On the Dynamics of HIV and Malaria Infection - Insights From Mathematical Models
Bernhard Paul Konrad
Mathematics
The University of British Columbia
4 September 2015
Overview
- Theory of branching processes
- Length of the eclipse phase for HIV
- Analysing Get Checked Online
- A fungus to fight malaria
Theory of Branching Processes
- Most results known in some form, but have not been applied to HIV in this way
Consider the birth-death process
birth
death
Want:
Simple: Forward and backward master equation
Helper: The probability generating function
Forward master equation leads to PDE
Backward master equation and branching property lead to ODE
Probability generating function
Knowing pgf means know everything (also: moments)
Probability generating function
Conditioning on non-extinction
When fitting model to HIV data, exposures that did not lead to infection can be recorded. Does this bias matter?
Coin-flip intuition
Heads: +1.1 points
Tails: - 1 point
A) Start with 20 points
B) Start with 1 point
Average "survivors" (> 0 points) only
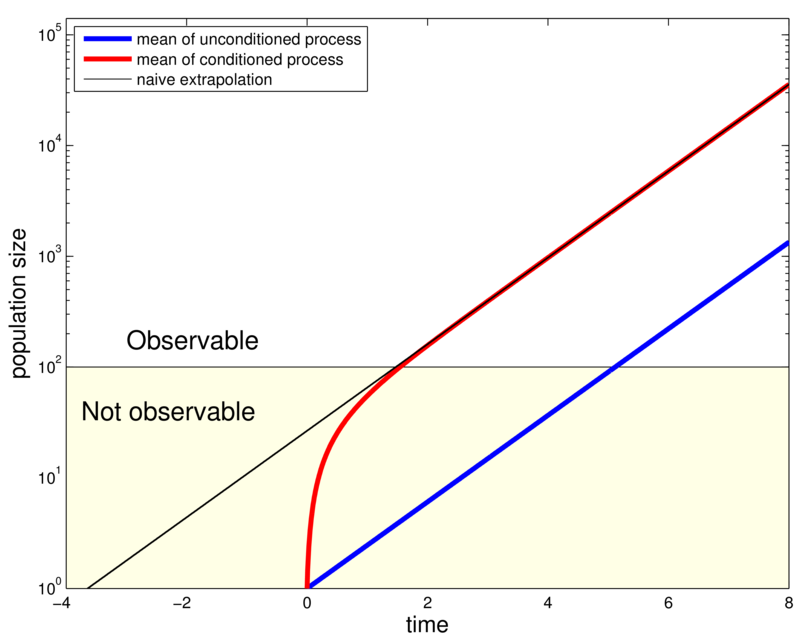
Fit conditioned process to match bias in the data! Using Bayes rule and the Markov property
So only a slight modification of the corresponding pgf is required.
Conditioning on non-extinction
Method naturally extends to multi-type processes.
Estimating the length of the eclipse phase
Clinical questions:
- How soon after exposure should I get tested for HIV infection?
- How reliable is an early negative result?
- When should I schedule a follow-up, confirmatory test?
