Fibroblastic Reticular Cells in Secondary Lymphoid Organs
Mechthild Lütge
Institute of Immunobiology
Kantonsspital St.Gallen, Switzerland
Lymph Nodes
Spleen
Peyer's patches
Secondary Lymphoid organs (SLO)
Dedicated sites where adaptive immunity is mounted to pathogens in the lymph, blood or intestine
Fibroblastic reticular cells orchestrate SLO organization
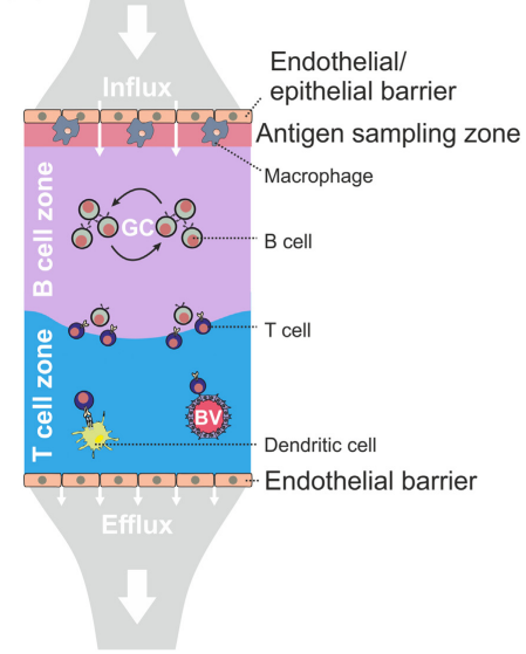
Acton et al. Trends in Immunology, 2021
Fibroblastic reticular cells orchestrate SLO organization

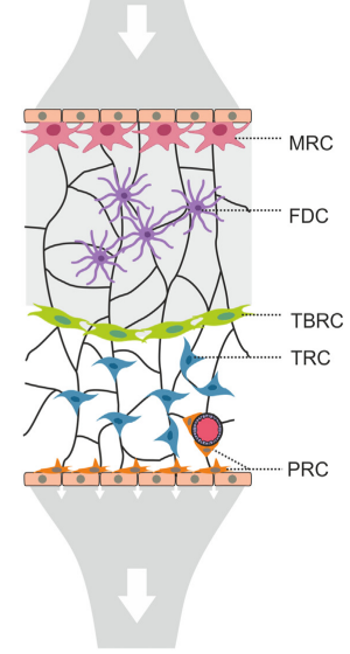
Acton et al. Trends in Immunology, 2021
Fibroblastic reticular cells orchestrate SLO organization
Fibroblastic reticular cells (FRC):
- provide structural foundation
- guide cellular interactions
- provide signals for immune cell survival, activation and differentiation


Acton et al. Trends in Immunology, 2021
Fibroblastic reticular cells orchestrate SLO organization
Fibroblastic reticular cells (FRC):
- provide structural foundation
- guide cellular interactions
- provide signals for immune cell survival, activation and differentiation


→ Decision making process (strength and specificity of immune response)
Acton et al. Trends in Immunology, 2021
Fibroblastic reticular cells orchestrate SLO organization
To what extend are FRC underpinned niches functionally conserved across:

Fibroblastic reticular cells orchestrate SLO organization
To what extend are FRC underpinned niches functionally conserved across:
(1.) SLOs?

Fibroblastic reticular cells orchestrate SLO organization
To what extend are FRC underpinned niches functionally conserved across:
(1.) SLOs?
(2.) Species?


Fibroblastic reticular cells orchestrate SLO organization
To what extend are FRC underpinned niches functionally conserved across:
(1.) SLOs?
(2.) Species?

→ What factors shape FRC subset identity and function?


Fibroblastic reticular cells orchestrate SLO organization
- Conserved stromal–immune cell circuits secure B cell homeostasis and function
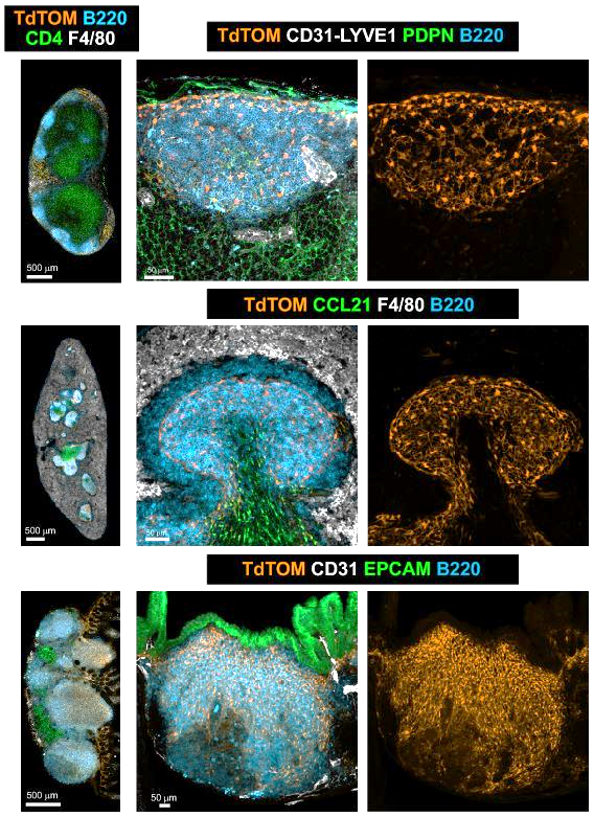
- PI16+ reticular cells support inflammation-induced remodeling in human lymph nodes
B cell zone reticular cells direct efficient humoral immunity
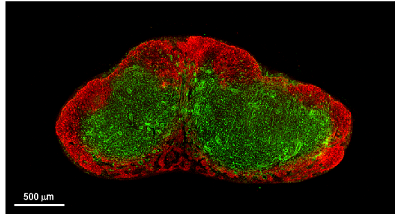
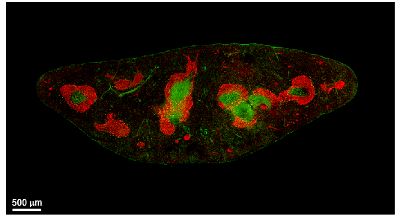
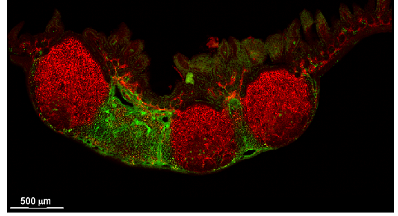
CXCL13 CCL19/CCL21
Peyer's patch
Spleen
Lymph node
CXCL13+ FRC = B cell zone reticular cells (BRCs)
B cell zone reticular cells direct efficient humoral immunity



CXCL13 CCL19/CCL21
Peyer's patch
Spleen
Lymph node
CXCL13+ FRC = B cell zone reticular cells (BRCs)
B cell zone reticular cells direct efficient humoral immunity
-
to what extend are BRC underpinned niches functionally conserved across SLO?



CXCL13 CCL19/CCL21
Peyer's patch
Spleen
Lymph node

CXCL13+ FRC = B cell zone reticular cells (BRCs)
B cell zone reticular cells direct efficient humoral immunity
-
to what extend are BRC underpinned niches functionally conserved across SLO?
-
Systemic humoral immunity?



CXCL13 CCL19/CCL21
Peyer's patch
Spleen
Lymph node

CXCL13+ FRC = B cell zone reticular cells (BRCs)
B cell zone reticular cells direct efficient humoral immunity
-
to what extend are BRC underpinned niches functionally conserved across SLO?
-
Systemic humoral immunity?
-
What are major pathways controlling BRC-immune cell interactions?



CXCL13 CCL19/CCL21
Peyer's patch
Spleen
Lymph node

CXCL13+ FRC = B cell zone reticular cells (BRCs)
B cell zone reticular cells direct efficient humoral immunity
-
to what extend are BRC underpinned niches functionally conserved across SLO?
-
Systemic humoral immunity?
-
What are major pathways controlling BRC-immune cell interactions?
-
Are these interactions functionally redundant across SLOs?



