Management of Low & Favorable Intermediate Risk Prostate Cancer
Clinical case
- 65-year-old man presents for discussion of management options
- Good performance status, no significant comorbidities
- PSA 7.5 ng/mL
- Digital rectal exam: T1c, 30g prostate, no nodules
Initial workup
- History & physical examination
- Family history
- Performance status
- Urinary symptoms (AUA score/IPSS)
- Sexual function (SHIM score)
- Laboratory studies
- PSA, 4K score (upgraded PSA)
- calculate PSA density to account for prostate volume >0.15-0.2 is abnormal
- Consider testosterone level
- PSA, 4K score (upgraded PSA)
IPSS Score
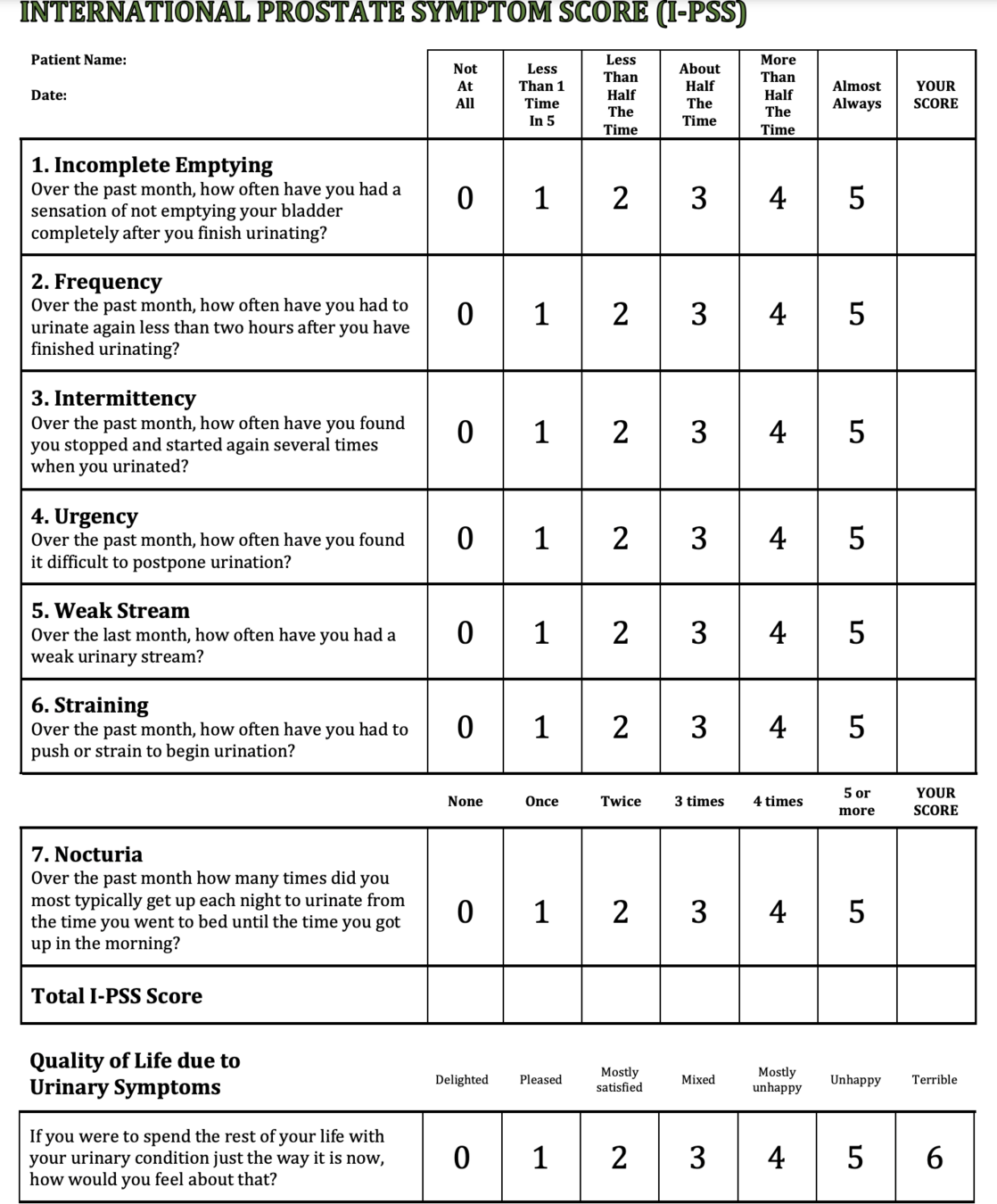
Mild (symptom score less than of equal to 7)
Moderate (symptom score range 8-19)
Severe (symptom score range 20-35)
SHIM Score