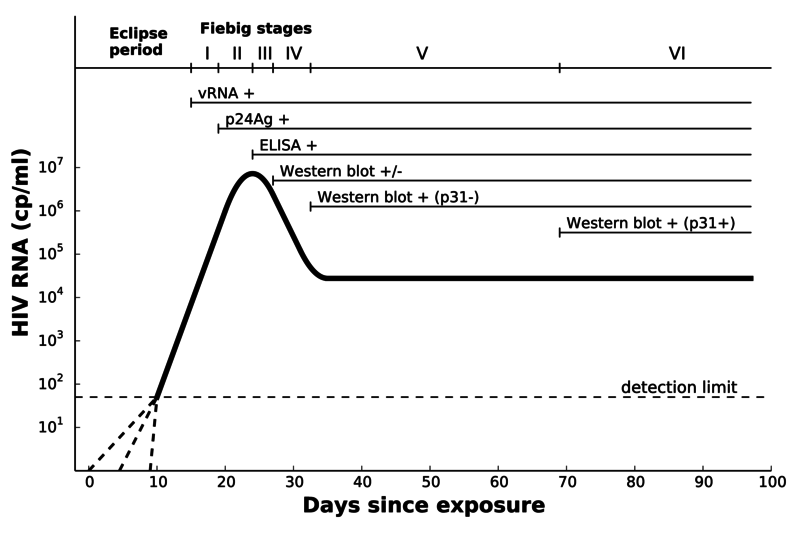
Data: seroconversion panels
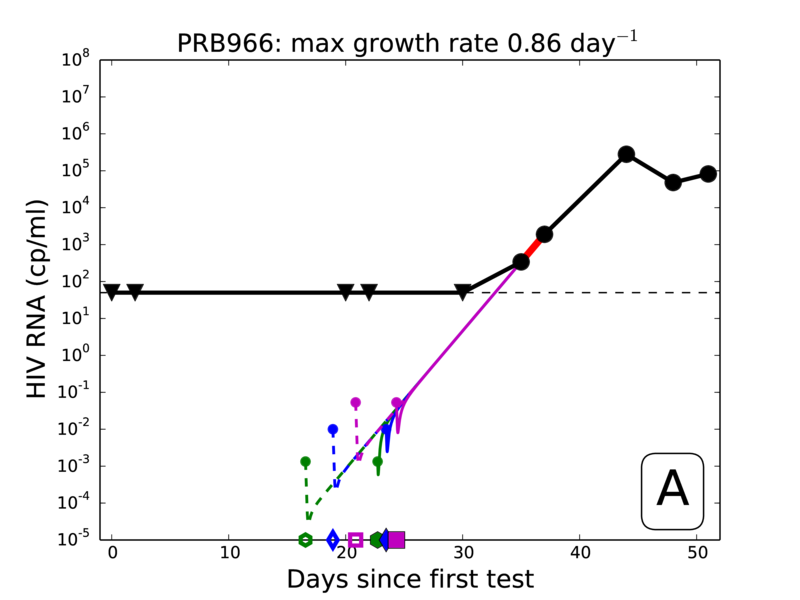
- Viral load growth data obtained from historic seroconversion panels (plasma donors)
- All donations removed from circulation if any sample tests positive
- More sensitive tests today can detect HIV in early samples
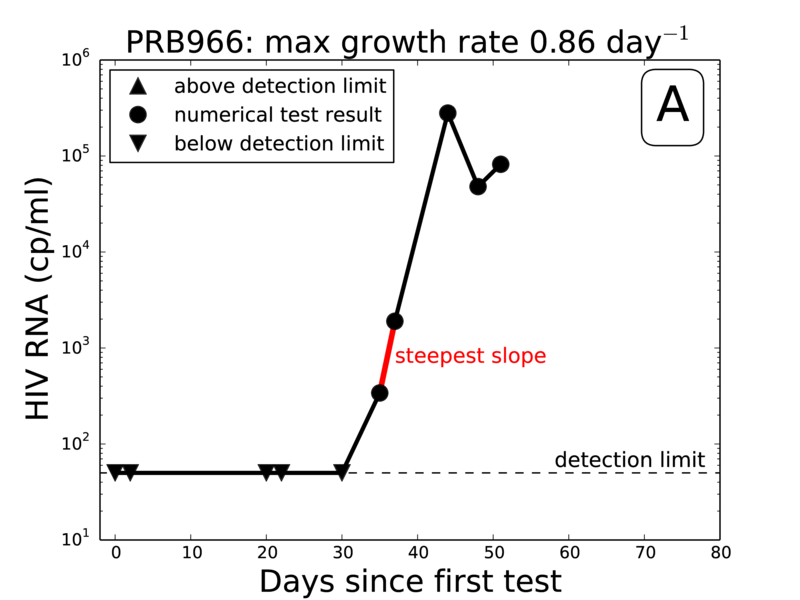
