Beyond Independence: Advances in Network Inference via Gaussian Graphical Models for Multimodal Data
Luisa Cutillo, l.cutillo@leeds.ac.uk, University of Leeds
in collaboration with
Bailey Andrew, and David Westhead, UoL
Sections
- Recap: Gaussian Graphical models and GmGM
- New: Non Central GmGM

- Practical tutorial in github codespaces

- New: Strong Product Model



Part 1

Background on Gaussian Graphical Models (GGM) and GmGM
Biological Networks
Examples:
- Genetic interaction networks
- Metabolic networks
- Signalling networks
- Gene regulatory networks
- Protein-protein interaction networks
Gaussian graphical model (GGM) estimation is one approach to estimating biological networks
undirected graphical model
X1
X3
X2
X4
X5
X6
X7
X8
As
Vertices V
Edges E
Global Markov Property:
conditional independence graph
the absence of an edge between nodes in A and in B corresponds to conditional independence of the random vectors A, B given the separating set C
What is a GGM?
special case of conditional independence graph where
Edges weights E ∝ Precision matrix
Vertices V
Partial correlations via
partial correlation
(Dempster, 72) it encodes the conditional independence structure
The sparsity pattern of Θ expresses conditional independence relations encoded in the corresponding GGM
Conditional Independence = SPARSITY!
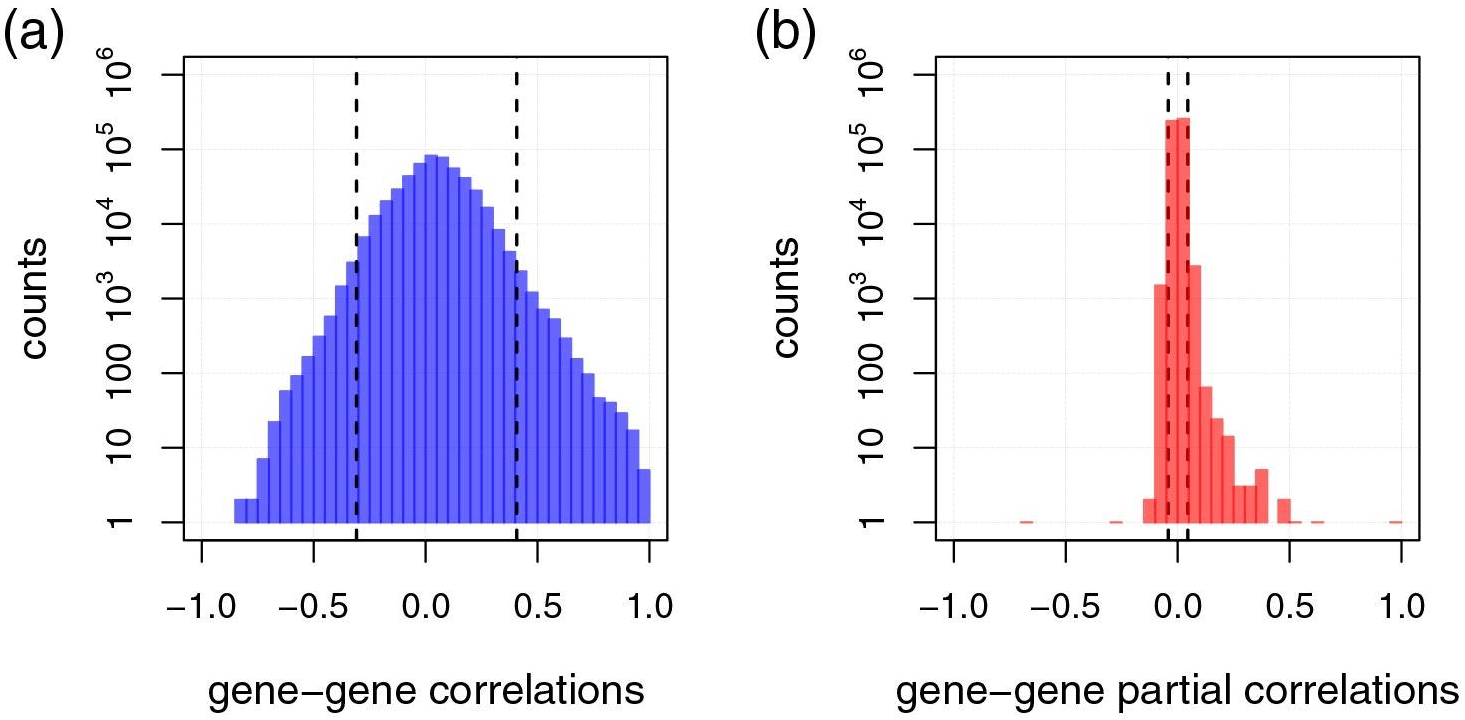
GGMs Networks VS Correlation Networks
Genes 1, 2, 3
GGMs Networks VS Correlation Networks
IEstimated gene-gene Pearson correlation coefficients (a) with their respective full order partial correlation coefficients (b) for single-cell RNA sequencing data of melanoma metastases (Tirosh et al., 2016).
About Sparsity...
- We want to study GGMs where is sparse
- This means more conditional independent variables
- Fewer elements to estimate -> Fewer data needed
Sparsity assumption => max graph degree d<<p
is reasonable in many contexts!
Example: Gene interaction networks
About Sparsity...
However the " Bet on Sparsity principle" introduced Tibshirani 2001, "In praise of sparsity and convexity":
(...no procedure does well in dense problems!)
- How to ensure sparsity?
Graphical Lasso (Friedman, Hastie, Tibshirani 2008):
imposes an penalty for the estimation of
Limitation
- Assumption of independence between features and samples
- Finds graph only between features
Features
Samples
Data
Single cell data
extract the conditional independence structure between genes and cells, inferring a network both at genes level and at cells level.
Cells
Genes
| 2 | ... | 10 |
|---|---|---|
| : | ... | : |
| 5 | ... | 7 |
Graph estimation without independence assumption
We need a general framework that models conditional independence relationships between features and data points together.
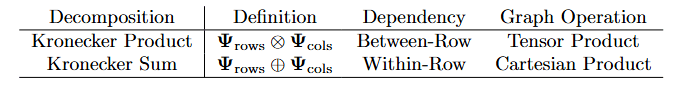
Bigraphical lasso: A different point of view
Preserves the matrix structure by using a Kronecker sum (KS) for the precision matrixes
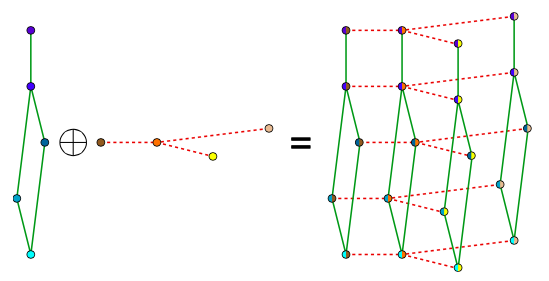
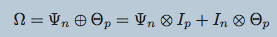
KS => Cartesian product of graphs' adjacency matrix
(eg. Frames x Pixels)

