Introduction to Astronomy
Radiation & Spectra
Introduction to Astronomy
Radiation and Spectra
Overview
Everything (!) we know about Astronomy to-date was learned by analyzing electromagnetic radiation.
Introduction to Astronomy
Radiation and Spectra
Overview
In this module, we examine:
The behavior of visible light and other EM radiation
Electromagnetic Spectra
The structure of atoms
Introduction to Astronomy
Radiation and Spectra
The behavior of electromagnetic radiation
The behavior of visible light and other EM radiation
Introduction to Astronomy
Radiation and Spectra
The behavior of electromagnetic radiation
In astronomy*, the term radiation is a general term used to describe electromagnetic signal that radiates outwards from a source.
This includes, for example:
Visible light
Radio waves
X-rays
Microwaves
Infra-red
Ultra-violtet
... etc
*Note that "radiation" has a different meaning in other contexts, for example in Medical Physics or Chemistry.
Introduction to Astronomy
Radiation and Spectra
The behavior of electromagnetic radiation
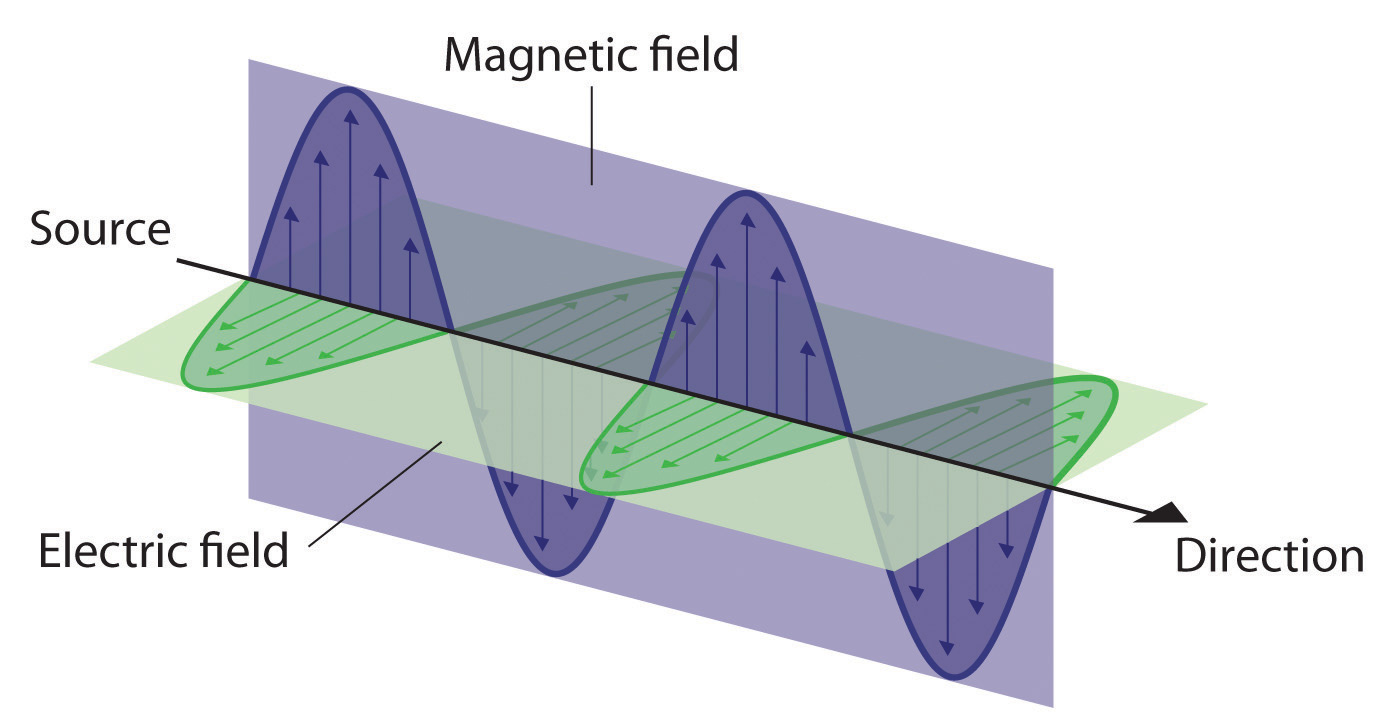
Physicists think of electromagnetic radiation in two complementary ways:
Introduction to Astronomy
Radiation and Spectra
The behavior of electromagnetic radiation
Physicists think of electromagnetic radiation in two complementary ways:

Wavelength: the distance of one oscillation
Frequency: number of oscillations per second
Amplitude: the power (intensity) of the wave.
Wave speed = Wavelength x Frequency

Introduction to Astronomy
Radiation and Spectra
The behavior of electromagnetic radiation
Physicists think of electromagnetic radiation in two complementary ways:
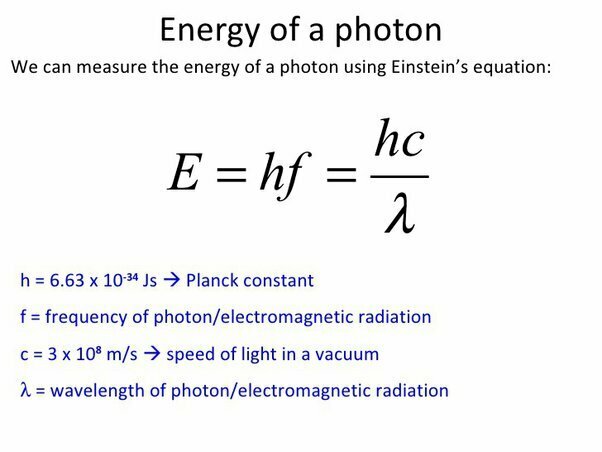
Energy of a photon
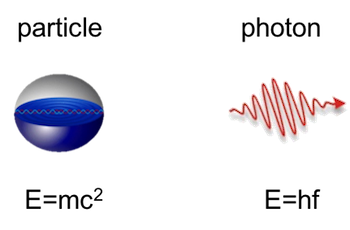

Summary!
Light travels w/ speed
Light can be thought of as an EM wave, w/ wavelength
Light can be thought of as photons (packets of energy)
The energy of a photon is inversely proportional to its wavelength
Introduction to Astronomy
Radiation and Spectra
The behavior of electromagnetic radiation
The intensity of the radiation decreases with distance (inverse square law)
The figure illustrates the
with increasing distance.
The intensity (energy per square)
decreases with the square (second power)
of the distance.
When the distance doubles,
the same energy is spread over 4 squares;
When the distance triples,
the same energy is spread over 9 squares;
The energy that passes through 1 square-meter per second is called the intensity
Introduction to Astronomy
Radiation and Spectra
The behavior of electromagnetic radiation (learn more...)
Introduction to Astronomy
Radiation and Spectra
The behavior of electromagnetic radiation (learn more...)
Watch this video to learn about the wider meaning of the term radiation, and what radiation is considered dangerous.
Introduction to Astronomy
Radiation and Spectra
Electromagnetic Spectra
Electromagnetic Spectra
Introduction to Astronomy
Radiation and Spectra
The Electromagnetic Spectrum
Electromagnetic waves are classified into different categories based on their wavelength
From shortest to longest (as shown in the figure)
Gamma rays
X-rays
Ultra-violet
Visible light
Infra-red
Microwave
Radio
Have a lot of energy / can be harmful to living tissue / mostly filtered by the atmosphere.
X for unknown / can penetrate flesh, but not bones/ harmful in large doses.
above violet in energy / can be harmful in large doses, especially to skin tissue
very small part of the spectrum / detectable by the part of our brain that is poking out of our skulls (eyes)
below red in energy / used in Wii controllers and other tech./ human body glows like an infra-red light bulb
used in cellphone communications and wifi, and of course in microwave ovens
Old school broadcasting, and v. important for astronomical observation.
Introduction to Astronomy
Radiation and Spectra
Atomic Spectra
In general,
Matter emits light
with different wavelengths
based on
composition & temperature

i.e. what kind of atom?

i.e. how vigorously do the atoms shake?

Introduction to Astronomy
Radiation and Spectra
Continuous Spectra
Have you ever noticed how different stars have different colors (yellow, orange, red, blue, etc..)?
So turns out we can tell the surface temperature of a star just by looking at the most dominant color of the light coming from it!
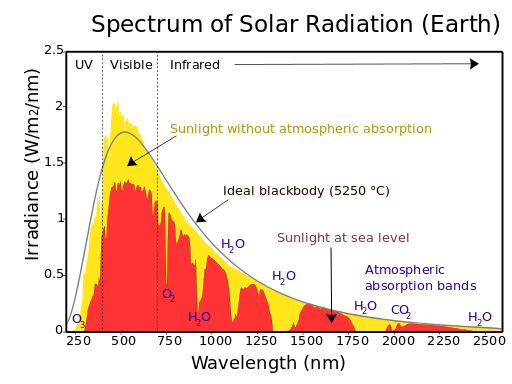
Turns out that stars emit electromagnetic radiation in all parts of the spectrum, but with varying intensities.
How much a star emits in a given part of the spectrum is determined by its surface temperature.

