My background

- Former Pharmacist (Lille)


-
2022: PhD degree at ENS Paris (bioinformatics)




-
MSc Bioinformatics - Paris Diderot
PharmD thesis
Hematopoietic Stem Cell transplants (HSCT)

The Idea: Predict mortality or GVHD events with machine-learning
- My own project from the beginning
- Worked with a national consortium and 2 clinical experts
Preliminary results +/- encouraging
Still ongoing
My PhD :
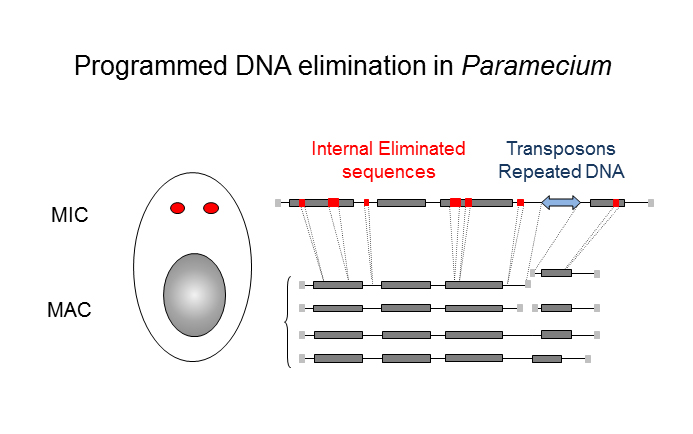
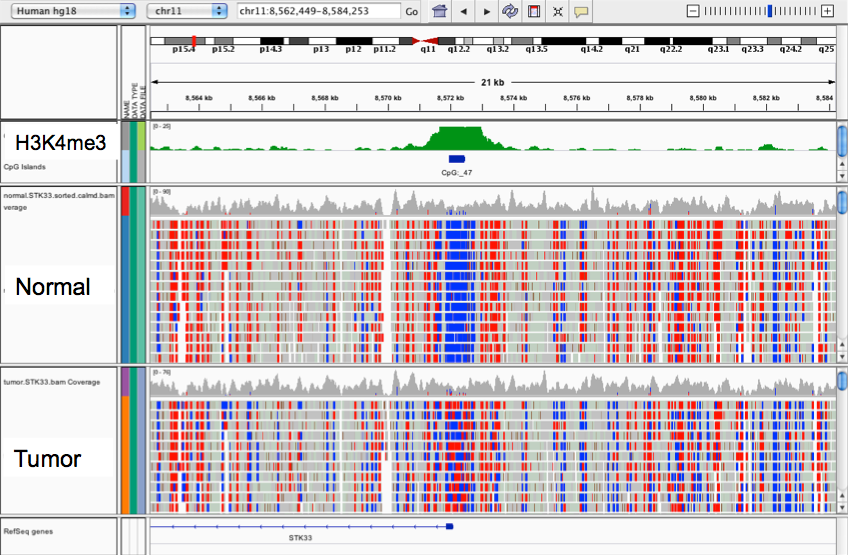
DNA methylation in P. tetraurelia (PacBio-seq)

PhD subject : P. tetraurelia
Unicellular eucaryote with 3 nuclei:
- 1xMAC nucleus (up to 800n)
- 2xMIC nuclei (2n)
DNA ratio: 1 MIC for 200 MAC
The difference ?
MAC =
- Transcriptionnally active
- Amplified
- Freed from TEs
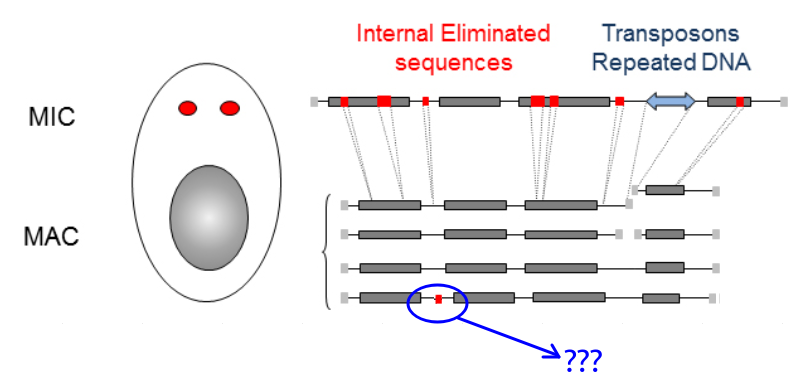
- Freed from >48.000 unique "IES"

Internal Excised Sequences (IES)
We understand the recognition of ~30% of IESs (small ncRNA)
...How are the other recognized ?
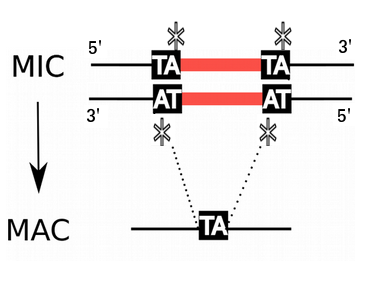
Constant 6mA pattern in the MIC (ONLY !)
Our hypothesis :
But, almost nothing was known about DNA Methylation in both the MIC, and the MAC
Main idea of the project:
- Characterize WT methylation (MIC and MAC)
- Candidate methyltransferases (MTases) were identified and silenced with interferent RNAs
- See whether or not IESs are recognized correctly in silenced conditions

Problems encountered during my work
Problem 1: The MIC cannot be purified
Total DNA was sequenced instead
IES
Other MIC
Other MIC
IES+
Mac Destinated Sequences (MDS)
IES-
Total DNA -> 99.5% of sequencing data = waste
Reminder : 1:200 comes from the MIC
Problem n°2 : Low amount of IES+ reads
- Remove contaminants
- 1 molecule out of 200 comes from the MIC
- ~ 1/6 of MIC inserts will carry an IES
- 30% don't interest us
- # of PacBio reads per sample : 150.000 to 300.000
- ...
That is,
-
Expected ~100 to 300 IES+ molecules per experiment only
- Got 49 to 310
This is not much, but if had been right, 100% of them had to be methylated