Bigraphical lasso: A different point of view
(Kalaitzis et al. (2013))
Limitations:

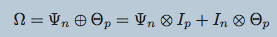
- Space complexity: There are dependencies but actually implemented to store elements
- Computational time: Very slow, could only handle low hundreds samples.
- Exploits eigenvalue decompositions of the Cartesian product graph and a more efficient version of the algorithm
- Reduces memory requirements from O(n^2p^2) to O(n^2+ p^2).
- Replaces Gaussianity with weaker ‘Gaussian copula’ assumption through preprocessing tools non-paranormal skeptic
- Introduces matrix non-paranormal distribution with a Kronecker sum structure
- Can be used on data with arbitrary marginals, as long as relationships between the datapoints ‘behave Gaussianly’).
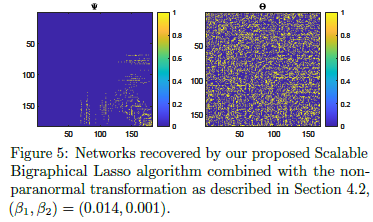
https://github.com/luisacutillo78/Scalable_Bigraphical_Lasso.git
Two-way Sparse Network Inference for Count Data
S. Li, M. Lopez-Garcia, N. D. Lawrence and L. Cutillo (AISTAT 2022)

- TeraLasso (Greenewald et al., 2019), EiGLasso (Yoon & Kim, 2020) better space and time complexity (~scLasso)
- Can run on low thousands in a reasonable amount of time
Related work for scalability
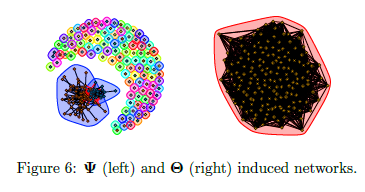
Limitations of prior work
-
Not scalable to millions of features
-
Iterative algorithms
-
Use an eigendecomposition every iteration (O(n^3) runtime - > slow)
-
O(n^2) memory usage
Do we need to scale to Millions of samples?