CXCL13 CCL19/CCL21
Peyer's patch
Spleen
Lymph node

CXCL13+ FRC = B cell zone reticular cells (BRCs)
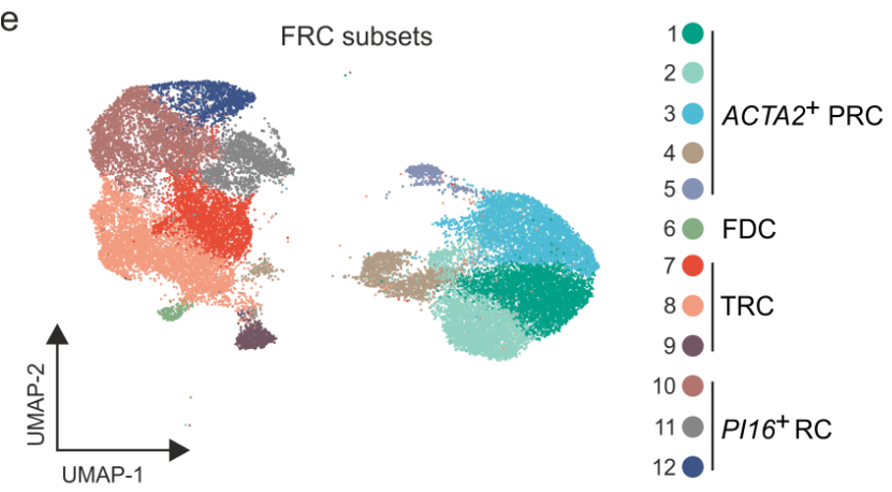
Shared B cell follicle and PI16+ BRC subset identity across SLOs
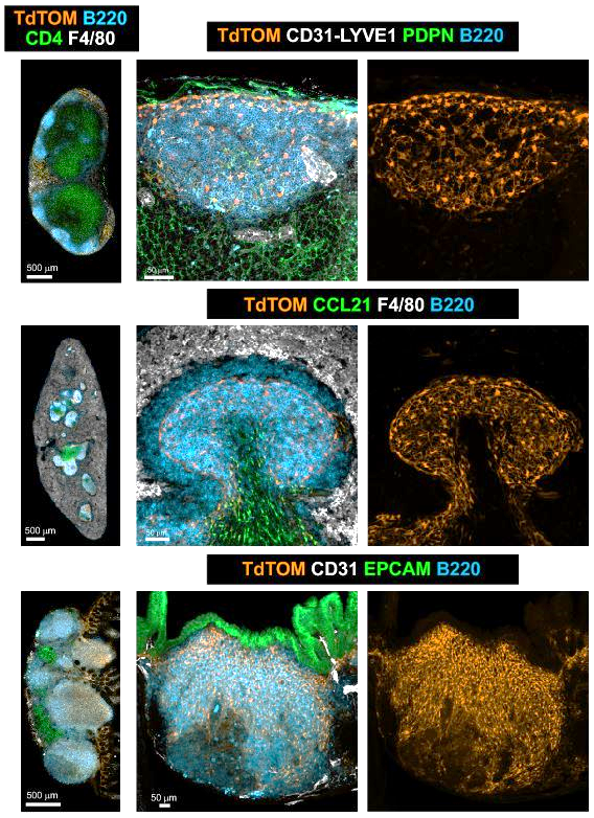

Onder L et al., Immunity, 2017
Lütge et al. Nat. Immunol., 2023
Shared B cell follicle and PI16+ BRC subset identity across SLOs


Onder L et al., Immunity, 2017
Lütge et al. Nat. Immunol., 2023
Shared B cell follicle and PI16+ BRC subset identity across SLOs



Onder L et al., Immunity, 2017
Lütge et al. Nat. Immunol., 2023
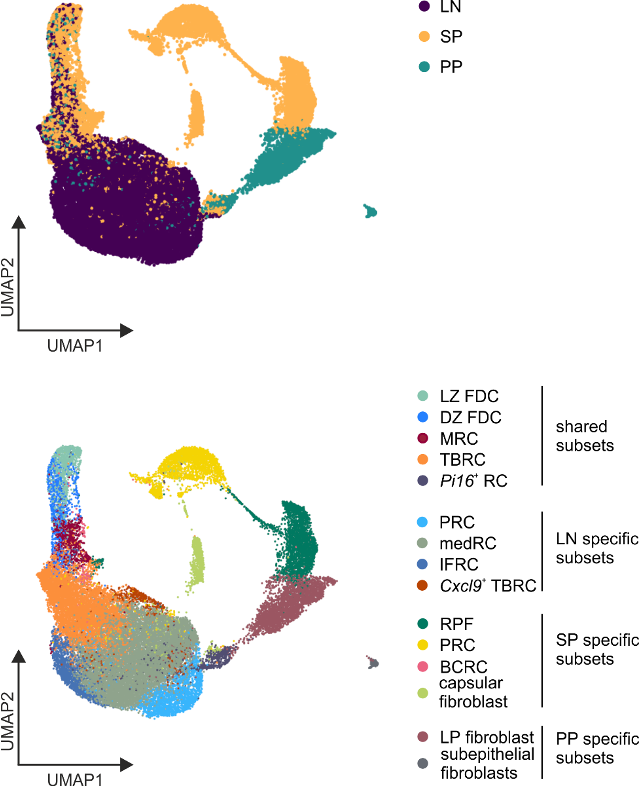
Developmental and anatomical gene sets imprint BRC identity
Lütge et al. Nat. Immunol., 2023
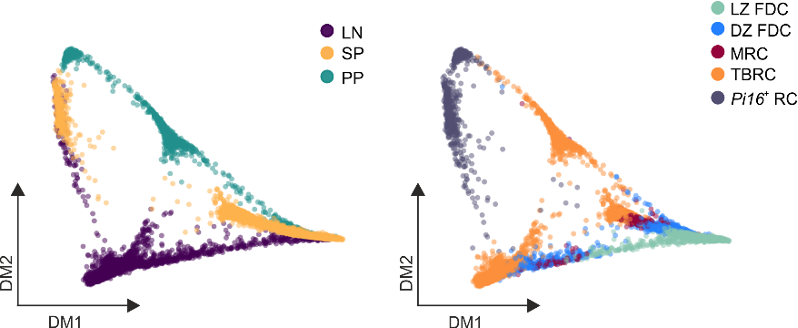
Developmental and anatomical gene sets imprint BRC identity
Lütge et al. Nat. Immunol., 2023


Organ
Developmental and anatomical gene sets imprint BRC identity
Lütge et al. Nat. Immunol., 2023


Organ
Subset identity
Developmental and anatomical gene sets imprint BRC identity
Lütge et al. Nat. Immunol., 2023


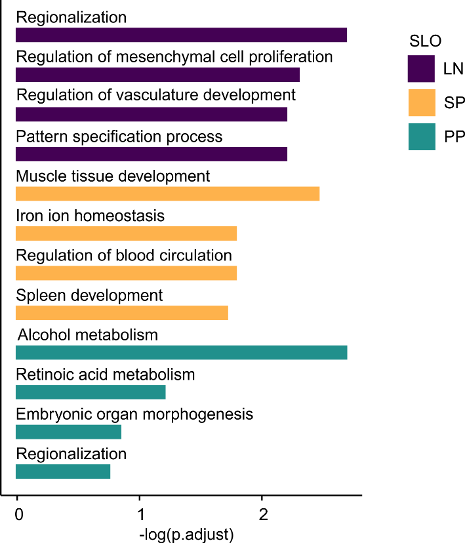
→ Organ-specific gene sets reflect developmental and anatomical imprints
Subset identity
Organ
Niche factors and signaling pathways define subset identity and function
Lütge et al. Nat. Immunol., 2023
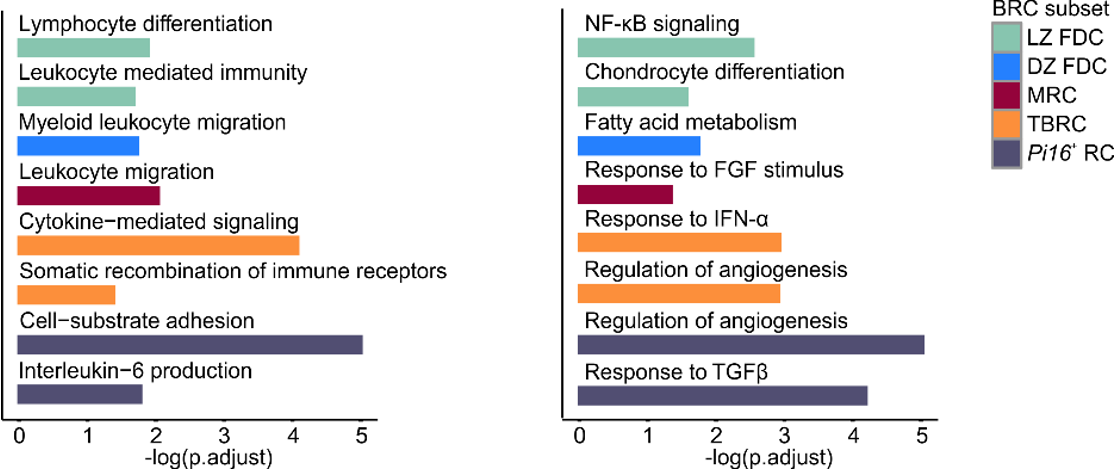
Subset-specific niche factors
Subset-specific signaling pathways
Niche factors and signaling pathways define subset identity and function
Lütge et al. Nat. Immunol., 2023
→ Subset-specific gene sets that are consistently found across SLOs point to BRC modulation by immune cells