Problem n°3 : PacBio SMRT-seq
PacBio SMRT sequencing
- PacBio SMRT-seq = 2 variants :
- Long inserts (several kb)
- Default
- Short inserts (~350bp)
- Very rare
- No analysis pipeline existed for our kind of data
- Long inserts (several kb)
- No analysis pipeline existed for us
- Much retro-ingineering of proprietary software was needed
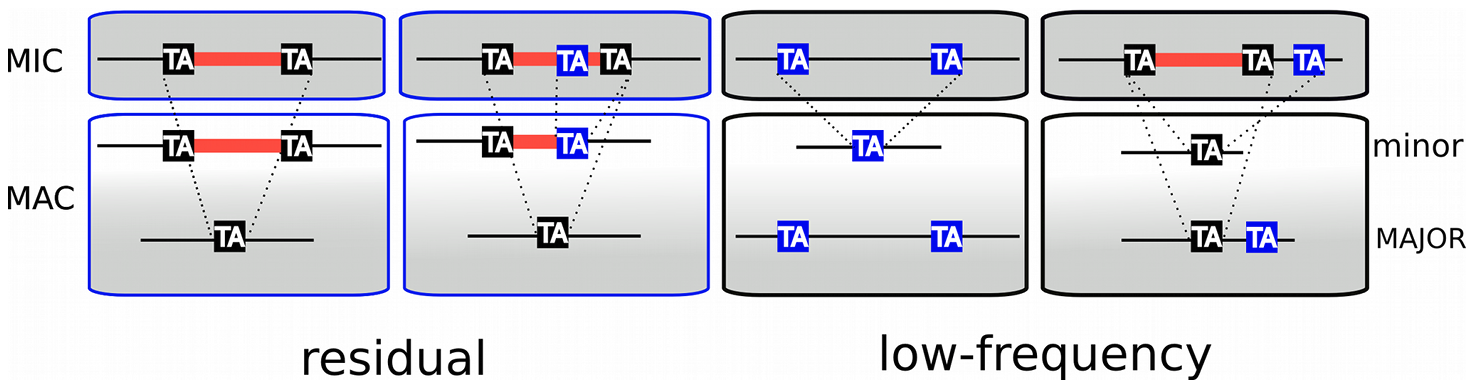
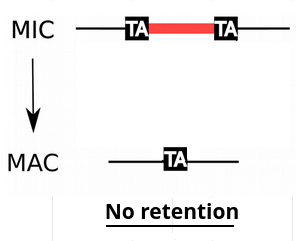
Problem n°4 : IES retention
-
IESs are sometimes retained in the MAC
-
Some IES are more retained than others
-
Danger ! >> These IES come from the MAC !
-
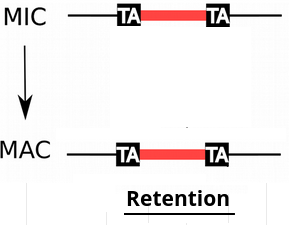
P(R)
1 - P(R)
No methodology existed to quantify R (and therefore P(MIC|IES+)) precisely
Results
The analysis pipeline
After a lot of retro-engineering :
- A reproducible pipeline
- Benchmarked (E. coli GATC sites)
- Python
- Conda install
- Tests
- Continuous integration (CircleCI)
- Clean command-line interface (CLI)
- FDR control procedures
Where do IES+ reads come from ?

Bayesian modelling
Hamiltonian Monte-Carlo with Stan
+ Old sequencing datasets

Surprising results :
-
Almost all IES+ reads actually come from the MAC (!)
- Impossible to study the MIC with our method !
-
The MAC ploidy is often said to be 800N
- Real value >1600N
- >50 years old mistake spotted !!
The real role of our MTases (1/2)
> It turns out that all the candidate methylases we silenced were actually active in the MAC !!
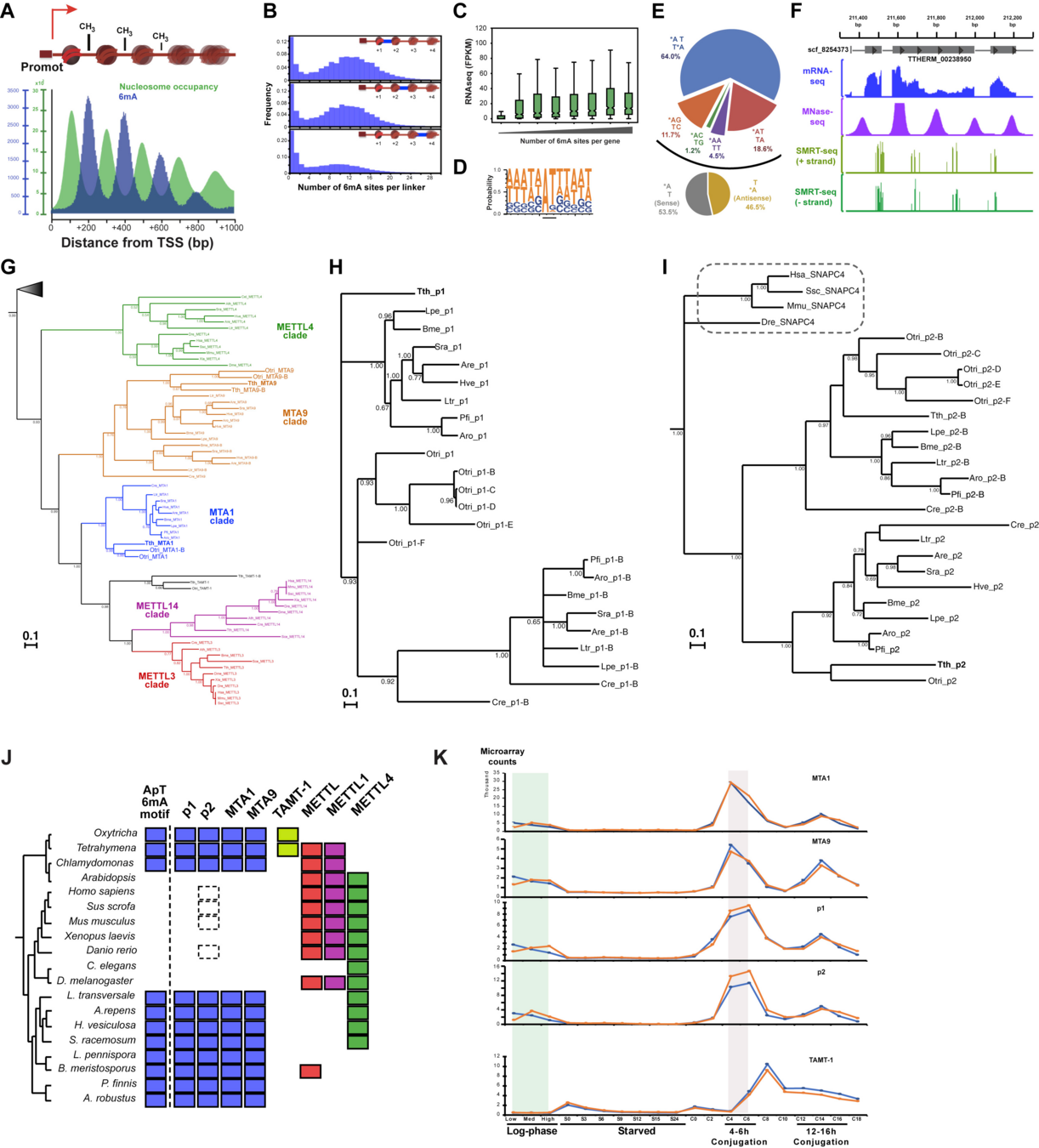
Similar to Oxytricha :
Beh et al. 2019
Therefore we were actually lucky to have so much MAC data to study it
Methylated symetrically in AT sites
The real role of our MTases (2/2)