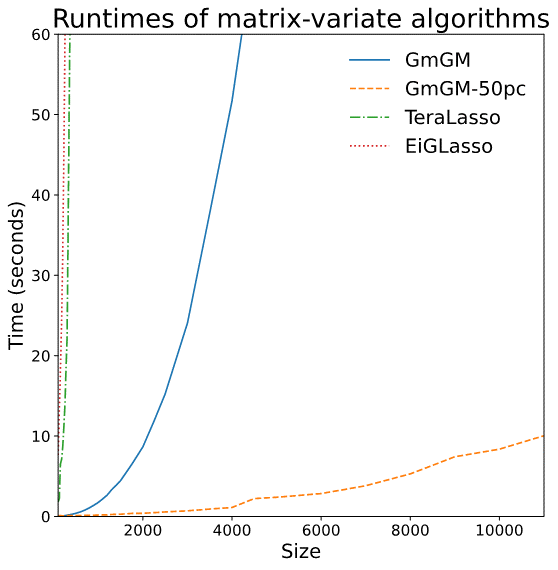
What is the improvement in GmGM?
In previous work graphical models an L1 penalty is included to enforce sparsity
-
Iterative algorithms
-
Use an eigendecomposition every iteration (O(n^3) runtime-slow!)
If we remove the regularization, we need only 1 eigendecomposition!
In place of regularization, use thresholding


Part 2
- A metagenomics matrix of 1000 people x 2000 species
- A metabolomics matrix of 1000 people x 200 metabolites
We may be interested in graph representations of the people, species, and metabolites.
GmGm addresses this problem!
GmGM: a fast Multi-Axis Gaussian Graphical Model ongoing PhD project (Andrew Bailey), AISTAT 2024
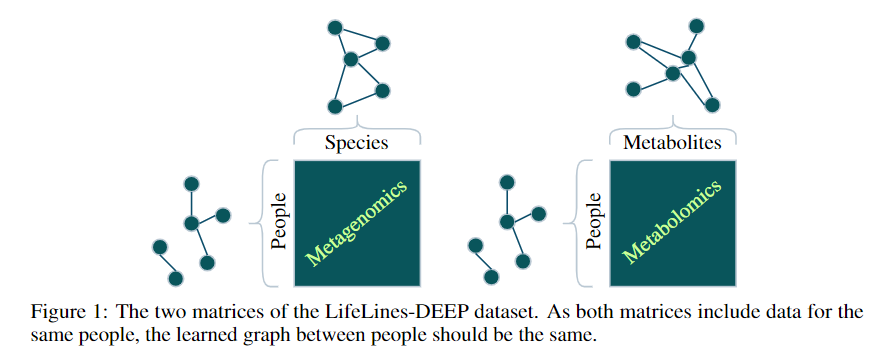
Tensors (i.e. modalities) sharing an axis will be drawn independently from a Kronecker-sum normal distribution and parameterized by the same precision matrix

Additional Assumptions!
- Both input datasets and precision matrices can be approximated by low-rank versions
Still some limitations:
- the initial eigen-decomposition is O(N^3) per axes anyway
- this can be unfeasible for large and complex datasets
- O(pn) memory
- O(pn^2) runtime
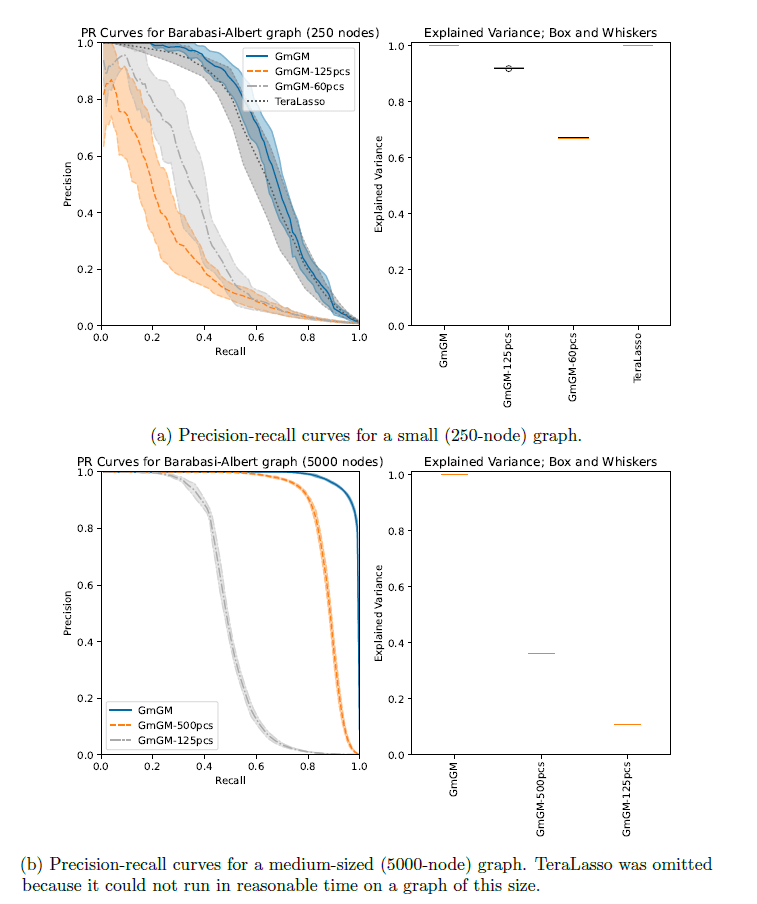
Is our GmGM much faster / good enough?