Subset-specific niche factors
Subset-specific signaling pathways
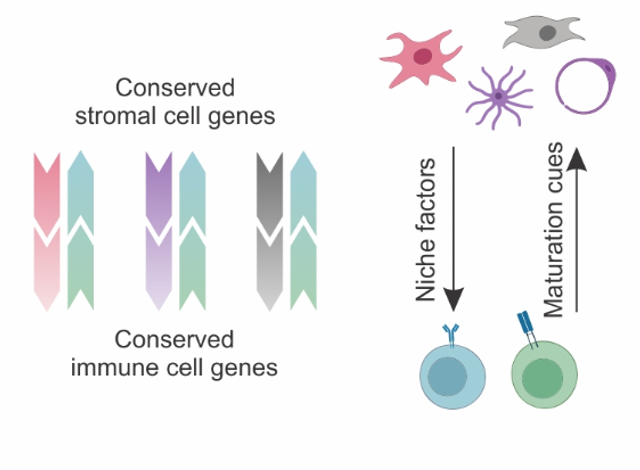
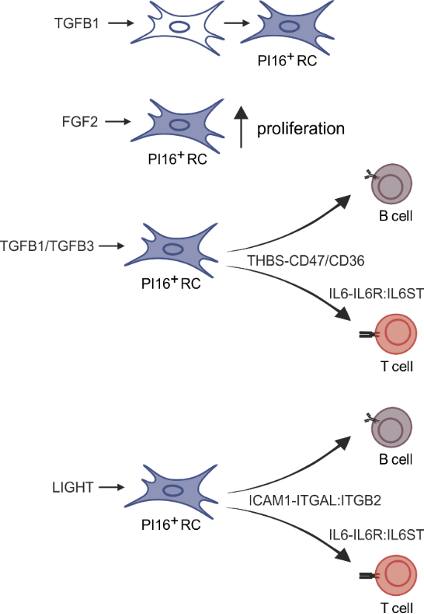
Conserved feedforward BRC-immune cell circuits sustain functional BRC niches
Lütge et al. Nat. Immunol., 2023
→ BRC-derived niche factors determine immune cell function
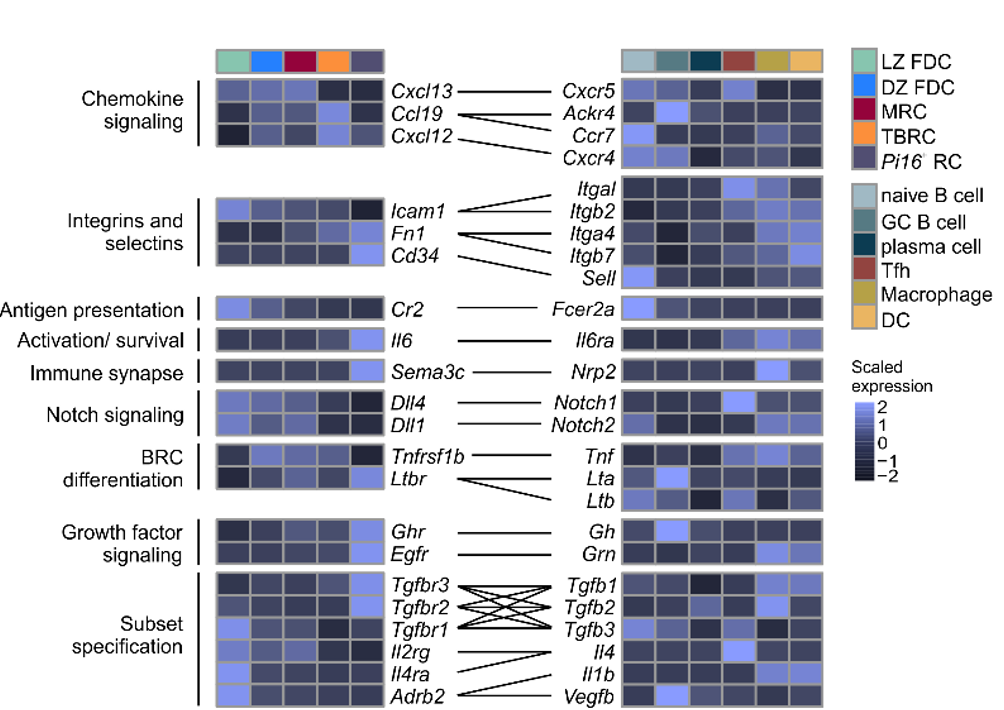
Conserved feedforward BRC-immune cell circuits sustain functional BRC niches
Lütge et al. Nat. Immunol., 2023
→ BRC-derived niche factors determine immune cell function
→ Leukocyte-derived maturation factors specify BRC subset identity
→ Conserved in humans


Conserved feedforward BRC-immune cell circuits sustain functional BRC niches
Lütge et al. Nat. Immunol., 2023
→ BRC-derived niche factors determine immune cell function
→ Leukocyte-derived maturation factors specify BRC subset identity
→ Conserved in humans

→ Validation?

Immune cell-derived maturation cues drive BRC differentiation and activation
Lütge et al. Nat. Immunol., 2023
In-vitro stimulation of CD45-CD31-EYFP+ cells:
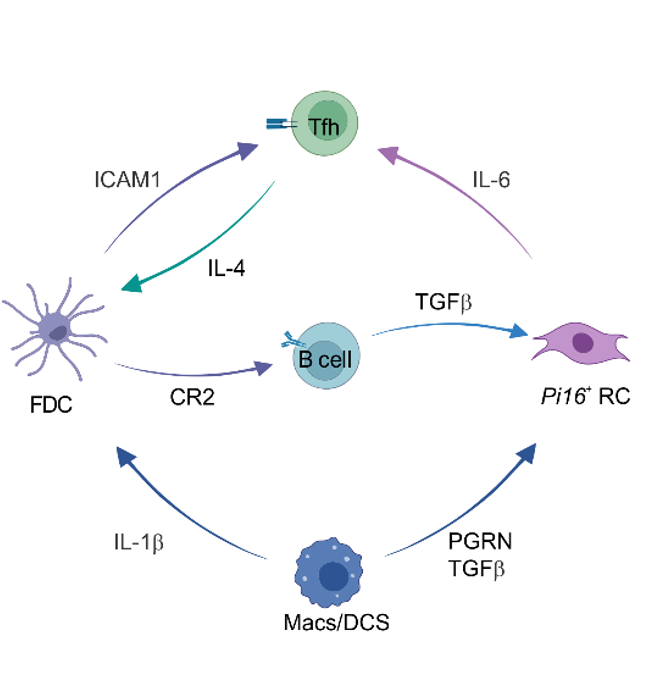
→ PGRN, TGFb, IL-4 and VEGF-B drive BRC differentiation
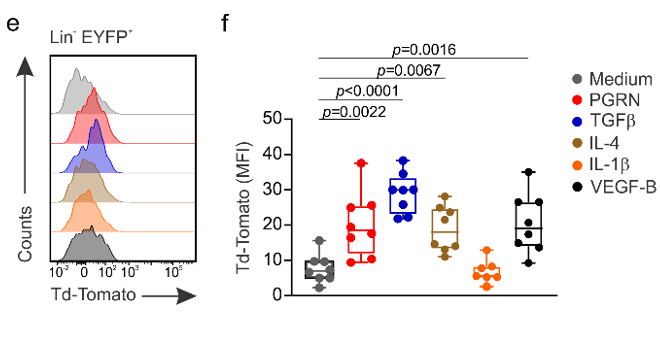


Immune cell-derived maturation cues drive BRC differentiation and activation
Lütge et al. Nat. Immunol., 2023
In-vivo stimulation of lymph node FRC:
→ IL-1b drives FDC subset specification