Extract steepest slope from each panel
Probability of false negative test
- Compare unconditioned and conditioned model
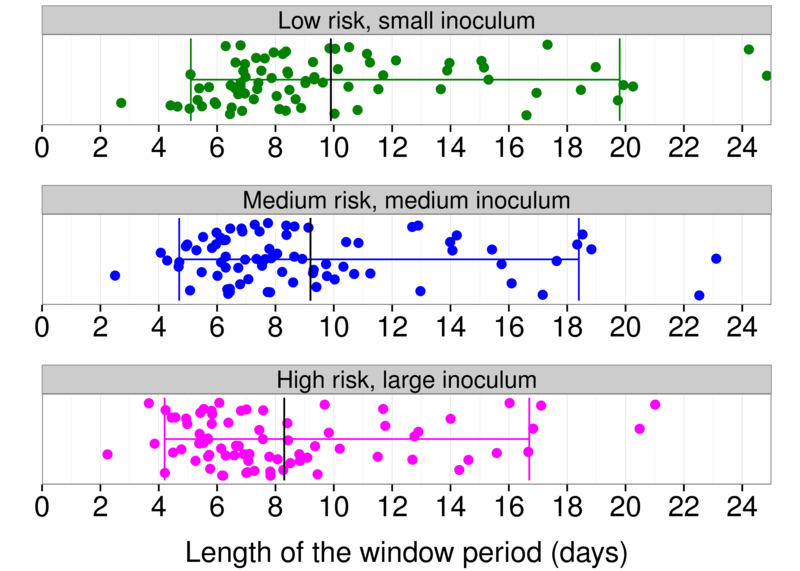
- Conditioning reveals shorter eclipse phase
- Consider different risk scenarios
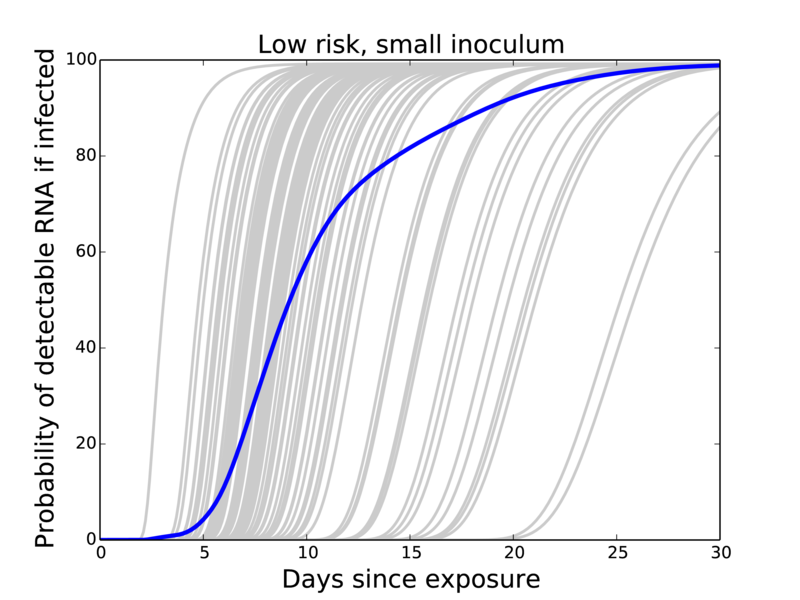
Have: Probability of negative test given infection
Want: Probability of infection given negative test
So use Bayes formula!