Is our GmGM much faster / good enough?
Part 2


2025
What’s the Problem with Uncentered Data?
In multi-axis case, inference is usually done in a one sample scenario
Zero mean assumption
can cause egregious modelling errors!
We relax the zero-mean assumption
we propose the
“Kronecker-sum-structured mean”
model with likelihood that
can be solved efficiently with coordinate descent.
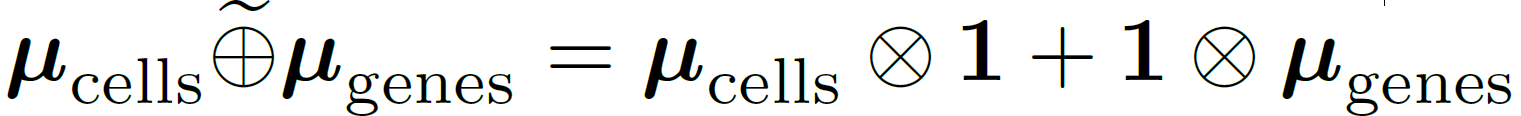
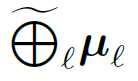
Remarks
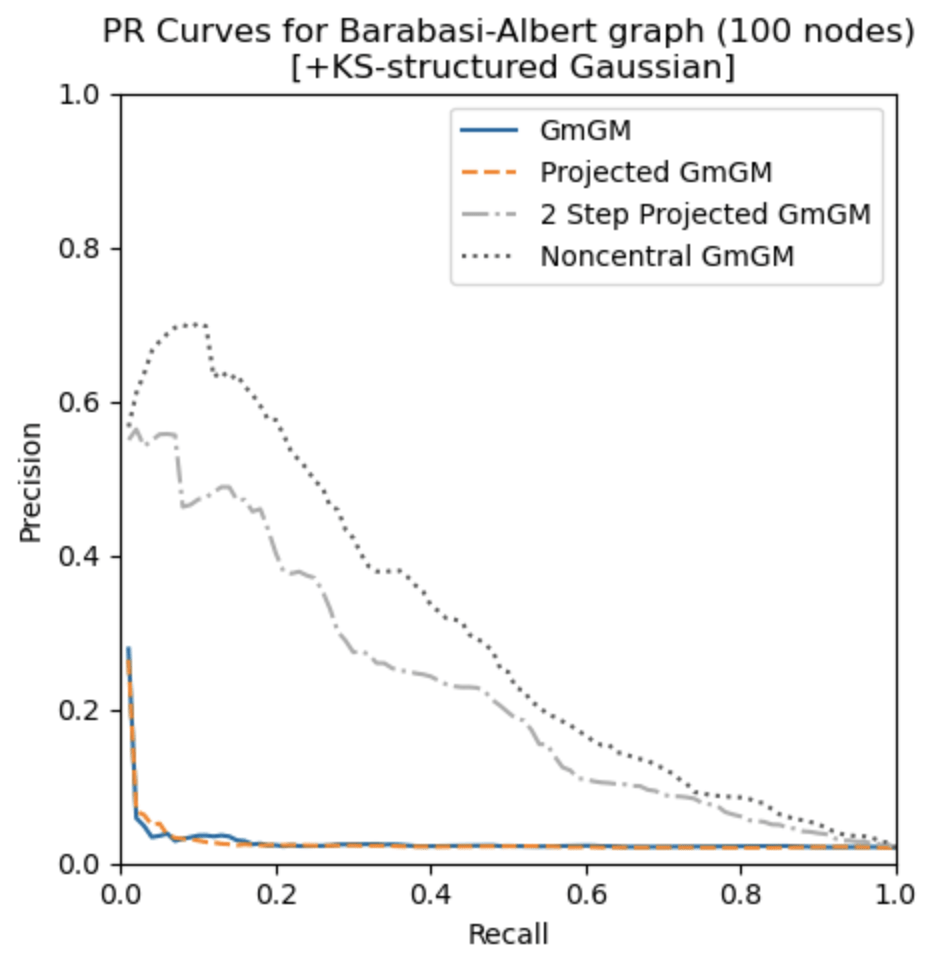
- noncentral GmGM is a ‘drop-in wrapper’ for pre-existing methods!
- Our estimator for the parameters is the global maximum likelihood estimator
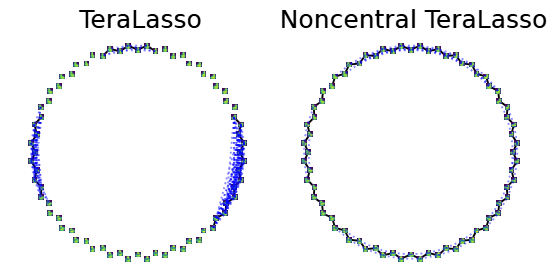
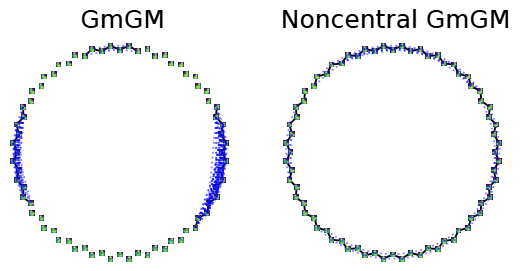
Applying our correction
Part 3