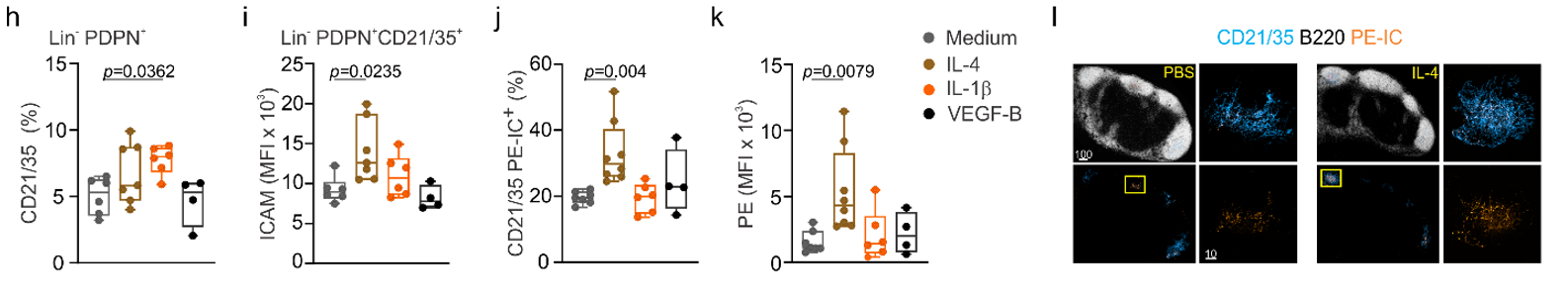

Immune cell-derived maturation cues drive BRC differentiation and activation
Lütge et al. Nat. Immunol., 2023
In-vivo stimulation of lymph node FRC:
→ IL-1b drives FDC subset specification
→ IL-4 shapes FDC function and activation





Immune cell-derived maturation cues drive BRC differentiation and activation
Lütge et al. Nat. Immunol., 2023

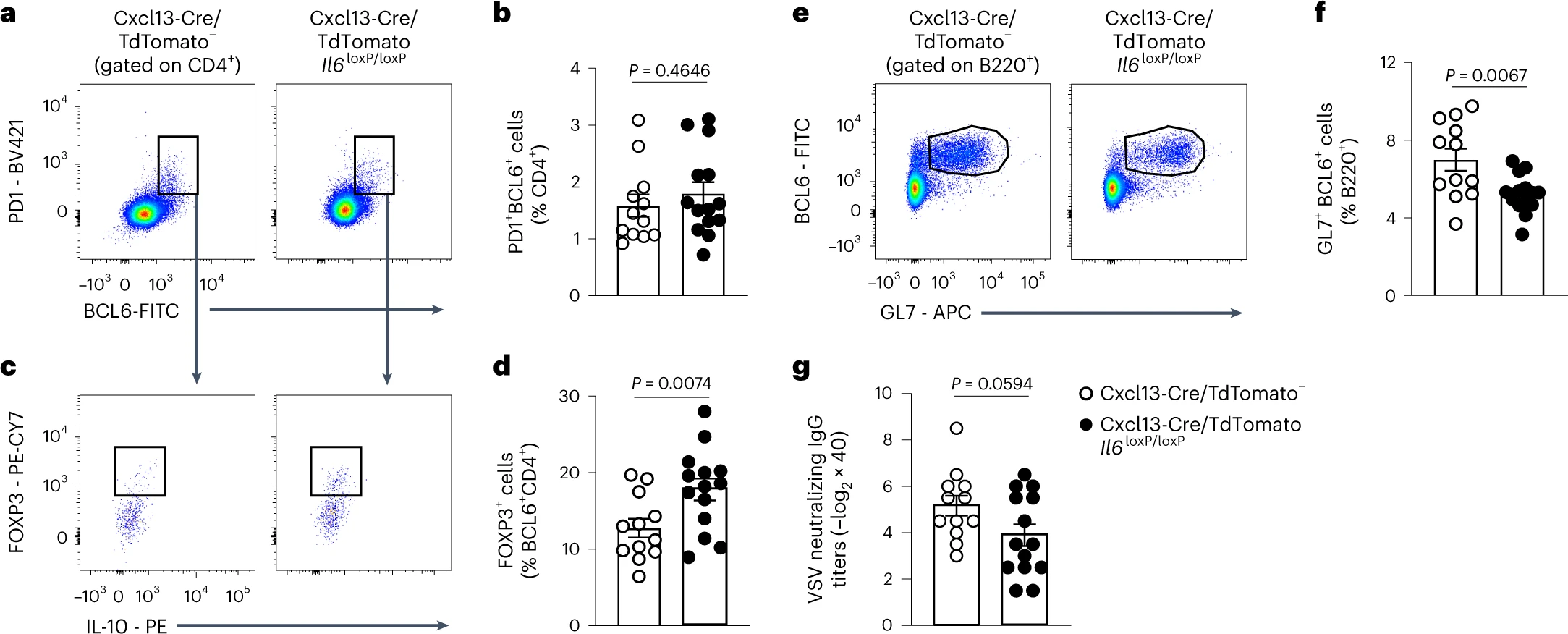
VSV-infected Cxcl13-Cre/TdTomato− or Cxcl13-Cre/TdTomato Il6loxP/loxP mice:
→ Cxcl13-Cre-provided Il6 controls TFH differentiation
Immune cell-derived maturation cues drive BRC differentiation and activation
VSV-infected Cxcl13-Cre/TdTomato− or Cxcl13-Cre/TdTomato Il6loxP/loxP mice:
→ Cxcl13-Cre-provided Il6 controls TFH differentiation to sustain GC responses



Immune cell-derived maturation cues drive BRC differentiation and activation
Lütge et al. Nat. Immunol., 2023
FDC
B cell
PI16+RC
Mph/DC
T cell
Immune cell-derived maturation cues drive BRC differentiation and activation
Lütge et al. Nat. Immunol., 2023

FDC
B cell
PI16+RC
Mph/DC
T cell
CR2
ICAM1
IL6
Immune cell-derived maturation cues drive BRC differentiation and activation
Lütge et al. Nat. Immunol., 2023

CR2
IL1b
IL4
ICAM1
TGFb
PGRN
TGFb
FDC
B cell
PI16+RC
Mph/DC
T cell
IL6
Immune cell-derived maturation cues drive BRC differentiation and activation
Lütge et al. Nat. Immunol., 2023

Summary - 1: Advanced understanding of systemic humoral immunity
Lütge et al. Nat. Immunol., 2023
Lymph node
Spleen
Peyer's patch
Organ-specific imprints
Summary - 1: Advanced understanding of systemic humoral immunity
Lütge et al. Nat. Immunol., 2023
Lymph node
Spleen
Peyer's patch
Organ-specific imprints

Functional convergence
Summary - 1: Advanced understanding of systemic humoral immunity
Lütge et al. Nat. Immunol., 2023
Lymph node
Spleen
Peyer's patch
Organ-specific imprints


Functional convergence
Feedforward paradigm: circulating immune cell imprint B cell follicle niches in an organ indiscriminate manner thereby securing efficient systemic humoral immunity
Fibroblastic reticular cells orchestrate SLO organization
To what extend are FRC underpinned niches functionally conserved across:
(1.) SLOs?
(2.) Species?

→ What factors shape FRC subset identity and function?


Fibroblastic reticular cells orchestrate SLO organization
To what extend are FRC underpinned niches functionally conserved across:
(1.) SLOs?
(2.) Species?

→ What factors shape FRC subset identity and function?


Fibroblastic reticular cells orchestrate SLO organization
- Conserved stromal–immune cell circuits secure B cell homeostasis and function