All weakly implied
All strongly implied
Function = Symmetry +++
- Our MTAses turn hemi-methylated sites into symetrically-methylated ones
- Total 6mA reduced by up to -50% when all MTAses are silenced
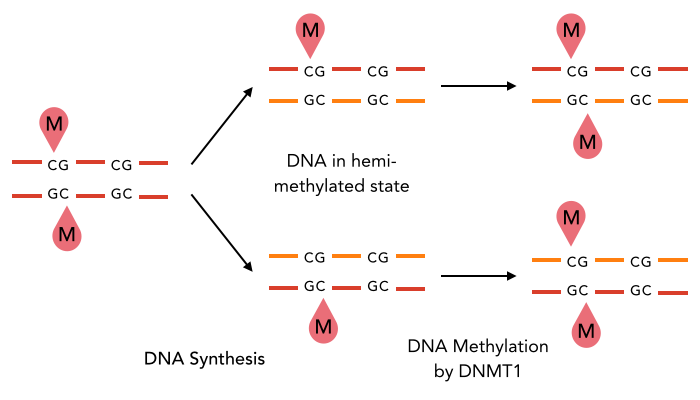
It is possible that hemi-methylation is actually preserved through DNA replication. We are still investigating this.
A "fun" fact about hemi-methylation
Statistical curiosity of our pipeline :
1) %6mA detected = True %6mA
2) % hemi-methylated AT sites = True % hemi-methylated AT sites
... But this is a coincidence !
In fact,
if Z = # of 6mA in an AT site
if D = # of detected 6mA in an AT site, then sometimes we have as low as :
P(Z=2 | D=2) ~ 20-50%
Even though P(D=2|Z=2) ~ 100%
this means that we cannot make good qualitative analysis on these detection : they are massively incorrect !
CONCLUSIONS
- New analysis pipeline for SMRT-seq (https://github.com/EMeyerLab/SMSN)
- Retro-engineering of Pacbio proprietary software (https://github.com/EMeyerLab/ipdtools)
- Quantification of false positive hemi-methylation (https://github.com/EMeyerLab/paired_detections)
- Long-time error spotted (MAC ploidy not 800N but >1600N)
- New method to quantify IES retention
- Demonstrated mathematically that it was impossible to answer to my problematic
- Identified the true role of our MTases
- Other secondary tools
- Eg: High-performance filtering of .bam files based on the CIGAR string
- (https://github.com/GDelevoye/cigarfilter)
More details :
https://drive.google.com/file/d/14Z2EEGpIRMeoXwIj4f8WhthG_x0AVF58/view
More about me

Dire Straits <3

4X - Strategy games

Chess

Memes

Climbing

lolcats
who doesn't ?

Our supreme leader
Guido Van Rossum
What is he ? A man ? A God ?
We'll never know.
(when I win)


Playing music
Internet
Hiking


Thanks :)


My scientific medley
Code
Stats
Hematology (HSCT)
ML
Ciliates
NGS
Structural biology
Ask me at the end !
Python C++ Bash R

Methylase candidates in Paramecium tetraurelia
Candidates identified:
- NM4, NM9, NM10
- Another family: MT1a, MT1b, MT2
2) PacBio sequencing with short inserts | 6mA ++
1) Grouped silencings by sequence homology
PacBio SMRT (short inserts)