2025
Genes
Cells
| 2 | ... | 10 |
|---|---|---|
| : | ... | : |
| 5 | ... | 7 |
Dependency between genes
Dependency between cells
How do these two types of dependency interact?
X
Y
X
Y
Z
The Strong Product differentiate and learns:
- within-row interaction
- between-row interaction
Model Choices without independence
single sample
parameters!
We can impose a specific structure on
Genes
Cells
| 2 | ... | 10 |
|---|---|---|
| : | ... | : |
| 5 | ... | 7 |
Strong Product model: We add these together!
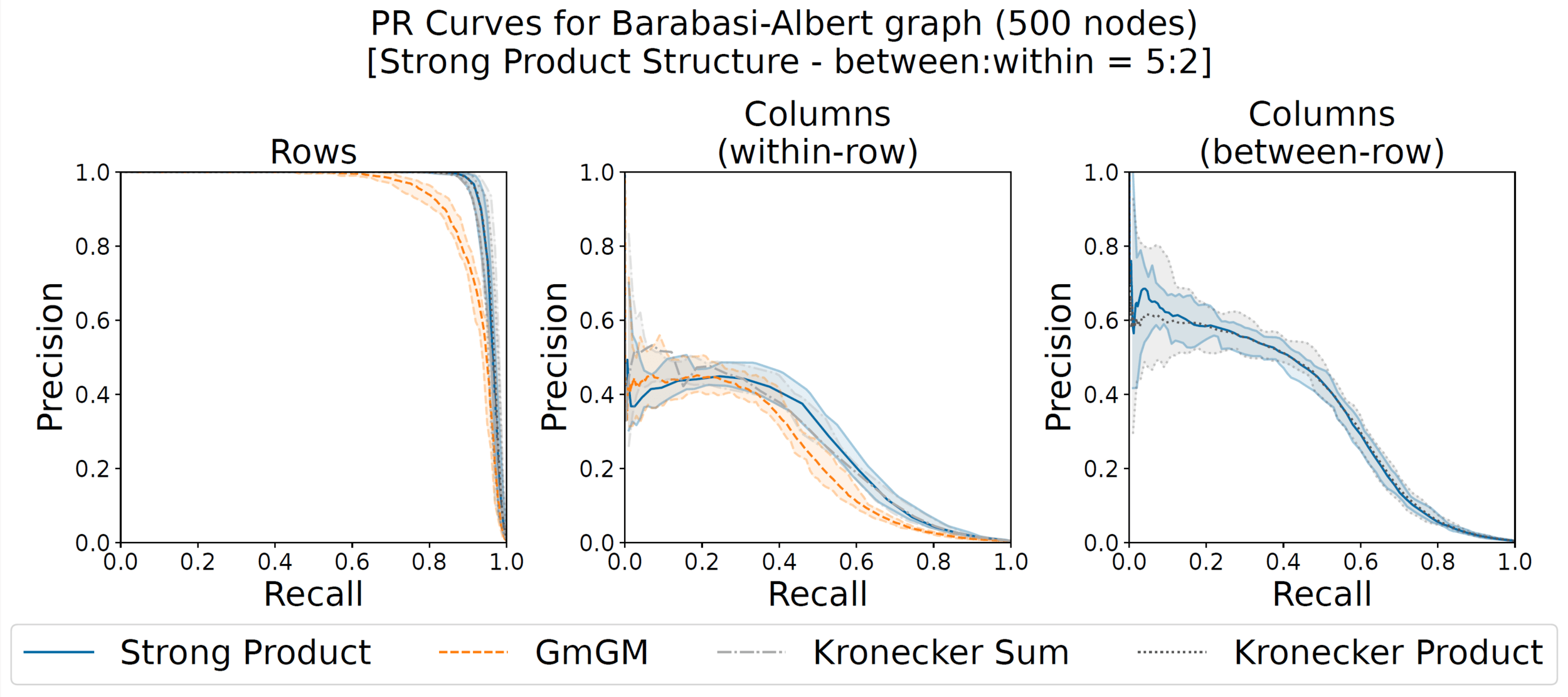