- PI16+ reticular cells support inflammation-induced remodeling in human lymph nodes
Repeated lymph node expansion and contraction throughout life
- Rapid expansion allows massive lymphocyte entry and proliferation
- Structural and functional tissue integrity is preserved
- FRC stretching and proliferation regulate network tension
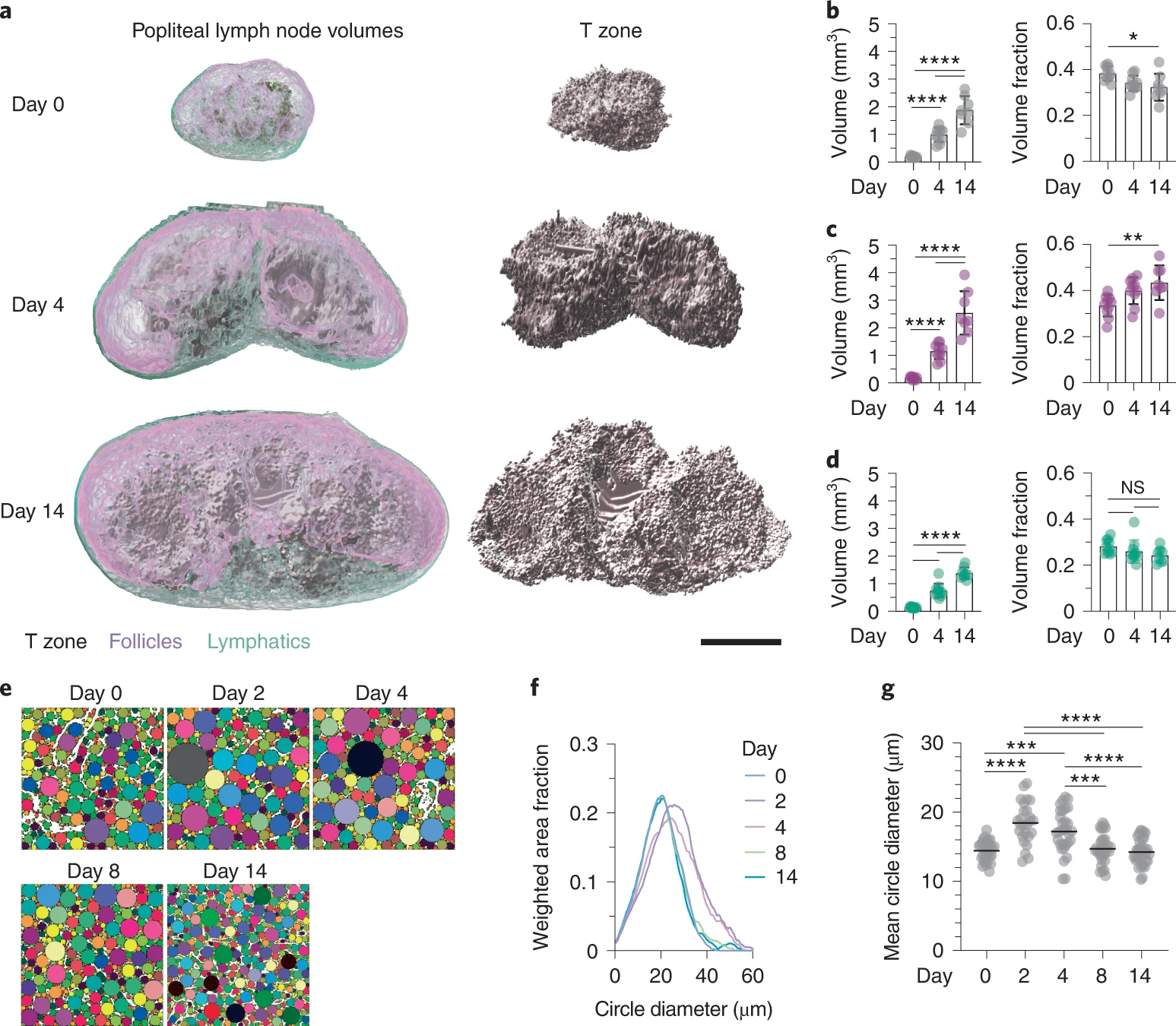
Assen et al. Nat. Immunol., 2022
Repeated lymph node expansion and contraction throughout life
- Rapid expansion allows massive lymphocyte entry and proliferation
- Structural and functional tissue integrity is preserved
- FRC stretching and proliferation regulate network tension

Assen et al. Nat. Immunol., 2022
→ How does repeated expansion and contraction in response to immunological stimuli shape the FRC network in human lymph nodes?
Repeated lymph node expansion and contraction throughout life
- Rapid expansion allows massive lymphocyte entry and proliferation
- Structural and functional tissue integrity is preserved
- FRC stretching and proliferation regulate network tension
→ How does repeated expansion and contraction in response to immunological stimuli shape the FRC network in human lymph nodes?
→ Stereotypic "resting" lymph node?

Assen et al. Nat. Immunol., 2022
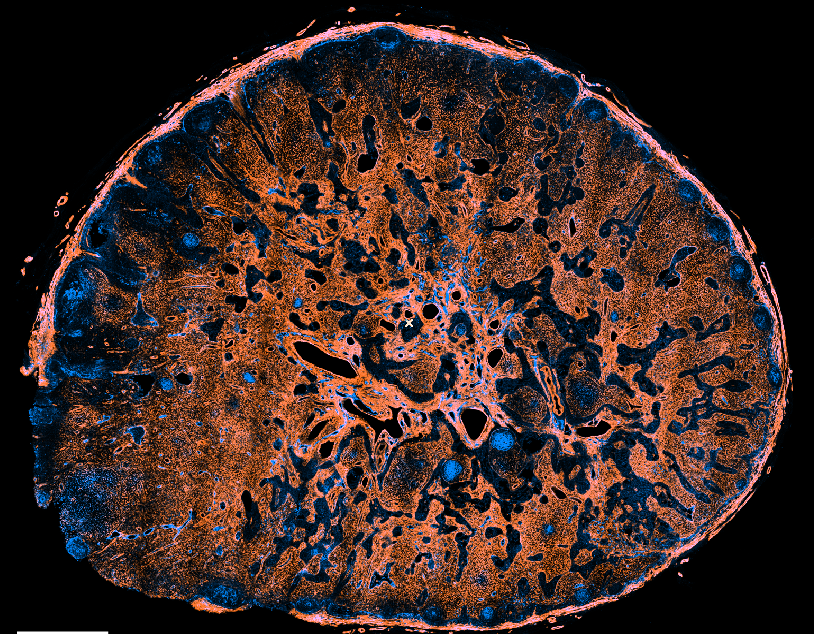
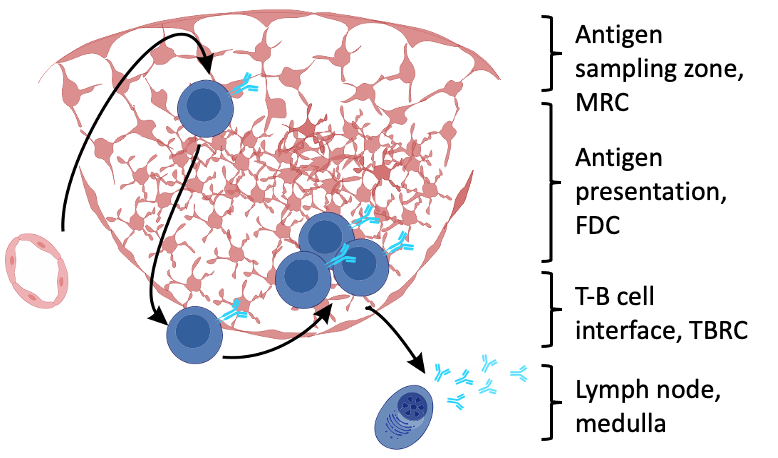
A detailed characterization of the human lymph node FRC landscape is lacking

The FRC landscape in human palatine tonsils
-
No marginal zone reticular cells (MRCs) in palatine tonsils; antigen sampling occurs in the “reticulated” epithelium
De Martin et al. Nat. Immunol., 2023
The FRC landscape in human palatine tonsils
-
No marginal zone reticular cells (MRCs) in palatine tonsils; antigen sampling occurs in the “reticulated” epithelium
-
Subepithelial PI16+ reticular cells form a distinct niche show the strongest inflammation induced remodeling
-
PI16+ reticular cells integrate immune cell-derived signals and govern T cell activation
De Martin et al. Nat. Immunol., 2023


The FRC landscape in human palatine tonsils
-
No marginal zone reticular cells (MRCs) in palatine tonsils; antigen sampling occurs in the “reticulated” epithelium
-
Subepithelial PI16+ reticular cells form a distinct niche show the strongest inflammation induced remodeling
-
PI16+ reticular cells integrate immune cell-derived signals and govern T cell activation
De Martin et al. Nat. Immunol., 2023