Inserts up to 350bp
Sequencing : unique molecule & both strands
Polymerase slowing ~ methylated adenine
The random sampling strategy
- Purify the MIC: Impossible
- Sequencing whole MIC from whole DNA : Impossible
-
Solution: Random sampling
- 1 among 200 molecules
- Not all with cary an IES at all
- ~20 to 100 IES only per experiment
Problem n°2
IESs are sometimes retained in the MAC
What is P(MIC|IES+) ?
Other analysis challenges
(skipped)
Details that I spare you (but don't hesitate to ask) :
- Random sampling of the MIC
- Sorting MIC and MAC
- Sorting IES specific and MIC non-IES sequences
- Filter IES retention
- Estimate Se and Sp
- Retro-ingineering PacBio's formats
- Recoding parts of the softwares for single molecules
- etc.
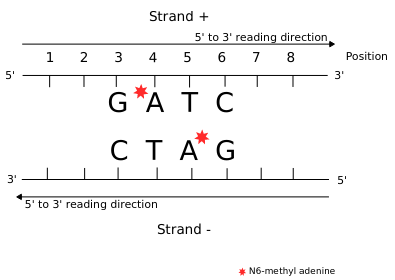
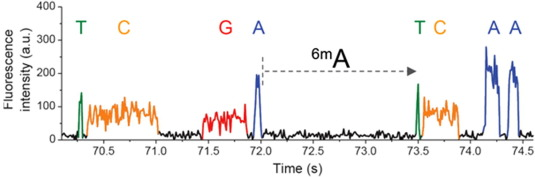
Detecting n6mA
Imperfect detections
No scientific method is perfectly reliable
In our case:
- Sensitivity = P(D | M) > 92%
- Specificity = P(ND | NM) > 99.8%
> Expect False Positives and False Negatives
Imperfect detections (2/2)
- Sensitivity = P(D | M) > 92%