Prostate MRI

Used for guidance of biopsy and treatment MRI screening and guided biopsy has become the standard of care
Multi-parametric
- multiple MR sequences required
Biopsy


Biopsy


Pathology - Gleason Score


Cribriform pattern is a highly predictive of early metastasis.... but requires confidence in your pathologist
Risk stratification

G: Because of the increased sensitivity and specificity of PSMA-PET tracers for detecting micrometastatic disease compared to conventional imaging (eg, CT, bone scan) at both initial staging and BCR, the panel does not feel that conventional imaging is a necessary prerequisite to PSMA-PET and that PSMA-PET/CT or PSMA-PET/ MRI can serve as an equally effective, if not more effective frontline imaging tool for these patients.
Risk Assessment Tools

risk assessment tools
Partin tables:
- Predicts pathologic stage based on:
- Clinical stage
- PSA
- Gleason score
- Helps estimate risk of:
- Organ-confined disease
- Extracapsular extension
- Seminal vesicle involvement
- Lymph node metastasis
Memorial Sloan Kettering nomogram:
- Predicts probability of:
- PSA recurrence after surgery
- Lymph node involvement
- Seminal vesicle invasion
- Available online at nomograms.mskcc.org


The Roach formula
Mack Roach III
UCSF
1990s - derived from the Partin nomogram to predict lymph node involvement.
- Risk of lymph node involvement:
- 2/3 PSA + (Gleason score - 6) × 10
- Used historically to guide pelvic nodal radiation
- Risk of extracapsular extension:
- 3/2 PSA + (Gleason score - 3) × 10
- Risk of seminal vesicle involvement:
- PSA + (Gleason score - 6) × 10
Historical context:
- Developed before widespread use of modern imaging
- May overestimate risk compared to modern series
- Still used in some clinical trial eligibility criteria
- Being replaced by more accurate tools like PSMA PET

CApra-S
UCSF
Risk of recurrence post-prostatectomy







Advanced risk assessment tools
Genomic classifiers: Evidence
Decipher:
- 22-gene expression classifier
- Predicts:
- Risk of metastasis
- Prostate cancer-specific mortality
- PREDICT-RT (ongoing)
- Trial to determine length of ADT for high risk patients
Oncotype DX GPS:
- 17-gene expression panel
- Predicts:
- Adverse pathology
- Biochemical recurrence
- Metastasis
Others:
- Prolaris
- ProMark
Notes:
- Most validated in post-prostatectomy setting
- Emerging data in radiation therapy
- May help guide active surveillance decisions
- Consider in borderline cases where treatment intensification is being considered

How to use Decipher


Management options
Low risk and Intermediate risk
Watchful Waiting
Active surveillance
- Preferred for very low & low risk
- Option for favorable intermediate risk
External beam radiation
- Conventional fractionation
- Moderate hypofractionation
- SBRT
- +/- ADT (for unfavorable)
Brachytherapy
- LDR monotherapy
- HDR monotherapy
- Combined EBRT + Brachytherapy (ASCENDE-RT)
Surgery
- Radical prostatectomy
- Nerve-sparing approach when possible
Treatment selection algorithm
Low risk disease:
-
First choice: Active surveillance
- Especially if life expectancy <10 years
- Must be compliant with monitoring
- If treatment desired:
- External beam RT: 60 Gy/20 fx or 70 Gy/28 fx
- SBRT if technically feasible: 36.25 Gy/5 fx
- LDR brachytherapy if good urinary function
- Surgery if patient preference
Favorable intermediate risk:
- Active surveillance reasonable option
- External beam RT without ADT
- 60 Gy/20 fx or 70 Gy/28 fx
- SBRT
- LDR/HDR monotherapy if eligible
- Surgery ± nerve sparing
Unfavorable intermediate risk:
- External beam RT + 4-6 months ADT
- Surgery with node sampling consideration
- Combined EBRT + Brachytherapy
- Not ideal for brachytherapy monotherapy