→ Are there PI16+ reticular cells in human lymph nodes? Location? Response to inflammation?
Patient cohort – clinically non-inflamed ("resting") cervical lymph nodes
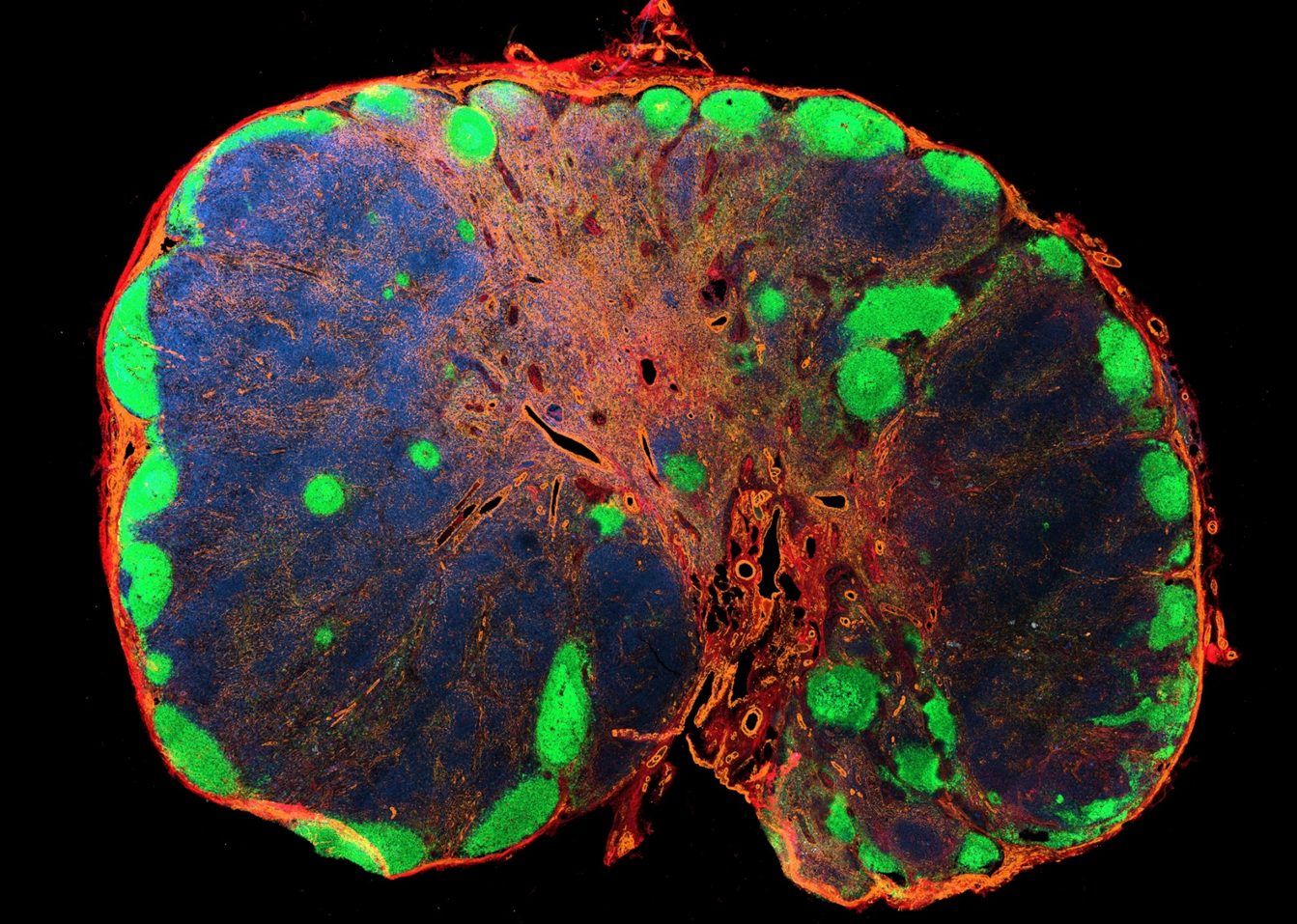
Baseline immunoanatomy of "resting" human lymph nodes
Baseline immunoanatomy of "resting" human lymph nodes

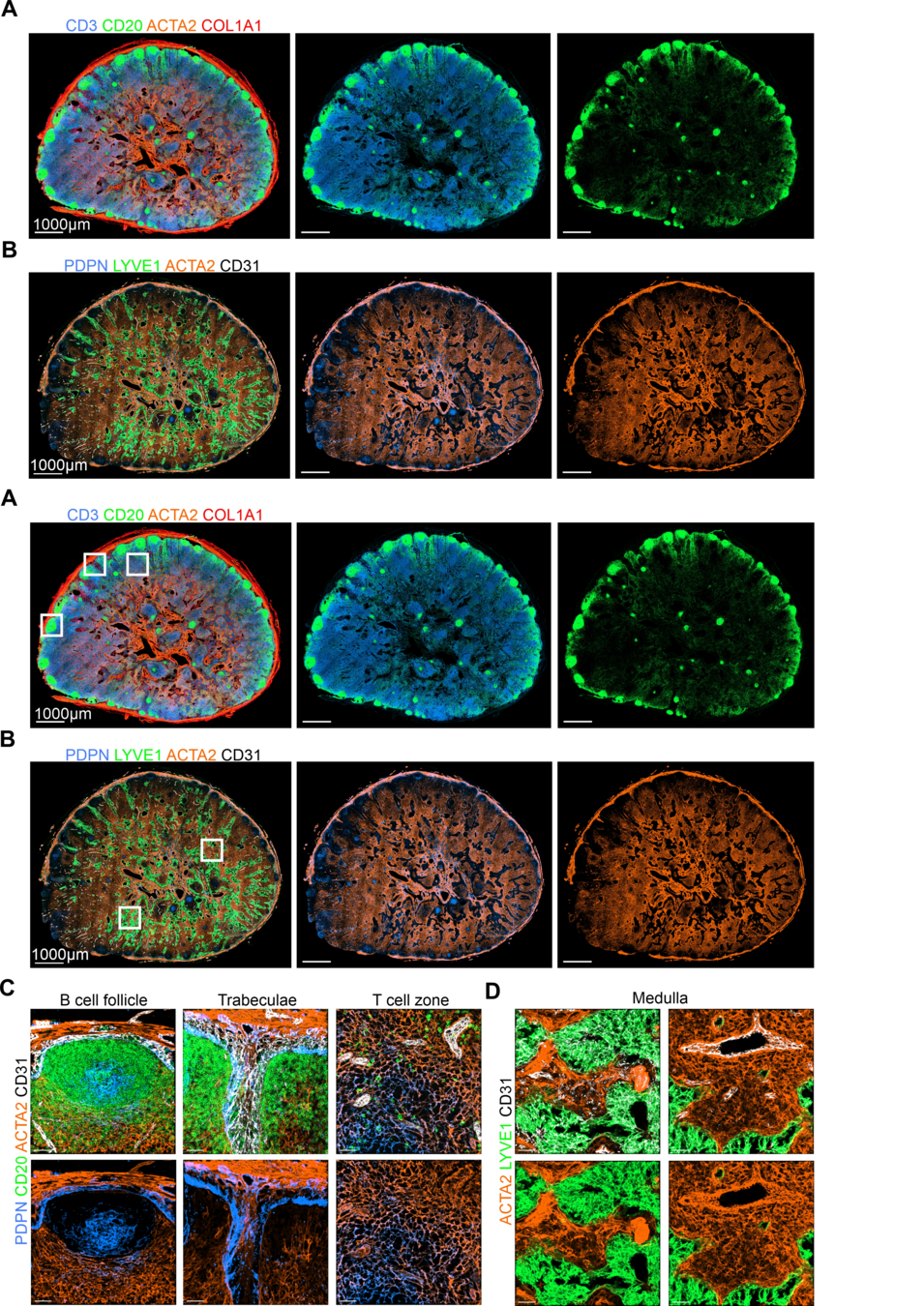
Baseline immunoanatomy of "resting" human lymph nodes

-
Extensive vasculature with a large perivascular space
-
PDPN high B cell follicles/ PDPN low T cell zone
-
B cell follicle are positioned throughout the LN
Baseline immunoanatomy of "resting" human lymph nodes