- Specificity = P(ND | NM) > 99.8%
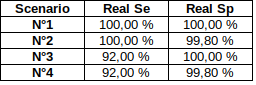
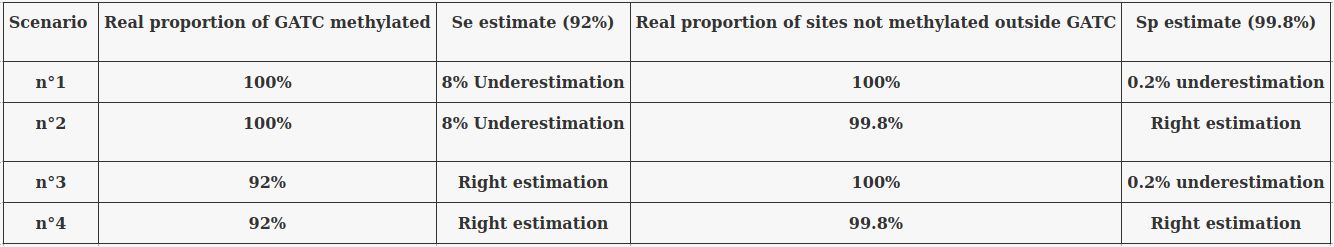
We can use 4 extreme scenarii :
... And see what is consistent no matter the scenario we place ourselves into
Finding unbiased estimators for and FDR
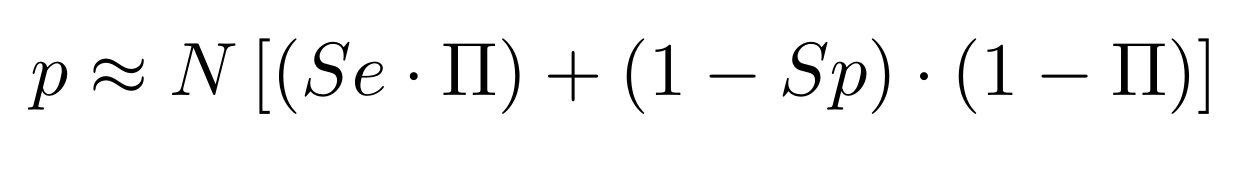
If p number of positive detections among N tests:
p = FP + TP
$$\pi$$
So,
Which means
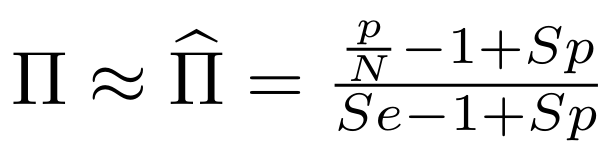
with fraction of 6mA
$$\pi =$$
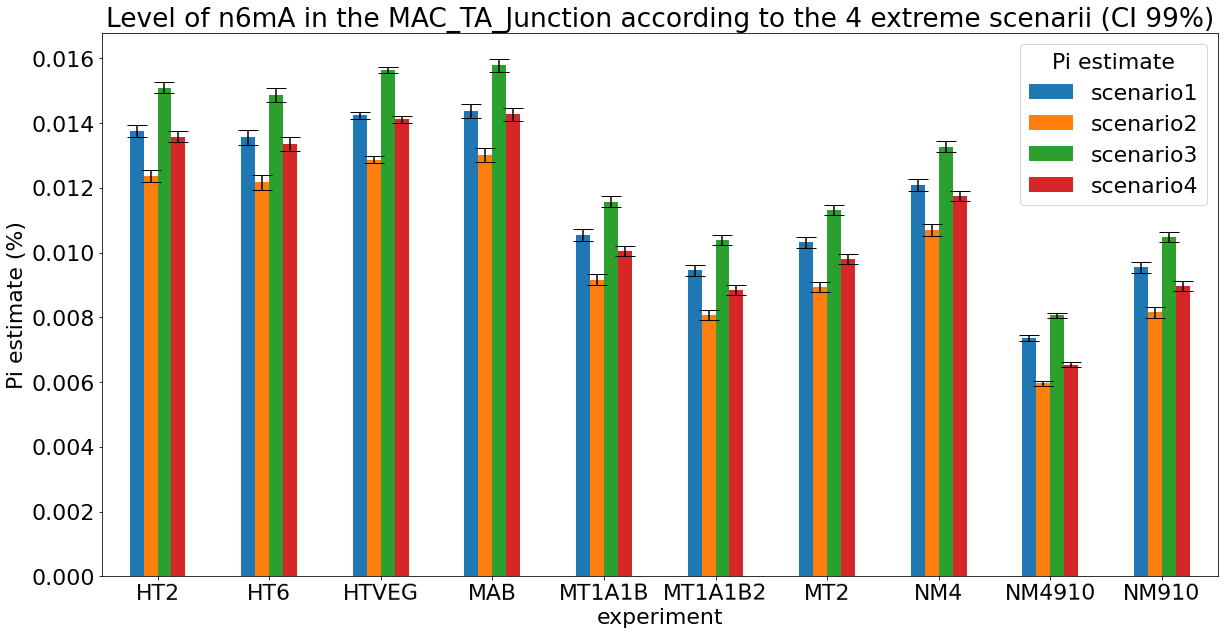
Debiased levels of m6A in the MAC
> 95% n6mA are located in AT sites
DNA n6-mA around/in the IESs
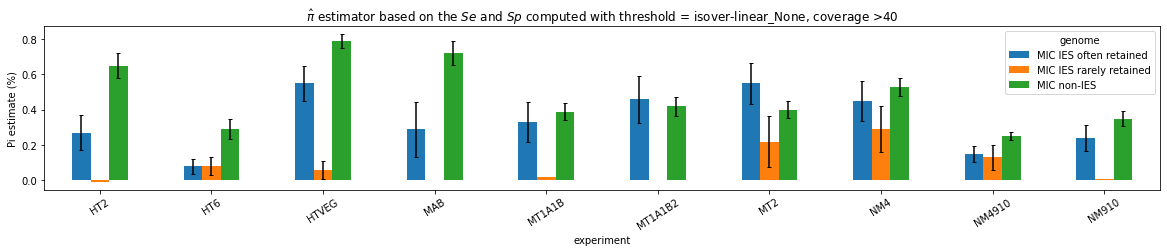
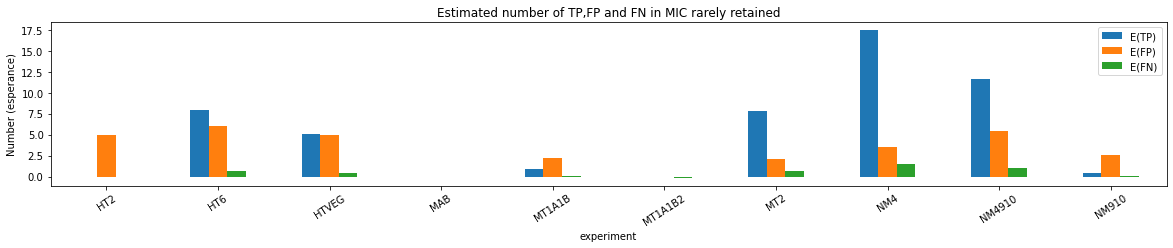
Results
Methodological development to correct hemi-methylation detection
Let FD1 and FD2 be resp:
- Fraction of AT sites detected hemi-methylated
-
Fraction of AT sites detected symmetrically methylated
PZ0, PZ1, PZ2: unbiased estimators of non, hémi, symetrically methylated AT sites
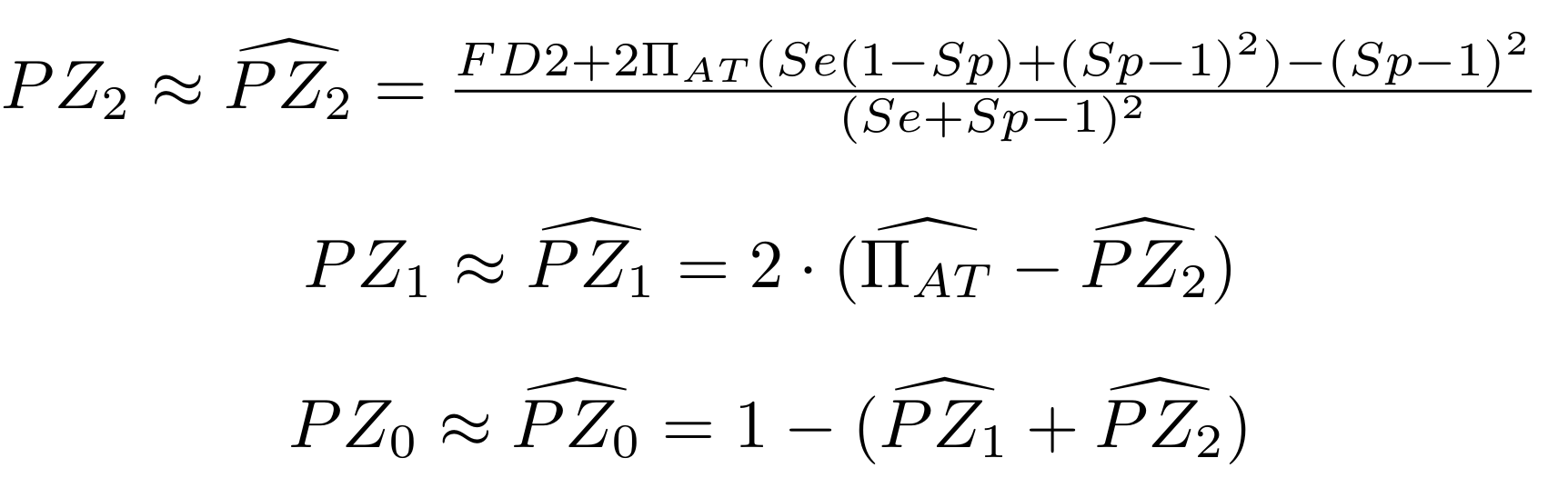
Then:
With
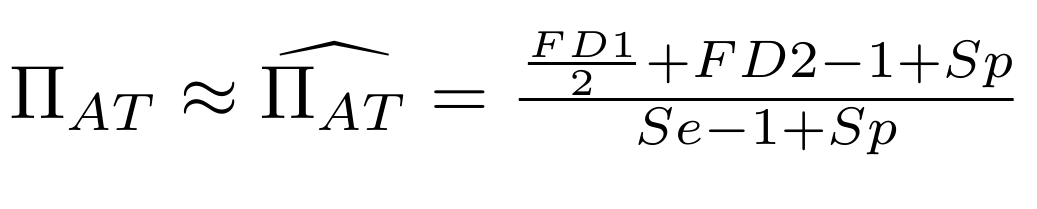
Debiased Hemi-methylation in MAC