Key factors in treatment selection
Patient factors:
- Age and life expectancy
- Comorbidities
- Urinary function (IPSS score)
- Sexual function priorities
- Ability to tolerate procedures
- Treatment preferences
Disease factors:
- Risk group classification
- Percent positive cores
- Prostate size
- MRI findings if available
Treatment considerations:
- Local expertise available
- Technical feasibility
- Prostate size for brachytherapy (<60cc)
- Image guidance capability
- Treatment duration impact
- Quality metrics of treating physician


Low & Favorable Intermediate Risk Prostate Cancer Treatment Algorithm
Monitoring schedule:
- Confirmatory testing in 6-12 months (if no prostate MRI performed prior to diagnosis)
- prostate biopsy,
- mpMRI with calculation of PSA density
- molecular tumor analysis
Then
- PSA every 6 months
- Digital rectal exam every 12 months
- Increased PSA or DRE change
- repeat mpMRI, no more often than every 12 months
- repeat prostate biopsy, if indicated by MRI or PSA change:
- No more than every 12 months
- Subsequent biopsies every 2-5 years
- transitioned to observation when life expectancy is <10 years
Triggers for intervention:
- Grade group progression (Gleason ≥7)
- Significant increase in tumor volume
- Patient preference
Active surveillance: Protocol

Management options overview
Treatment modalities:
- Active surveillance
- Preferred for low risk (category 1)
- Option for favorable intermediate risk
- Radical prostatectomy
- Open, laparoscopic, or robotic
- Consider nerve-sparing approach
- External beam radiation
- Conventional fractionation
- Moderate hypofractionation
- SBRT in selected patients
- Brachytherapy
- LDR or HDR monotherapy
Which option is best for our 65-year-old with Gleason 3+4=7 cancer?
Radical prostatectomy:
Patient selection
Ideal candidates:
- Life expectancy >10 years
- Good performance status
- No major comorbidities
- Acceptable surgical risk
- Motivated to undergo surgery
Relative contraindications:
- Prior extensive pelvic surgery
- Morbid obesity
- Large prostate size (>100g)
- Prior pelvic radiation
- Significant medical comorbidities
Special considerations:
- Patient preferences regarding side effects
- Surgeon experience/volume
- Nerve-sparing feasibility

Radical prostatectomy:
Patient selection
Surgical approach options:
- Open retropubic
- Robotic-assisted
- Laparoscopic
- Similar oncologic outcomes across approaches
Keys to successful surgery:
- Good surgical exposure
- Careful apex dissection
- Preservation of urethral sphincter
- Water-tight anastomosis
- Nerve-sparing when appropriate
Quality metrics:

Pelvic lymph node dissection ?
Current evidence:
- No proven therapeutic benefit in low/favorable intermediate risk
- Risk of lymph node involvement <5%
- NCCN guidelines: Not recommended
Supporting data:
- German trial (Steiner 2008)
- N=100 low/intermediate risk
- No positive nodes found
- SEER analysis (Bhuller 2020)
- No survival benefit
- Increased complications
Potential complications:
- Lymphocele
- Deep vein thrombosis
- Increased operative time
- Lymphedema

Radiation Treatment
Treatment planning guidelines
Dose options:
- Moderate hypofractionation:
- 60 Gy/20 fx (PROFIT/CHHiP)
- 70 Gy/28 fx (RTOG 0415)
- Conventional:
- 78-79.2 Gy/39-44 fx
Planning priorities:
- 95% of PTV to prescribed dose
- 99.5% of CTV to prescribed dose
- Minimize hot spots >107-115%
- Daily IGRT required