Introduction to Astronomy
Radiation and Spectra
Continuous Spectra

Stars emit different colors because they have different surface temperatures!
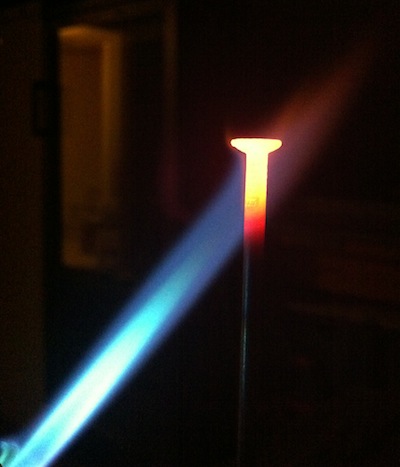
Just like the flame and the nail emit different colors because they are at different temperatures!
Have you heard of the phrase "red-hot"?
Well, there is also "orange-hot" and "blue-hot"
Introduction to Astronomy
Radiation and Spectra
Continuous Spectrum
This simulation shows the spectrum produced by any object based on its temperature.
slide the arrow up and down to change the temperature
As the object's temperature go up, does the peak move to longer wavelength (right) or shorter (left)?
As the object's temperature go up, does the total power (area under the curve) increase or decrease?
Introduction to Astronomy
Radiation and Spectra
Continuous Spectra
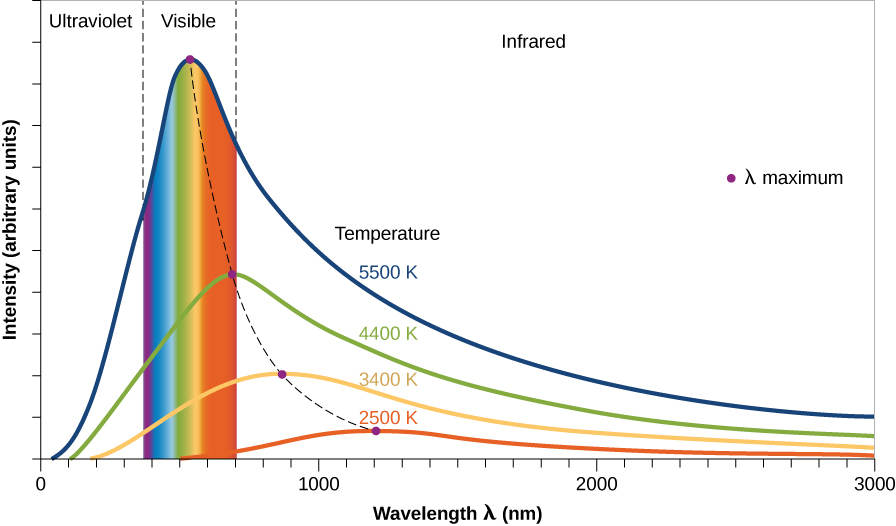
As the object's temperature goes up, the peak of the spectrum moves to shorter wavelengths!
As the object's temperature goes up, the total power emitted by the object (area under the curve) increases!
By the way, this is the working-principle of the no-contact thermometer
Introduction to Astronomy
Radiation and Spectra
Continuous Spectra