2) Transcient in the new forming MAC
1) Constant pattern in the MIC
Transcient ?
Our hypothesis:
Analysis showed that:
~2.5% of n6mA in Paramecium (MIC) : Cummings 1974
?
> Actually way lower (if any)
> Unexpectedly, NM and MT proteins play a whole another role in the MAC,
not the MIC
Conclusion
IGEM2014
MT and NM families :
In the MAC
Convertase activity DNMT1-like
But: Hemi-methylated AT sites kept after many mitosis
New hypothesis:
Symmetrical methylation of AT sites = Mitotic clock or maturity indicator for the cell to be allowed to go enter meiosis
A bit more about me...
I don't like
Red socks

Fun

Windows 10 update of May 2021
Some keywords for my PhD
1) Paramecium tetraurelia
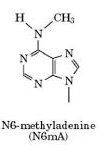
3) DNA methylation


4) PacBio sequencing
2) Transposable elements / IESs
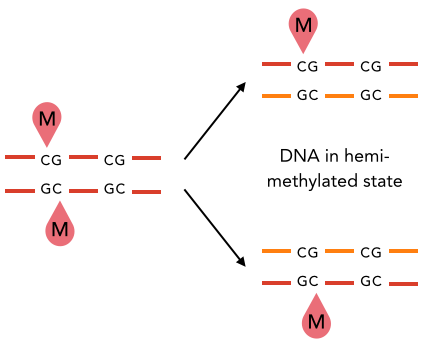
5) hemi-methylation of
palindromic motifs
(AT sites)

P. tetraurelia

Unicellular eucaryote with 3 nuclei:
- 1xMAC nucleus (up to 800n)
- 2xMIC nuclei (2n)
DNA ratio: 1 MIC for 200 MAC
The difference ?
MAC genome compared to MIC =
- Amplified+++
- Free from TEs
- Transcriptionnally active
- Not transmitted to progeny
Genome invaders in Paramecium tetraurelia
In the MIC:
- 45.000 unique sequences
- Small (<27bp), Non-coding
- Lots present in the CDS
- Remnants of TE ?
- 100% TA-bounded


--> How ?
Present in the MIC
Absent in the MAC
= Excised generation after generation




2) Transcient in the new forming MAC
1) Constant pattern in the MIC
Transcient ?
Recognition of IESs: Our hypothesis
Our hypothesis:
~2.5% of n6mA in Paramecium (MIC&MAC) : Cummings 1974
Methylase candidates
Candidates:
- NM4, NM9, NM10
- Another family: MT1a, MT1b, MT2
2) PacBio sequencing with short inserts | 6mA ++
1) Grouped silencings by sequence homology
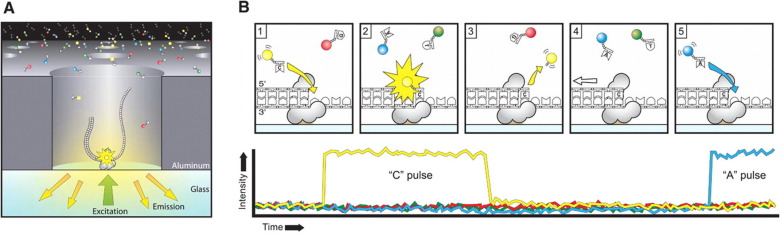
PacBio SMRT principle


Reads up to 80 kbp
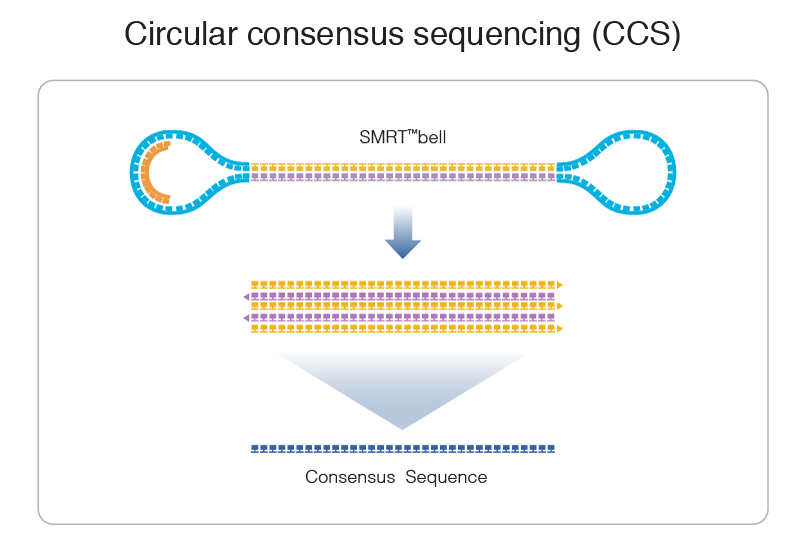
1) Sequencing (unique molecule)
2) High fidelity consensus
3) DNA methylation analysis
What are the results ?