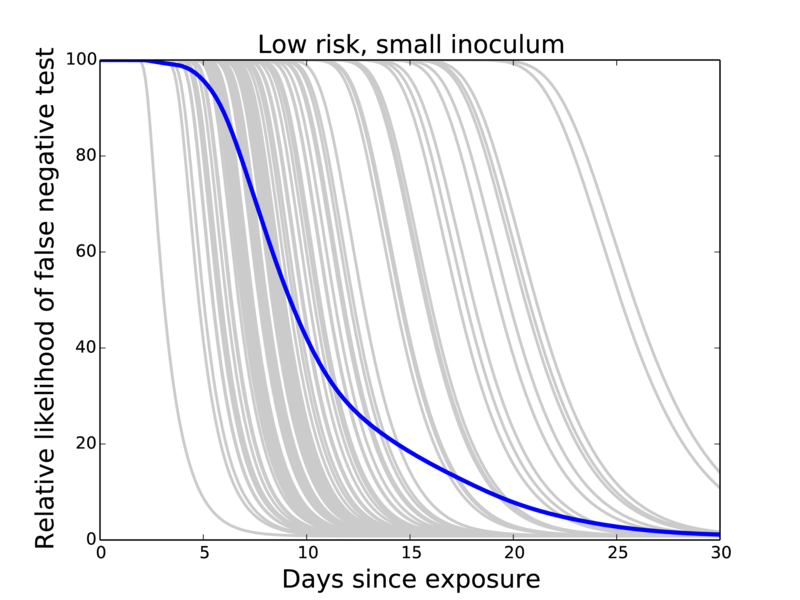
Probability of false negative test
Get Checked Online
Collaboration with BCCDC to understand, predict and optimize impact of MSM-focused health program
HIV prevalence:
- 0.2% in Canada
- 15% in Vancouver MSM
MSM = Men who have sex with men
BCCDC = British Columbia Centre for Disease Control
23% of young MSM under 30 never tested for HIV
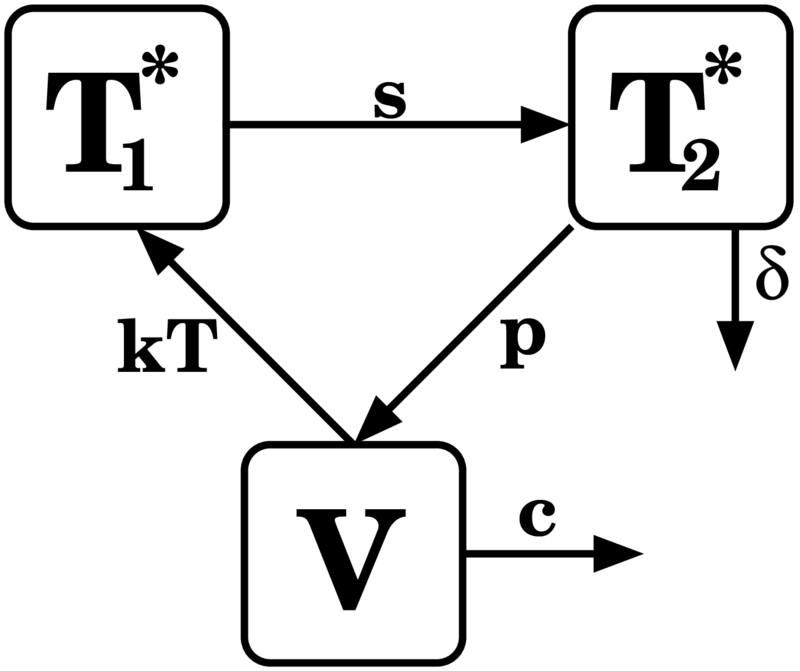
Basic Mathematical Model
- Susceptible
- Infected Unaware
- Infected Aware
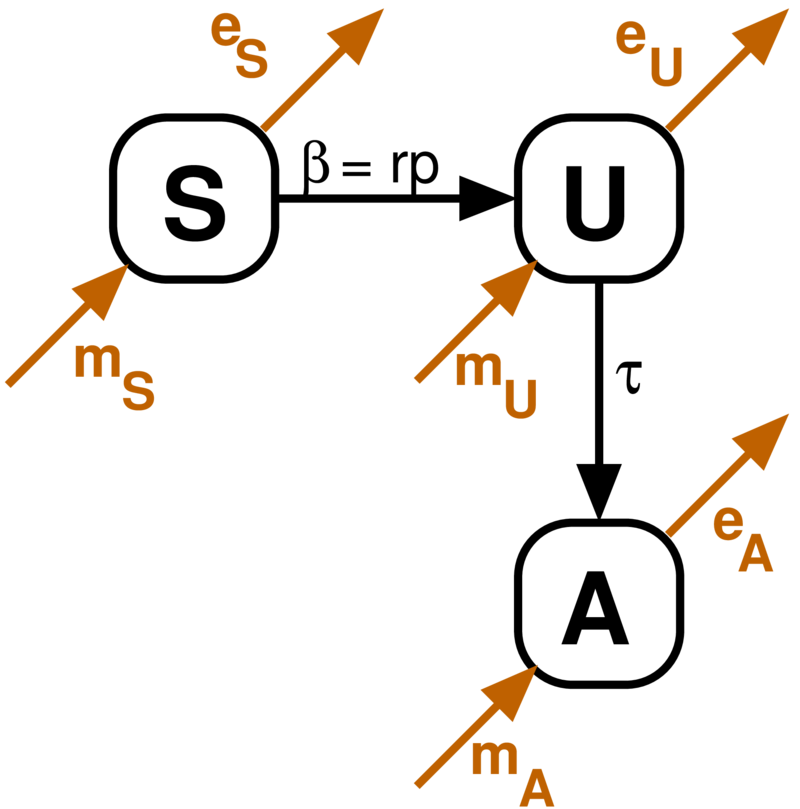
Main parameters to estimate:
- r - rate of risky events
- p - per-encounter risk
- - testing rate
Data and parameters
r - rate of risky events
p - per-encounter risk
- testing rate
Two data sources (N = 166):
- Online surveys on, e.g.