CT simulation and patient setup
Patient preparation:
- Bladder filling protocol
- Drink half a liter of water 30-60 min before
- Hold for simulation
- Bowel protocol
- Empty rectum before simulation
- Consider fleet enema
Setup:
- Supine position
- Custom immobilization
- Vacuum lock bag or comparable
- Consider fiducial markers
- At least 3 non-coplanar
- Wait >1 week post placement
Imaging:
- CT slice thickness ≤3mm
- Consider MRI fusion
- Consider spacer placement
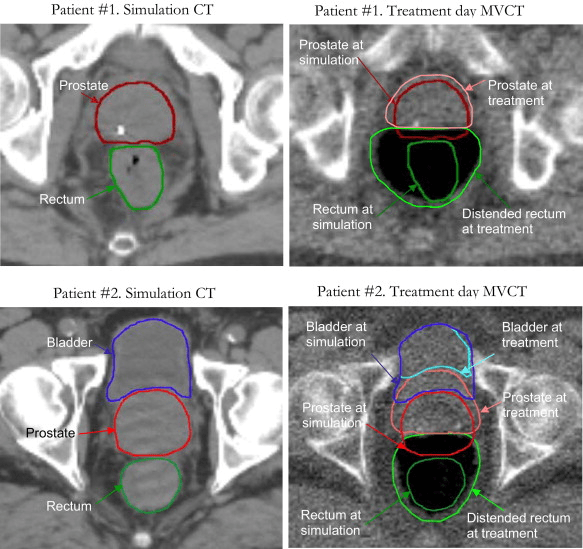
Target volumes and contouring guidelines
Target volumes:
- GTV = prostate only
- CTV determination:
- If SV risk >15% : prostate + proximal 1cm SV
- Otherwise: prostate only
- PTV margins:
- 7mm posterior (towards rectum)
- 10mm all other directions
- 5mm posterior reasonable off-trial
OAR delineation:
- Rectum: whole organ from ischial tuberosities to rectosigmoid junction
- Bladder: whole organ
- Penile bulb: best seen on sagittal view
- Femoral heads: to ischial tuberosities
Special considerations:
- Prostate apex at GU diaphragm
- MRI fusion helps delineation
- PROFIT used 3mm wall vs. whole organ for bladder

ADT in intermediate risk: Who benefits?
Unfavorable intermediate risk factors:
- Primary Gleason pattern 4
- ≥50% positive cores
- Multiple intermediate risk factors
Key evidence:
- D'Amico (DFCI):
- 6 months ADT improved OS in intermediate risk
- Gleason 7 showed most benefit
- PROFIT:
- Excellent outcomes without ADT
- Favorable intermediate only
- RTOG 0815:
- 79.2 Gy ± 6 months ADT
- Results pending
Current recommendations:
- Consider 4-6 months ADT for unfavorable
- Can omit for favorable intermediate
- Safe with hypofractionation
Short-term ADT: Duration & timing
Duration:
- 3 vs 6 months:
- No randomized comparison
- Most modern trials use 4-6 months
- Timing options:
- Neoadjuvant + concurrent
- Concurrent + adjuvant
- Similar outcomes in retrospective data
- Testosterone recovery:
- ~12 months after 6 months ADT
- Faster with shorter duration
PSA monitoring after treatment
After external beam RT:
- PSA q3-6 months for 5 years, then annually
-
Phoenix definition of failure:
- Nadir + 2 ng/mL
- Requires confirmation
- PSA bounce phenomenon:
- Common in years 1-2
- More common in younger patients
- Usually <2 ng/mL rise
- Returns to nadir within 6-12 months
After brachytherapy:
- Similar schedule to EBRT
- PSA decline more gradual
- Bounces more common
- Can take 3-5 years to reach nadir
Management of rise:
- Consider timing/magnitude
- Rule out bounce in first 2 years
- Consider imaging if confirmed failure
Evidence
Active surveillance: Key evidence
PROTECT Trial
Performed in the UK with the support the NHS
- 15-year outcomes comparing active monitoring vs. surgery vs. radiation
- No difference in prostate cancer mortality
- 15-year results:
- PCM: 2.2% vs. 1.5% vs. 2.1%
- Metastasis: 7.1% vs. 3.5% vs. 3.7%
- Overall mortality: Similar (~15%)


Study Design: PROTECT
15-year outcomes:
- Prostate cancer mortality
- Active monitoring: 2.2%
- Surgery: 1.5%
- Radiation: 2.1%
- No significant differences
- Distant metastases
- Active monitoring: 7.1%
- Surgery: 3.5%
- Radiation: 3.7%
- p<0.05 for both vs monitoring
- Overall mortality
- Similar across all arms (~15%)
Clinical progression at 10 years:
- Active monitoring: 20%
- Surgery: 5.9%
- Radiation: 6.6%