2) Crosstalks in the new forming MAC
1) Constant pattern in the MIC
Transcient ?
Our hypothesis:
Prelimary analysis shows that:
~2.5% of n6mA in Paramecium (MIC&MAC) : Cummings 1974
Details invegetative MAC
-
~95% of n6mA locates in AT dinucleotides in the MAC
-
75% of the methylation in an AT dinucleotide is actually symetrically modified
Bulk of methylation:
Total = 1.2 to 1.5% of adenines
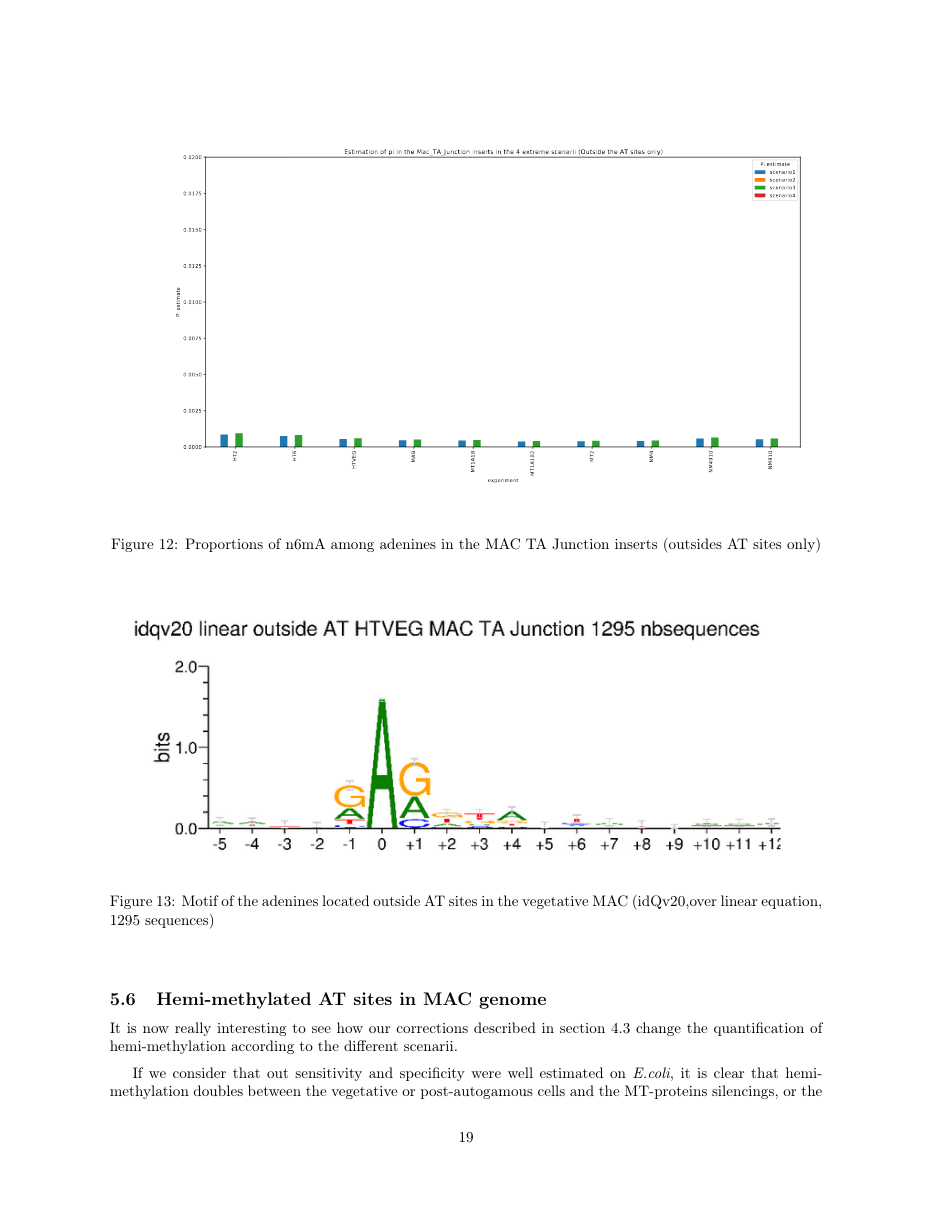
0.6% of the adenines outside AT sites:
Now a bit more details...
Imperfect detections
No scientific method is perfectly reliable
- We don't care about Se and Sp
- We care about the fact that eventual mis-estimations of them doesn't really change anything
In our case:
- Sensitivity = P(D | M) > 92%
- Specificity = P(ND | NM) > 99.8%
Finding unbiased estimators for and FDR
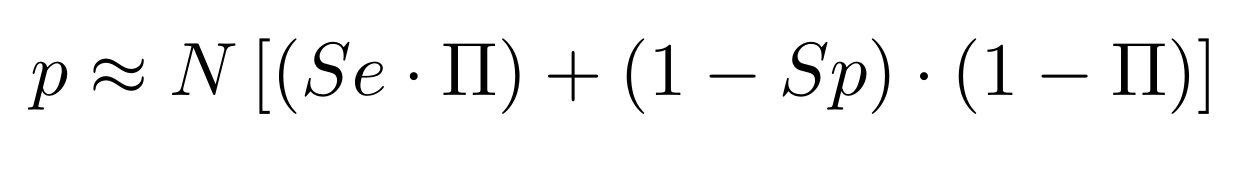
If p number of positive detections among N tests:
p = FP + TP
$$\pi$$
So,
Which means
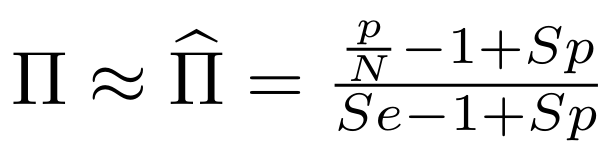
with fraction of 6mA
$$\pi =$$