When I first saw this image on a lighting-fixture website, I was very confused!
As a physicist, I know that hotter objects radiate shorter wavelengths, so an object radiating blue light is actually hotter than an object radiating red light ...
Why is the reddish light labeled as warmer than the blueish light?
and when I look at the temperatures above the light fixtures, they seem correct from what I know about the peaks of continuous spectra ...
So, what is going on here?
(let me know what you think on YellowDig)
Introduction to Astronomy
Radiation and Spectra
Continuous Spectra (Section 5.2 )
Introduction to Astronomy
Radiation and Spectra
The structure of atoms; emission & absorption
The structure of atoms
Emission Spectra
Absorption Spectra
Introduction to Astronomy
Radiation and Spectra
The structure of atoms
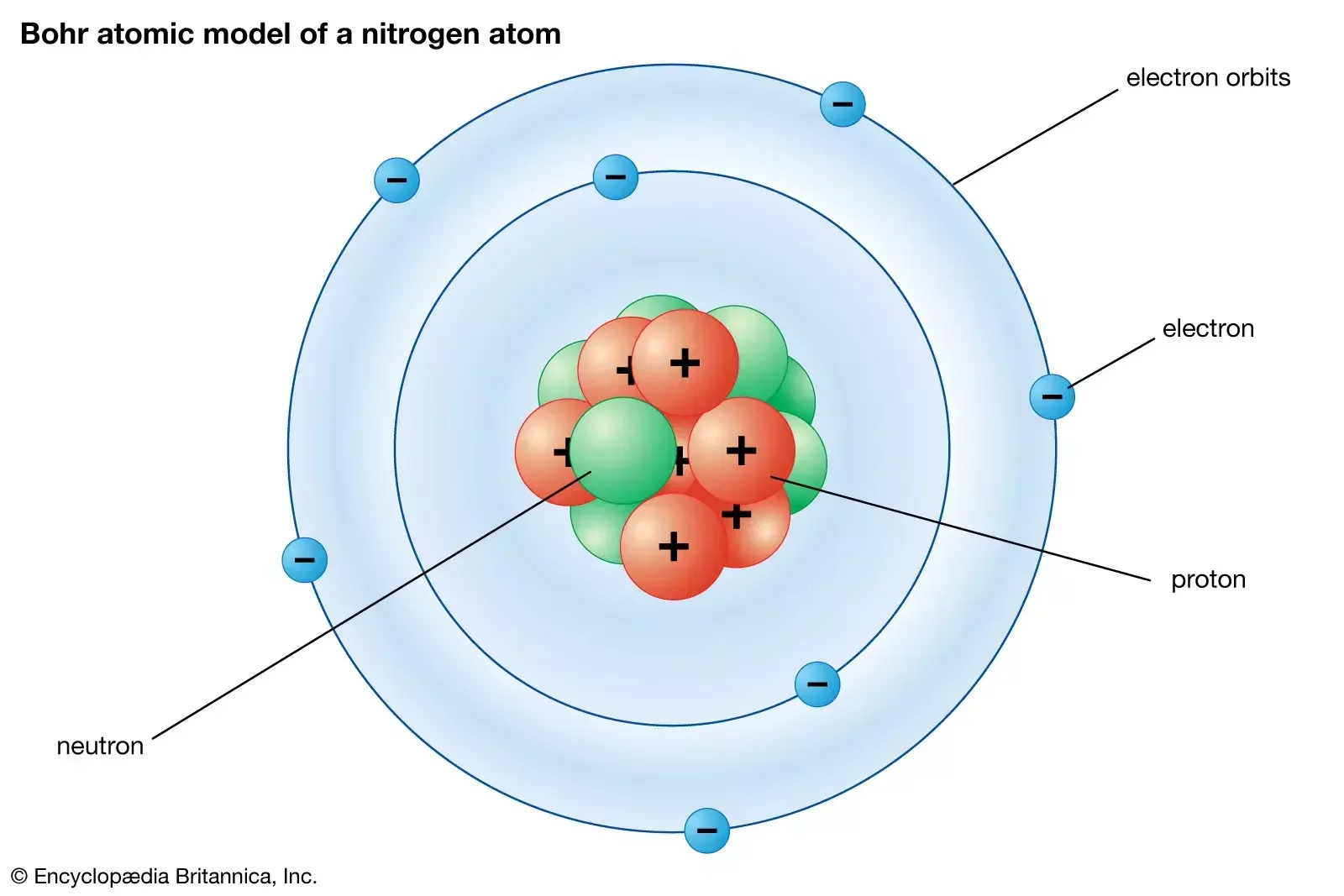
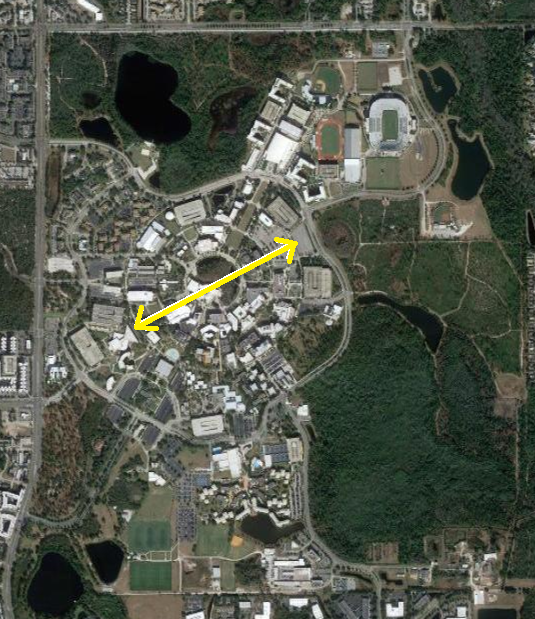
The nucleus is tiny compared to the atom: The comparative spatial extension of the atomic nucleus to the spatial extension of the electronic cloud in an atom is of the same order as the ratio of the size of your thumb compared to the size of UCF campus.