- HIV testing history
- Network grid interviews
- recent partners, relationships
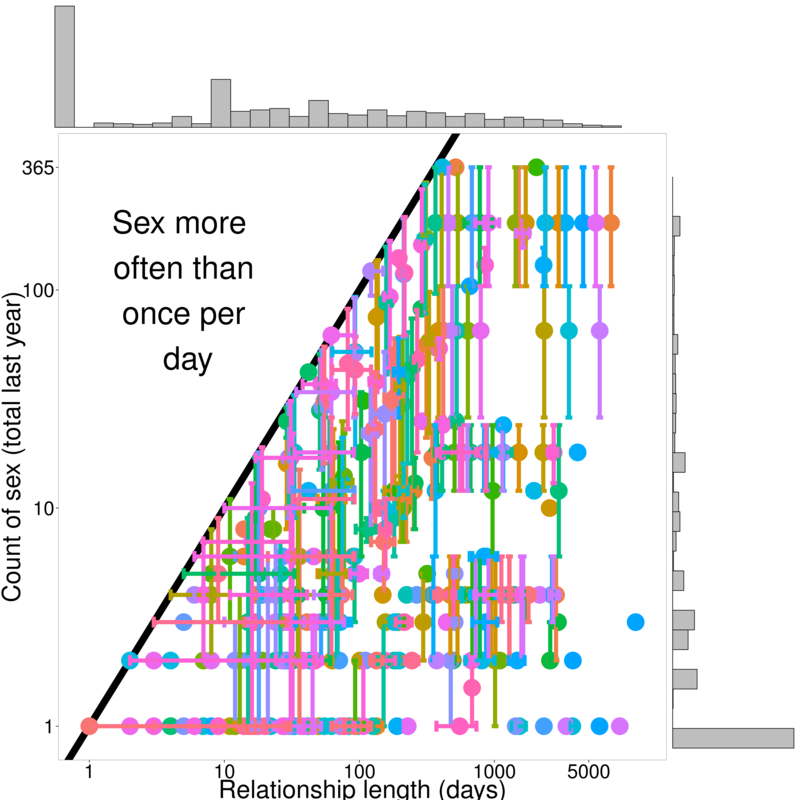
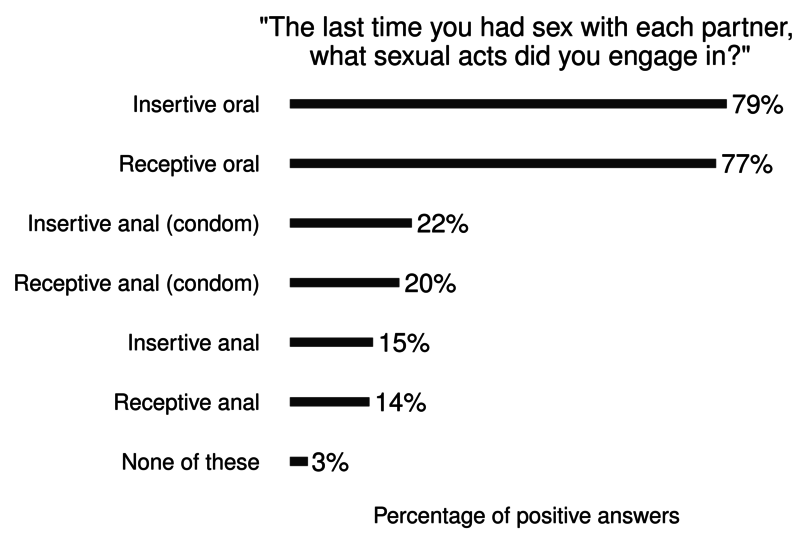
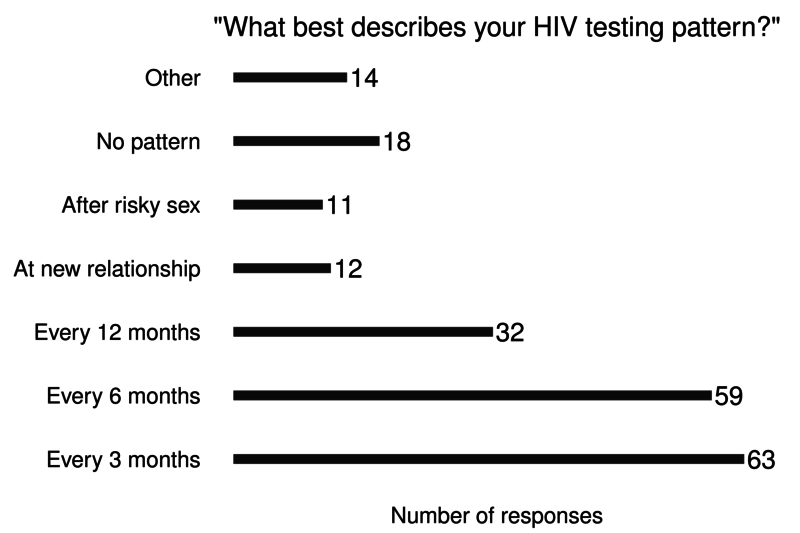
Additional: Sex Now 2011
Full model
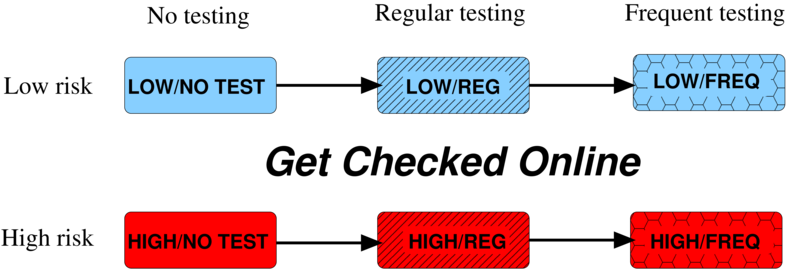
- 3 x 6 = 18 equations
- Consider both extremes of contact mixing
Parameter estimation
- Define testing groups from survey answers
- Use Sex Now data to calculate transition rate from "no testing" to "regular testing"
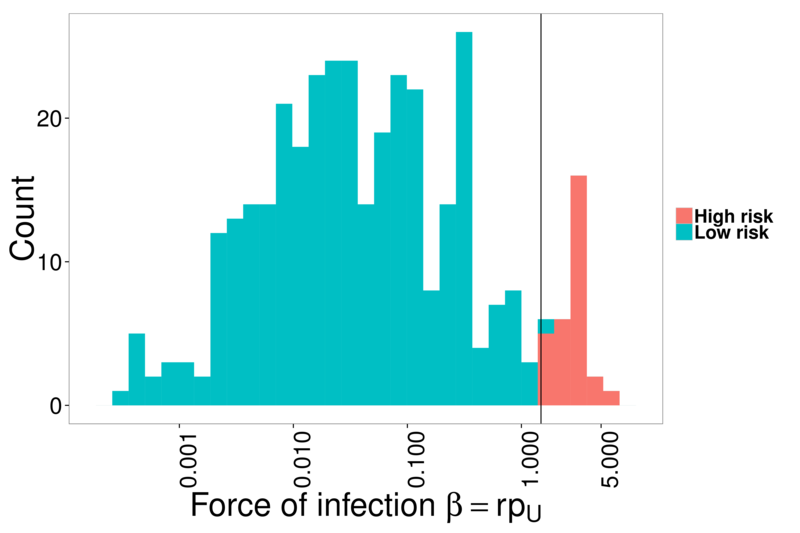
- Calculate per-person force of infection
- Use 2-means clustering to find cut-off (result: 8.3% high risk)
Results
BCCDC: 806 newly detected infections
| Homogeneous | 5 years projection | Heterogeneous |
| 2.73 | R0 | 2.50 |
| 2314 | New infections | 1878 |