PROTECT trial: Quality of life outcomes
Urinary function:
- Surgery
- Worst incontinence (20% pad use)
- Persists long-term
- Radiation
- More irritative symptoms
- Peaks at 6 months
- Improves over time
Sexual function:
- Surgery
- 85% ED at 6 months
- Worst long-term function
- Radiation
- 74% ED at 6 months
- Improves after ADT cessation
- Active monitoring
- Natural age-related decline
Bowel function:
- Worse with radiation
- 6% bloody stools
- Minimal impact with surgery
Active surveillance: Additional evidence
Johns Hopkins Experience (Tosoian 2015)
- Very low risk cohort
- 15-year outcomes:
- Overall survival: 69%
- Cancer-specific survival: 99.9%
- Metastasis-free survival: 99.4%
- Grade reclassification: 31%
Moderate Hypofractionation
CHHiP trial: Study design
Patient population:
- 3216 patients
- T1b-T3aN0M0
- 15% low risk, 73% intermediate risk, 12% high risk
- PSA <30
Treatment arms:
- Conventional: 74 Gy/37 fx
- Hypofractionated: 60 Gy/20 fx
- Hypofractionated: 57 Gy/19 fx
Key aspects:
- Non-inferiority design
- Most patients received 3-6 months ADT
- Primary endpoint: biochemical/clinical failure
- Dose constraints:
- Rectum V20<85%, V30<57%, V40<38%
- Bladder V60<5%, V48.6<25%
CHHiP trial: Key results
Efficacy outcomes:
- 5-year biochemical control
- 74 Gy: 88%
- 60 Gy: 91% (non-inferior)
- 57 Gy: 86% (not non-inferior)
- 10-year outcomes
- BC: 76% vs 80% vs 73%
- OS: ~80% all arms
Toxicity:
- Acute effects peaked earlier with hypofractionation
- Late GI Grade 2+: ~12% all arms
- Late GU Grade 2+: 6.6-11.7%
Conclusions:
- 60 Gy/20 fx non-inferior to conventional
- 57 Gy/19 fx not recommended
- Similar late toxicity profile
Moderate Hypofractionation
PROFIT trial: Study design
Patient population:
- 1206 intermediate risk patients
- T1-T2
- No ADT allowed
Treatment arms:
- Conventional: 78 Gy/39 fx
- Hypofractionated: 60 Gy/20 fx
Important features:
- Non-inferiority design
- Strict dose constraints used
- Rectal wall constraints (not whole organ):
- D30% <46 Gy
- D50% <37 Gy
- Treatment time: 8 weeks vs 4 weeks
Primary endpoint:
- Biochemical-clinical failure
PROFIT trial: Key results
Efficacy:
- 5-year biochemical failure: 15% both arms
- Met non-inferiority endpoint
- No difference in patterns of failure
Toxicity:
- Acute GI:
- Worse with hypofractionation
- Resolved by 6 months
- Late toxicity:
- No difference in GI or GU
- Grade ≥2 GI: ~14% both arms
- Grade ≥2 GU: ~22% both arms
Key implications:
- Established 60 Gy/20 fx as standard option
- Important role of strict dose constraints
- Safe without ADT in intermediate risk


Moderate Hypofractionation
RTOG 0415: Study design
Patient population:
- 1092 low risk patients
- T1-2a
- PSA ≤10
- Gleason ≤6
Treatment arms:
- Conventional: 73.8 Gy/41 fx
- Hypofractionated: 70 Gy/28 fx
Protocol details:
- Non-inferiority design
- ~80% used IMRT
- Daily IGRT required
- No ADT allowed
- 5mm margins (3mm posterior)
Dose constraints:
- Rectum: V75<15%, V70<25%, V65<35%, V60<50%
- Bladder: V80<15%, V75<25%, V70<35%, V65<50%
- Femoral heads: V50<5%
RTOG 0415:
RTOG 0415: Outcomes
Efficacy:
- 5-year disease-free survival
- Conventional: 85%
- Hypofractionated: 86%
- Met non-inferiority endpoint
- 12-year outcomes
- DFS: 56% vs 62%
- BF: 83% vs 90%
- No OS difference
Toxicity:
- Late grade ≥2 GI: 15.4% vs 23.8%
- Late grade ≥2 GU: 26.8% vs 33.4%
- Late grade ≥3 GI: 3.2% vs 4.4%
- Late grade ≥3 GU: 3.4% vs 4.4%
Patient-reported outcomes:
- No difference in:
- EPIC GI/GU domains
- Sexual function
- Anxiety/depression