Synthetic data ground truth
(here y=1, x=2.5)
with A, B, C are Barabasi-Albert random graphs with 500 nodes each
Our Strong Product is the only one that learns both types of column graphs
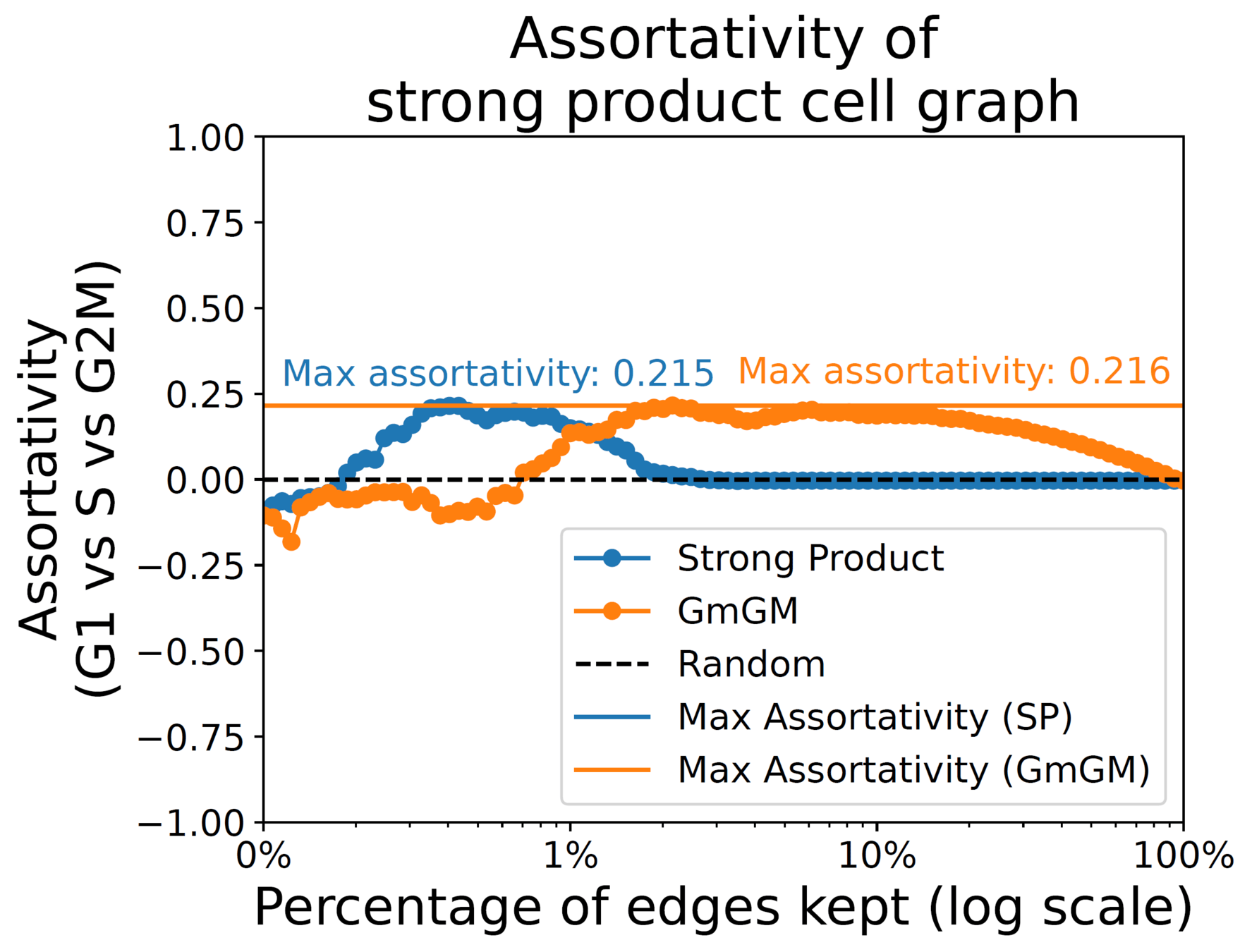
288 mouse embryo stem cell scRNA-seq dataset from Buettner et al. (2015), limited to 167 genes mitosis-related genes cell cycle labelled (G1,S,G2M).
Assortativity:
measures tendency of cells within a stage to connect
1 (tend to connect)
0 (no tendency)
-1 (tend not connect)
Results