| 2063 | Newly detected | 1757 |
| 66.4 | Averted infections | 36.4 |
Core group effect
Future work:
- Add partnerships
- Include acute infection and treatment
- Testing rate may depend on risk activity
- Agent-based model
Malaria

- Malaria endemic in 100 countries
- Transmitted by mosquitoes
- Traditional: Decrease mosquito count
- Problem: Toxic, mosquito resistance
- Recently proposed: Fungus that neutralizes malaria parasite in mosquito
Research question:
What is the optimal fungal virulence?
biopesticide effect vs. competition effect
Model and objective
Mosquito competition
Fungus spraying rate
Fungal virulence
Basic model
Goal: Minimize endemic prevalence in humans
Results
Results:
- Increasing fungus-spraying rate decreases human malaria prevalence
- There is a worst-case virulence
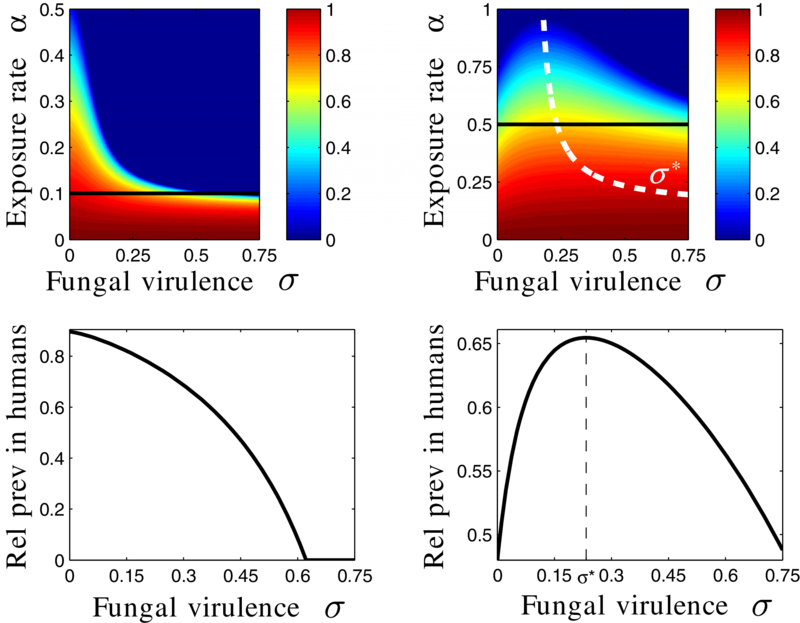
Life-structured model with with vertical transmission and competition in larvae stage
Vertical transmission
- unlikely: choose high virulence
- likely: choose low, but not too low virulence
Summary
Projects that show the usefulness of mathematical biology to reveal practical insights into current problems of global scale
- Derive theory of branching processes from first principles, focus on the application and numerical implementation
- Quantify uncertainty of early HIV tests and predict that eclipse phase may be shorter than previously assumed
- Estimate population-level impact of increased HIV testing and reveal core-group dynamics
- Optimize fungus characteristics to minimize human malaria prevalence
defence
By Bernhard Konrad
defence
- 530