Brachytherapy Boost
ASCENDE-RT Trial: Study Design
Inclusion Criteria:
- Population: Intermediate- or high-risk prostate cancer (NCCN-defined).
- Intermediate-risk: T2b-T2c, PSA 10-20 ng/mL, or Gleason Score = 7.
- High-risk: T3a+, PSA >20 ng/mL, or Gleason Score ≥8.
- Performance Status: ECOG 0-2.
- Eligible for Treatment: Whole-pelvis radiation therapy + ADT + either DE-EBRT or LDR-PB.
- Informed Consent: Signed before enrollment.
Exclusion Criteria:
- Disease Characteristics:
- T3b+ disease, PSA >40 ng/mL.
- Prior pelvic RT or surgery (e.g., TURP).
- Prostate volume >75 cm³ post-ADT.
- Health Factors: Ineligibility for anesthesia or contraindications to ADT/RT.
- Advanced Disease: Evidence of nodal or distant metastases on imaging.
ASCENDE-RT Trial: Results
Clinical Implications:
- Benefits: Significant improvement in biochemical control with LDR-PB.
- Challenges: Increased GU toxicity and no significant difference in OS, CSS, or DMFS.
- LDR-PB may be suitable for patients prioritizing biochemical control and delaying ADT.
- Alternative modalities, such as SBRT or HDR, offer reduced toxicity with similar survival outcomes.
| Endpoint | LDR-PB | DE-EBRT | p-value |
|---|---|---|---|
| 10-Year bPFS | 85% | 67% | < 0.001 |
| 10-Year DMFS | 88% | 86% | 0.56 |
| 10-Year OS | 80% | 75% | 0.51 |
| 10-Year CSS | 95% | 92% | 0.26 |

ASCENDE-RT Trial: Results
Toxicity Findings:
- Genitourinary (GU): 5-year grade 3 GU toxicity: 18% (LDR-PB) vs. 5% (DE-EBRT), p < 0.001.
- Gastrointestinal (GI): 5-year grade 3 GI toxicity: 8% (LDR-PB) vs. 3% (DE-EBRT), p = 0.12.
- Erectile Function: Preservation at 5 years: 45% (LDR-PB) vs. 37% (DE-EBRT), p = 0.30.

LDR brachytherapy: Evidence base
MDACC experience (Frank 2018):
- 300 intermediate risk patients
- Technical details:
- I-125: 145 Gy
- Pd-103: 125 Gy
- Cs-131: 115 Gy
- Outcomes:
- 5-year biochemical PFS: 97.3%
- 5-year overall survival: 95%
- Late grade 3 GU: 4 patients
- Late grade 3 rectal: 2 patients
- No grade 4-5 events
RTOG 9805:
- 101 low risk patients
- I-125 monotherapy
- 5-year outcomes:
- Biochemical failure: 6%
- Overall survival: 97%
- Grade 3 acute: 8 patients
- Grade 3 late GU: 2 patients
HDR monotherapy: Current evidence
Key advantages:
- Optimal dose distribution
- Real-time planning
- No seed migration
- Reduced radiation exposure
Common regimens:
- 27 Gy/2 fx
- 19 Gy/1 fx (not recommended)
- 31.5 Gy/3 fx
- Similar biologic doses to LDR
Available data:
- Mount Vernon experience:
- Phase II data
- Low toxicity rates
- Good early biochemical control
- William Beaumont single fraction:
- 19% local failure
- Higher than expected
- Single fraction not recommended
Limitations:
- Limited long-term data
- Multiple procedures for multi-fraction
- Resource intensive
Treatment Options for Low & Favorable Intermediate Risk Prostate Cancer - Part 1
By RadMedSkiier
Treatment Options for Low & Favorable Intermediate Risk Prostate Cancer - Part 1
Evidence-based review of management options - surgical management and early trials
- 78