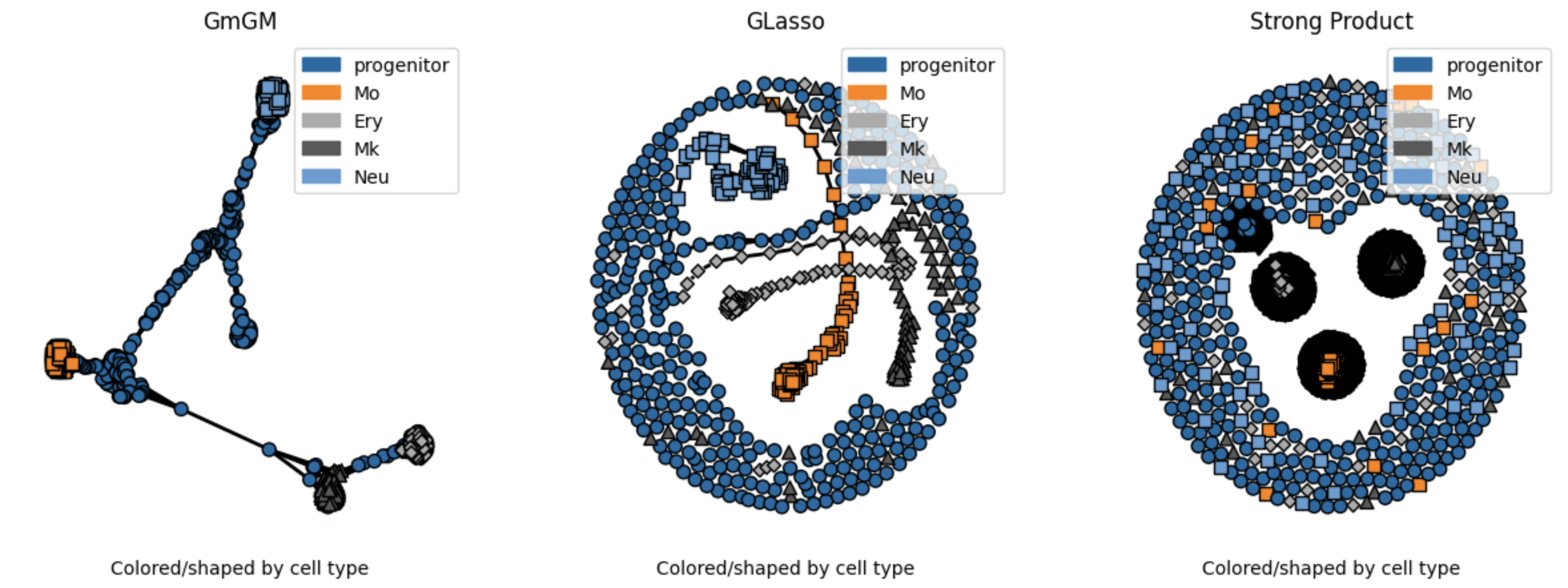
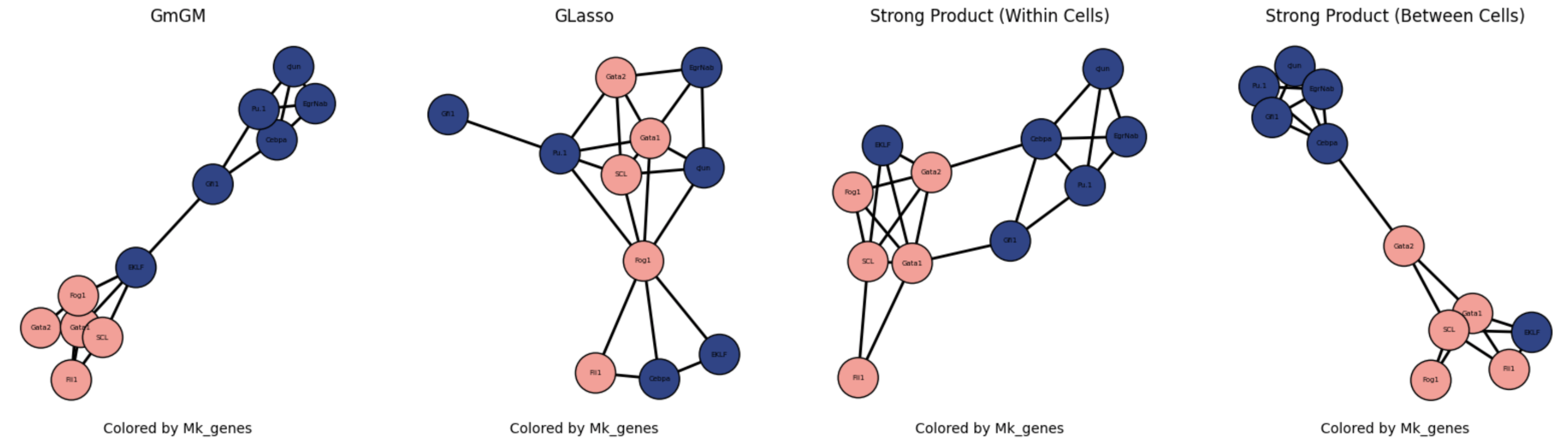
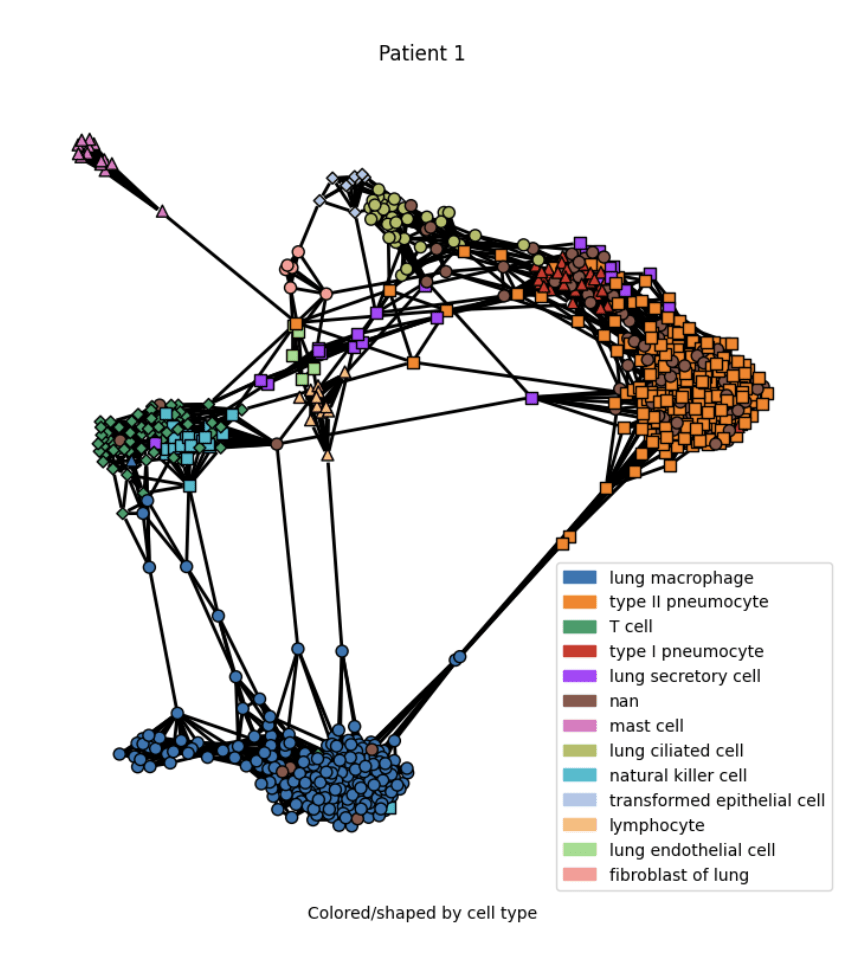

Practical tutorial in github codespaces

Part 4
Instructor: Bailey Andrew, University of Leeds
Practical
- Synthetic data (15min)
- Getting the most out of GmGM (15min)
- Real data (15min)
- Open-ended challenges
Ask questions if stuck or curious!
Ellis_Summer_School2025
By Luisa Cutillo
Ellis_Summer_School2025
- 266