Baseline immunoanatomy of "resting" human lymph nodes
-
Extensive vasculature with a large perivascular space
-
PDPN high B cell follicles/ PDPN low T cell zone
-
B cell follicle are positioned throughout the LN
Transcriptome analysis - Patient characteristics
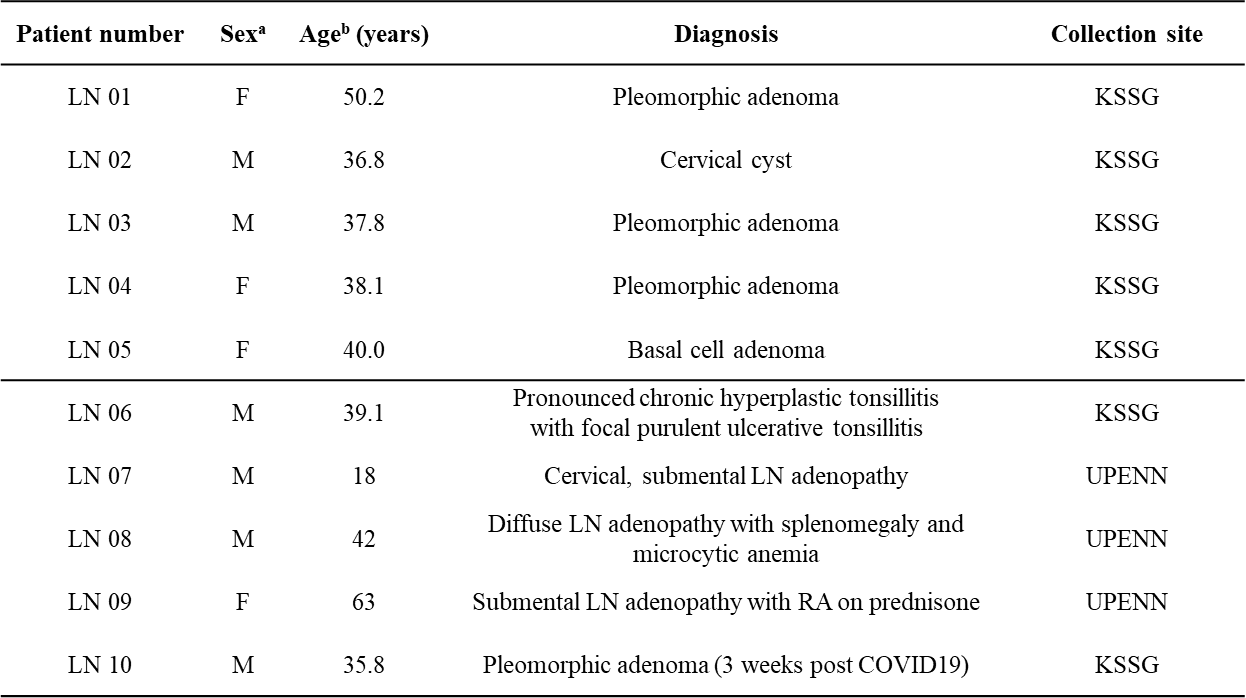
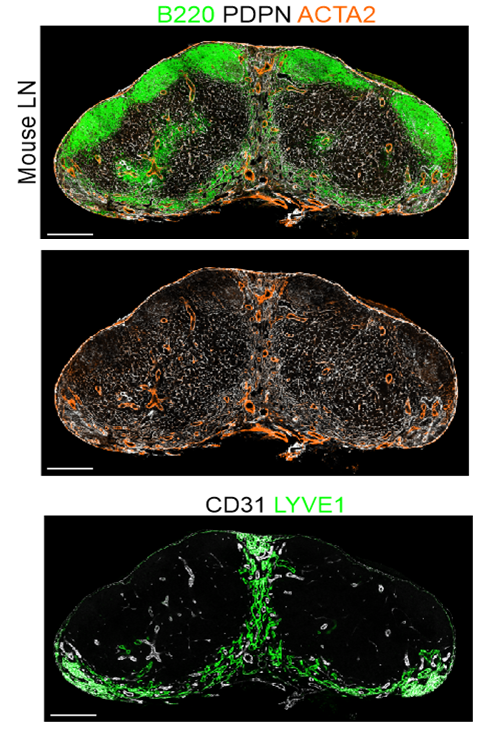
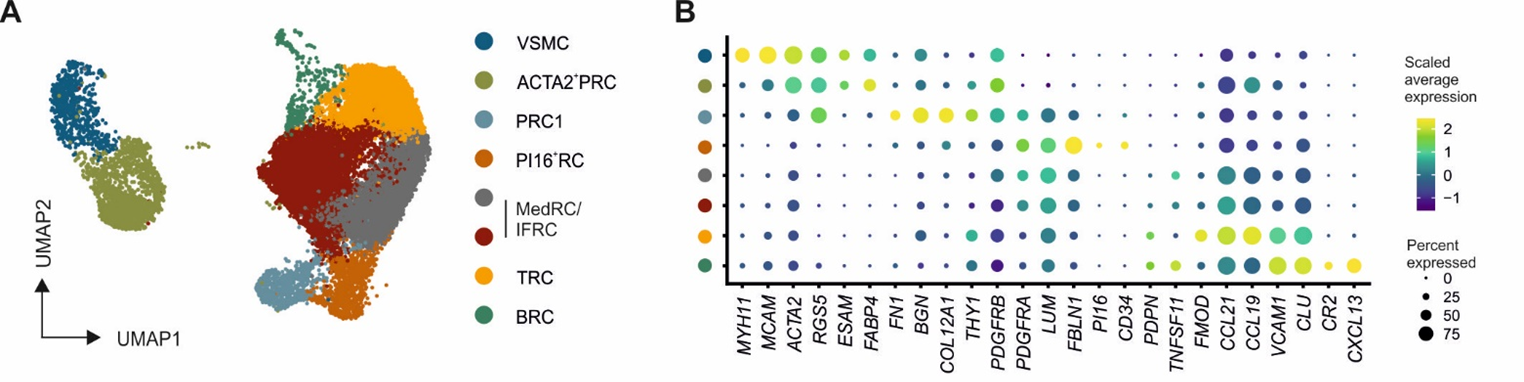
Distinct FRC subsets form the perivascular niche in human lymph nodes
-
Strong overlap with FRC landscape in human tonsils
-
Distinct perivascular FRC subsets
-
PI16+ reticular cells
→ Localization?
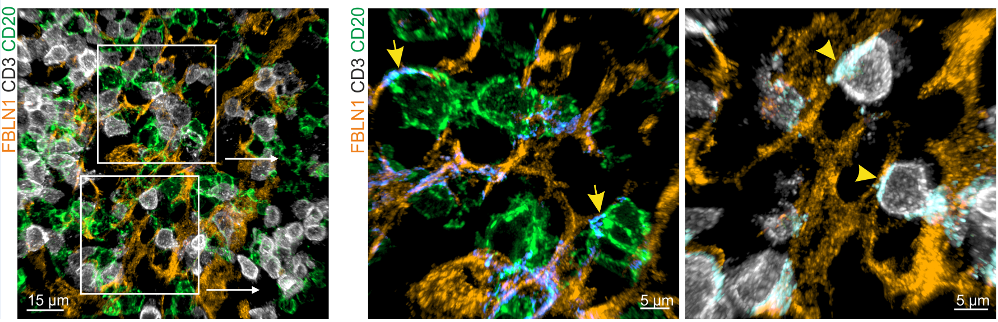
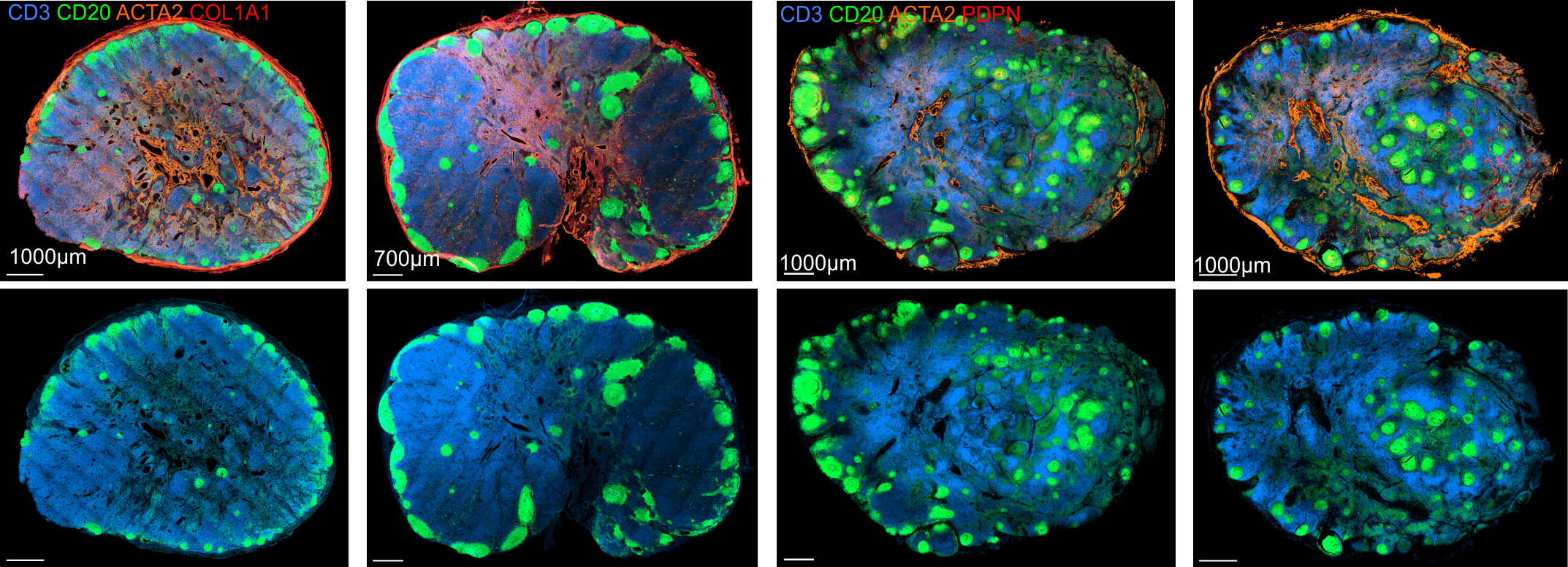
Distinct FRC subsets form the perivascular niche in human lymph nodes

Distinct FRC subsets form the perivascular niche in human lymph nodes



PI16+RC co-localize with plasma cells. Extrafollicular B cell guidance? Plasma cell survival?