In Bohr's model of the atom, a heavy nucleus containing positively charged protons and neutral neutrons is surrounded by a cloud of negatively charged electrons in definitive states that are sometimes referred to as orbitals. The number of protons determines the type of chemical element.
Introduction to Astronomy
Radiation and Spectra
The structure of atoms
In the periodic table of the elements, each element is uniquely identified by the number of protons, which is typically written on top of its symbol.
Clicking on each element will take you to its page, where you can see a list of its isotopes. isotopes are variants of the same chemical element but with different number of neutrons in the nucleus.
A neutral atom has the same number of electrons as protons. But if a neutral atom loses or gains extra electrons, it is known as an ion, and it is said that the atom is ionized!
Introduction to Astronomy
Radiation and Spectra
The structure of atoms; emission spectra
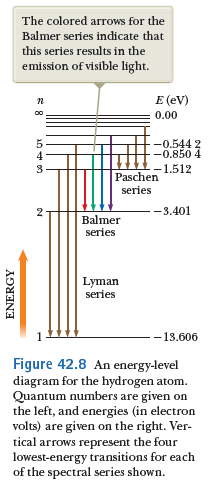
When an electron transitions from higher orbitals to lower orbitals, the atom emits a photon.
The energy of the emitted photon is exactly equal to the amount of energy lost by the atom.
Introduction to Astronomy
Radiation and Spectra
Emission Spectra
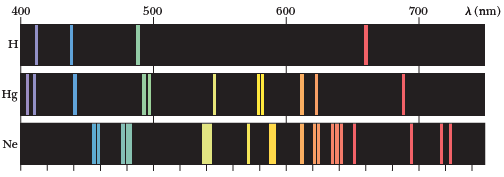
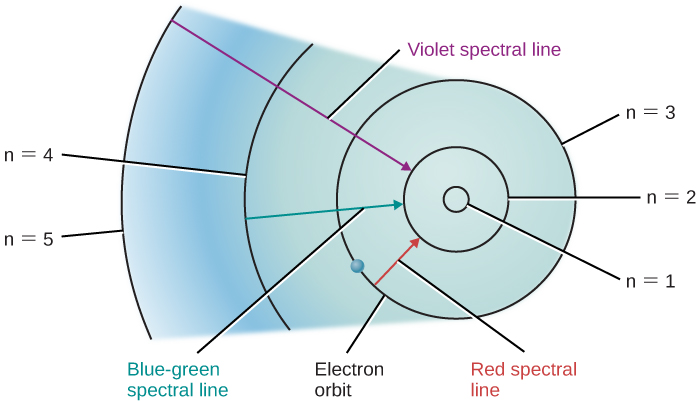
Each atom has a signature of unique photon energies that it emits based on the transition energies of the electrons.
Introduction to Astronomy
Radiation and Spectra
Emission Spectra



