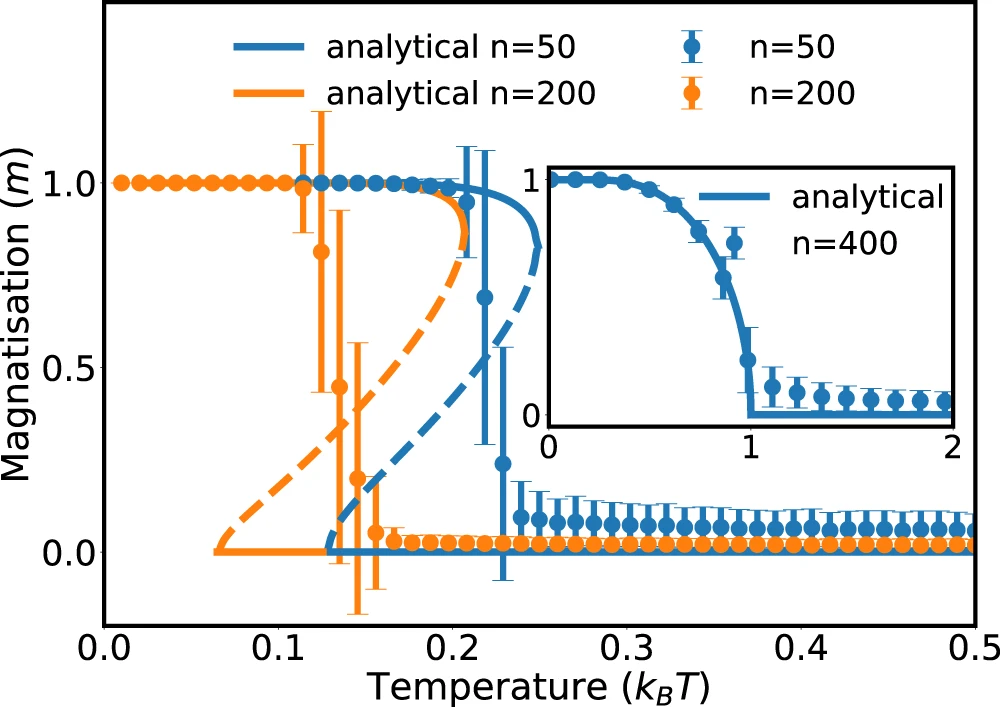
Stochastic thermodynamics of structure-forming systems
Detailed fluctuation theorem
$$\frac{P(\Delta \sigma)}{\tilde{P}(-\Delta \sigma)} = e^{\Delta \sigma}$$
where
$$\Delta \sigma = \Delta s_i + \log j_0 - \log j_f$$
Stochastic thermodynamics of non-linear systems
(with D. Wolpert - New J. Phys. doi:10.1088/1367-2630/abea46)
- Many complex, long-range systems are non-linear
- Question: can we use stochastic thermodynamics?
Requirements:
- Non-linear Markov dynamics $$ \dot{p}_i(t) = \frac{1}{C(p)} \sum_{j} [w_{ij} \Omega(p_{j}(t)) - w_{ji} \Omega(p_i(t)) ]$$
- Detailed balance $$\frac{w_{ij}}{w_{ji}} = \frac{\Omega(p_i^{eq})}{\Omega(p_j^{eq})} $$
- Second law of thermodynamics:
- For some (generalized) entropy \(S = f(\sum_i g(p_i))\): \(\dot{S}_i \geq 0\).
Stochastic thermodynamics of non-linear systems
Theorem: Requirements 1-3 imply
1) \(\Omega(p_m) = \exp(-g'(p_m))\)
2) \(C(p) = f'(\sum_m g(p_m))\)
Stochastic entropy: \(s(t) = \log\left(\frac{1}{\Omega(p_x(t))}\right) = g'(p_x(t))\)
Detailed fluctuation theorem holds:
$$\frac{P(\Delta s_i)}{\tilde{P}(-\Delta s_i)} = e^{\Delta s_i}$$
Interested in stochastic thermodynamics?
Here's more!
1) CSH Talk: Friday 12th March 3pm - Gülce Kardes
Thermodynamic uncertainty relations for multipartite processes
2) WOST II: Virtual workshop on stochastic thermodynamics
Tutorials 13th May, Conference 17th - 21st May
Web: https://wiki.santafe.edu/index.php/Stochastic_Thermodynamics_II

Stochastic thermodynamics of complex systems
By Jan Korbel
Stochastic thermodynamics of complex systems
- 425