Transcriptome analysis - Patient characteristics
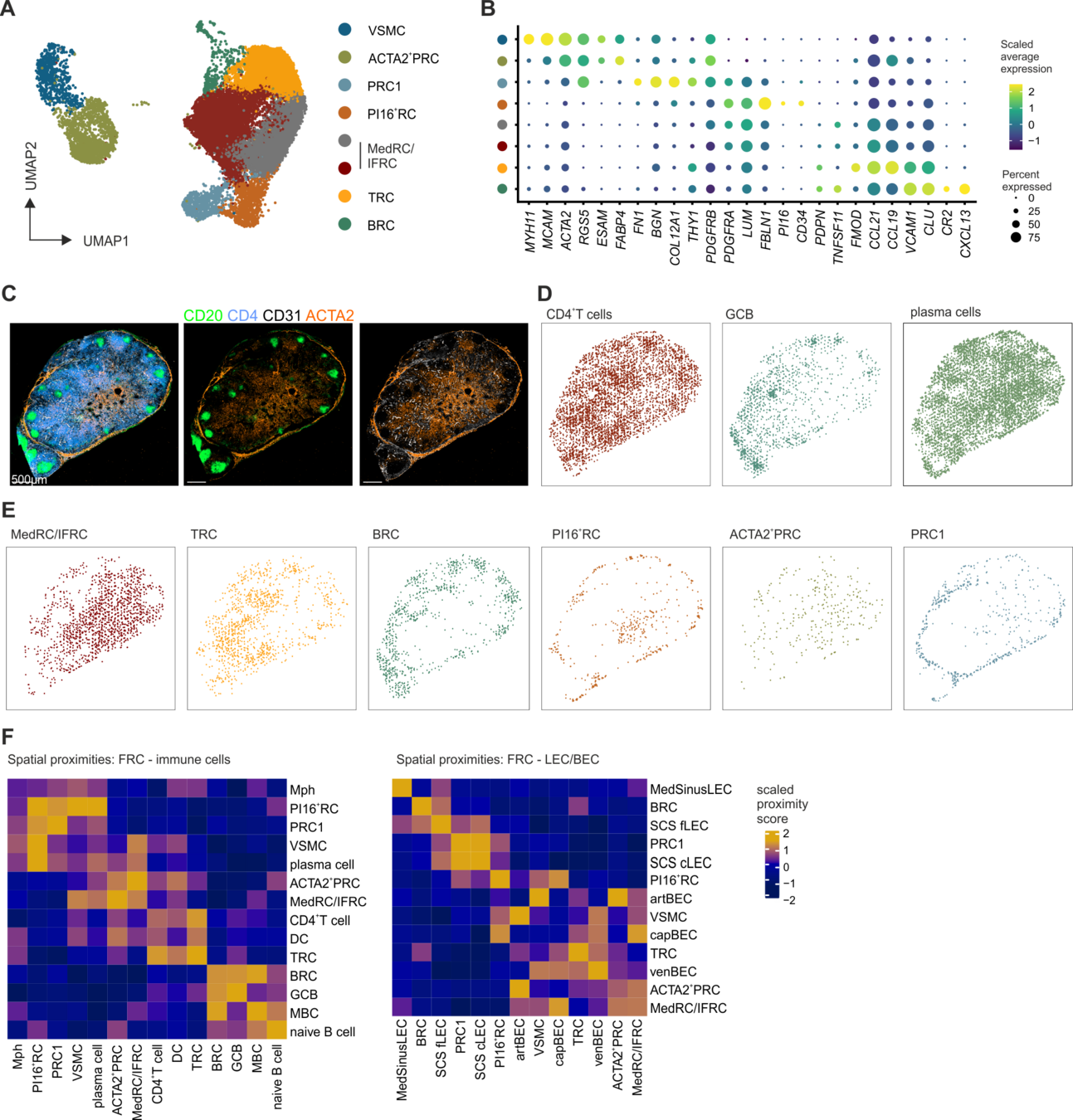
PI16+ RCs support inflammation-induced remodeling in human lymph nodes
-
All FRC subset are conserved upon chronic activation
PI16+ RCs support inflammation-induced remodeling in human lymph nodes


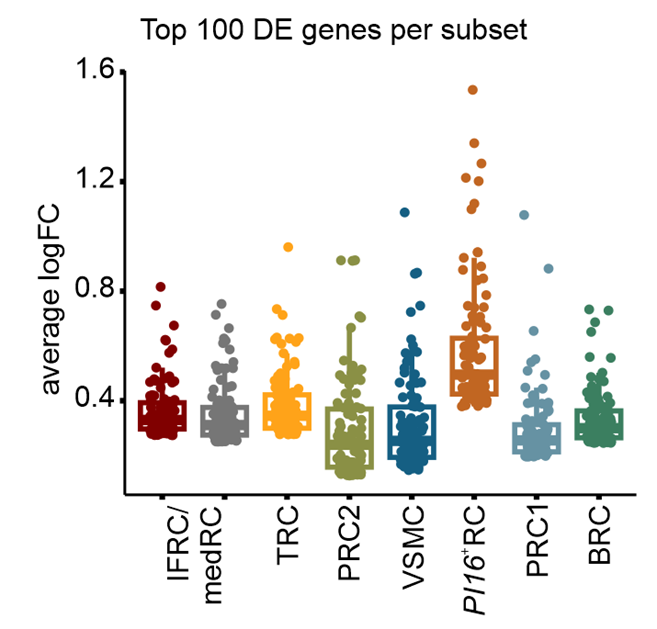
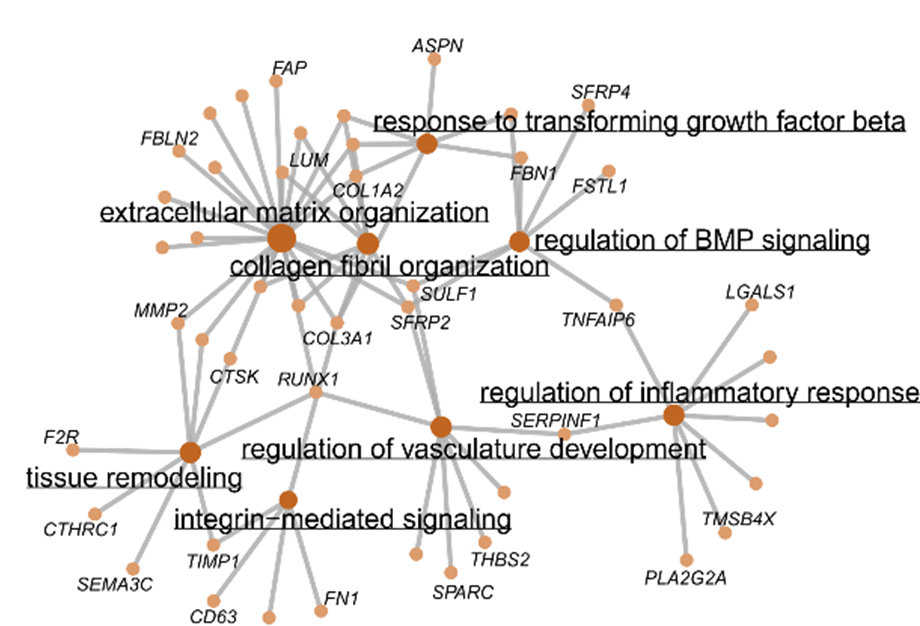
Summary II: Fibroblastic reticular cells in human lymph nodes
Stereotypic «resting» human lymph node:
- extensive vasculature with a large perivascular space formed by distinct FRC subsets
- large number of B cell follicles positioned throughout the lymph node

Summary II: Fibroblastic reticular cells in human lymph nodes
Stereotypic «resting» human lymph node:
- extensive vasculature with a large perivascular space formed by distinct FRC subsets
- large number of B cell follicles positioned throughout the lymph node
PI16+ reticular cells:
- PI16+ RCs are located in the interfollicular and medullary region
- PI16+ RCs support inflammation-induced remodeling in human lymph nodes



Burkhard Ludewig Group
Angelina De Martin
Yves Stanossek
Lisa Kurz
Samuel Meili
Nadine Cadosch
Christian Perez-Shibayama
Cristina Gil-Cruz
Hung-Wei Cheng
Lucas Onder
Acknowledgements

Natalia Pikor Group
Sarah Grabherr
Department of
Otorhinolarnygology
Sandro Stöckli
University of Pennsylvania
Ivan Maillard
Joshua Brandstadter
University of Zurich
Mark Robinson
Charlotte Soneson
Talk UPENN
By Almut Luetge
Talk UPENN
- 89