Each atom has a signature of unique photon energies that it emits based on the transition energies of the electrons.
Introduction to Astronomy
Radiation and Spectra
The structure of the atom; emission and absorption spectra
There are three categories of spectra that we observe:
- A continuous spectrum (formed when a solid or very dense gas gives off radiation) is an array of all wavelengths.
- A dark line, or absorption spectrum, consists of a series or pattern of dark lines—missing colors—superimposed upon the continuous spectrum of a source.
- A bright line, or emission spectrum, appears as a pattern or series of bright lines; it consists of light in which only certain discrete wavelengths are present.
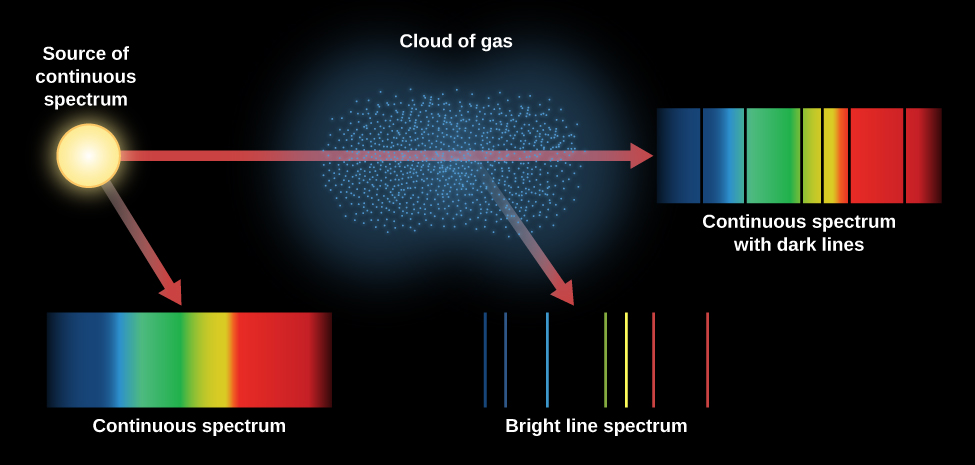
This is the most important video in this module.
Introduction to Astronomy
Radiation and Spectra
The structure of the atom; emission and absorption spectra
There are three categories of spectra that we observe:
- A continuous spectrum (formed when a solid or very dense gas gives off radiation) is an array of all wavelengths.
- A dark line, or absorption spectrum, consists of a series or pattern of dark lines—missing colors—superimposed upon the continuous spectrum of a source.
- A bright line, or emission spectrum, appears as a pattern or series of bright lines; it consists of light in which only certain discrete wavelengths are present.

Sodium Absorption lines (ASMR)
Introduction to Astronomy
Radiation and Spectra
Absorption Spectrum
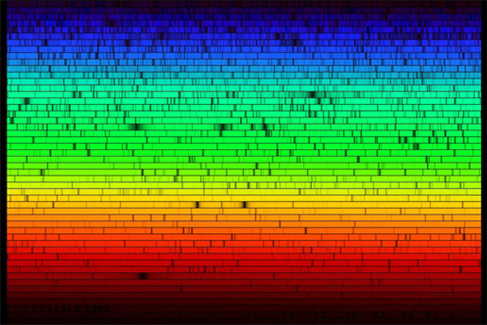
Visible Spectrum of the Sun. Our star’s spectrum is crossed by dark lines produced by atoms in the solar atmosphere that absorb light at certain wavelengths. (credit: modification of work by Nigel Sharp, NOAO/National Solar Observatory at Kitt Peak/AURA, and the National Science Foundation)
Introduction to Astronomy
Radiation and Spectra
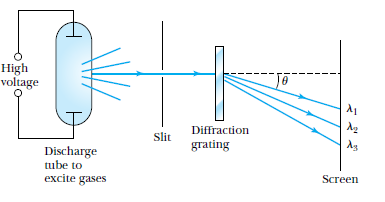
Make your own spectrum
Introduction to Astronomy
Radiation and Spectra
Example Star
Introduction to Astronomy
Radiation and Spectra
Line Spectra
Introduction to Astronomy
Radiation and Spectra
The Doppler effect
The Doppler effect
Introduction to Astronomy
Radiation and Spectra
The Doppler effect
Introduction to Astronomy
Radiation and Spectra
The Doppler effect
Introduction to Astronomy
Radiation and Spectra
Summary
Dr. Maria Weber Summarizes the module.
1/5
Introduction to Astronomy
Radiation and Spectra
Summary
Dr. Maria Weber Summarizes the module.
2/5
Introduction to Astronomy
Radiation and Spectra
Summary
Dr. Maria Weber Summarizes the module.
3/5
Introduction to Astronomy
Radiation and Spectra
Summary
Dr. Maria Weber Summarizes the module.
4/5
Introduction to Astronomy
Radiation and Spectra
Summary
Dr. Maria Weber Summarizes the module.
5/5
AST - Radiation and Spectra
By omoussa
AST - Radiation and Spectra
- 173