Debiased levels of m6A in the MAC_TA inserts
Methodological development to correct hemi-methylation detection
Let FD1 and FD2 be resp:
- Fraction of AT sites detected hemi-methylated
-
Fraction of AT sites detected symmetrically methylated
PZ0, PZ1, PZ2: unbiased estimators of non, hémi, symetrically methylated AT sites
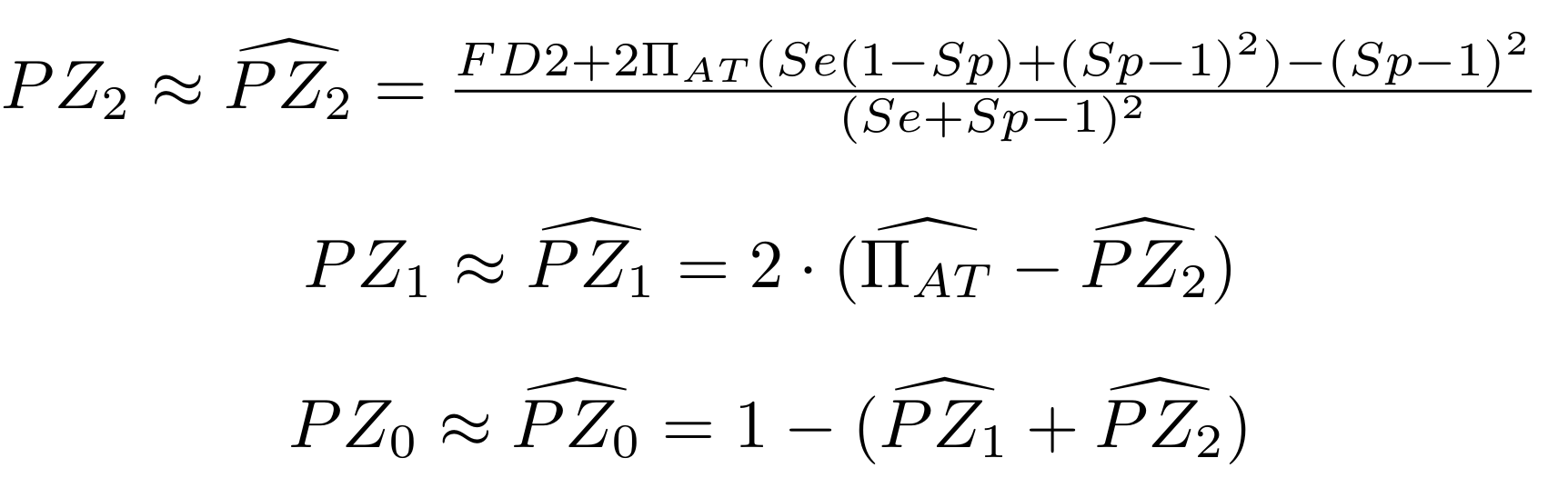
Then:
With
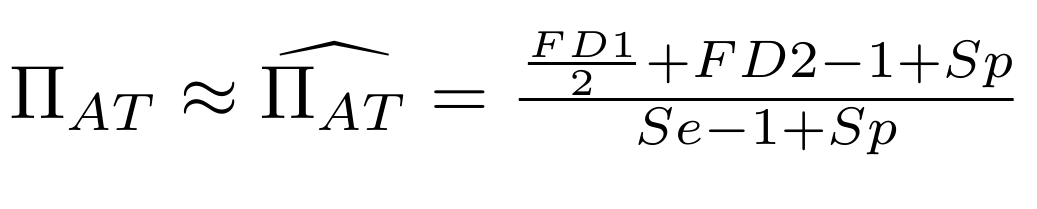
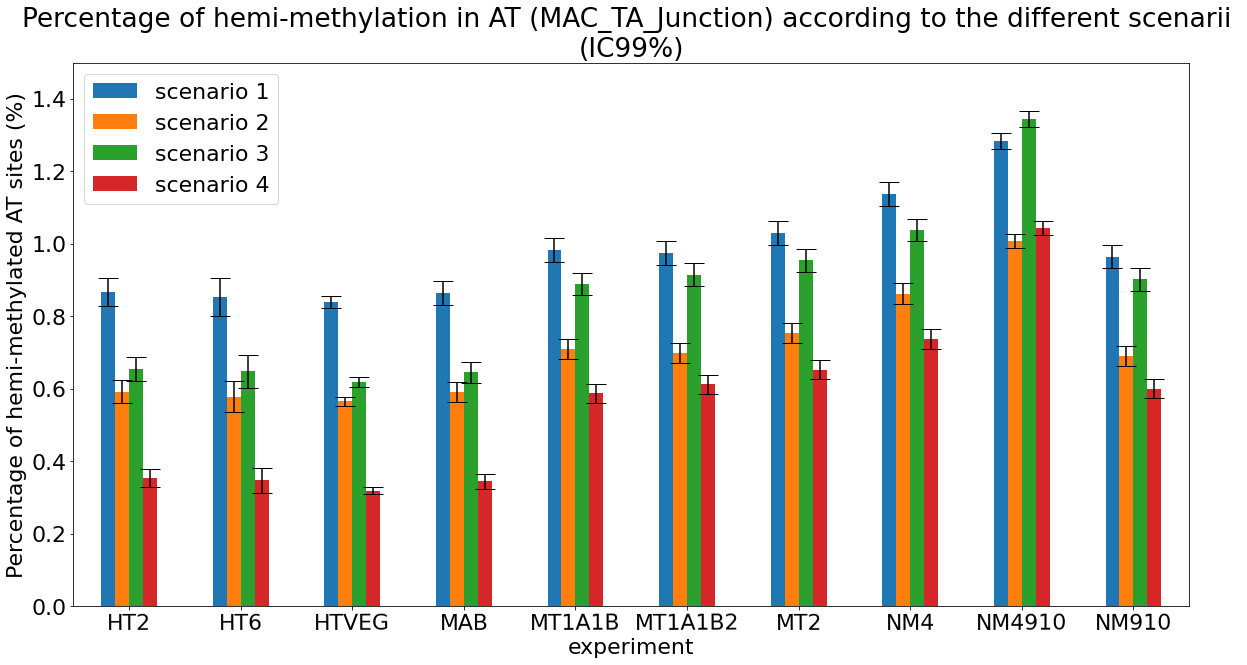
Debiased Hemi-methylation in MAC_TA

Conclusion
- In the MIC:
- Probably no m6A after all
- Hard to imagine a role in TE/IES excision
-
In the MAC
-
Our 6 genes are implied in the bulk of the MAC m6A
- Sym-methylated -> Hemi-methylated
-
MT and NM families: Functional analogs of DNMT1 ?
- Not really: Not kept after replication
-
Lots of questions raised by the MAC methylation:
- TSS ?
- IES excision Junctions ?
- Nucleosome positionning ?
-
Our 6 genes are implied in the bulk of the MAC m6A
-
One important phenotype:
- NM4-9-10 somehow makes the cell unable to go into autogamy

Thanks :)
Role of DNA-6mA in Paramecium tetraurelia






Guillaume DELEVOYE
3rd Year PhD student
Bioinformatics
P. tetraurelia: Genomic architecture

Unicellular eucaryote with 3 nuclei:
-
2xMIC nuclei (2n)
- Germline nucleus
-
Contains: TE & IES
- No transcription outside meiosis
-
1xMAC nucleus (up to 800n)
- Somatic nucleus
- Amplified and "fixed" version of the MIC
- Free from TE and IES
- Transcriptionnally active
DNA ratio: 1 MIC for 200 MAC
+ DIfficult to purify the MIC DNA
Profiling the IESs
- Non-coding
- Excised after sexual processes
- Remnant of TE ?
- Most recent IES are very short (< 27bp), and are the majority of IESs
45.000 Unique sequences
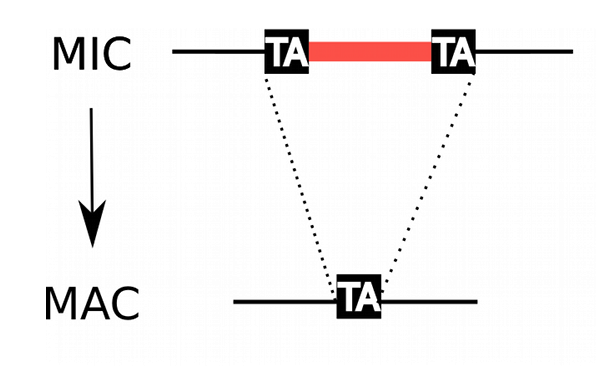
How are IESs recognized ?
(1/2)
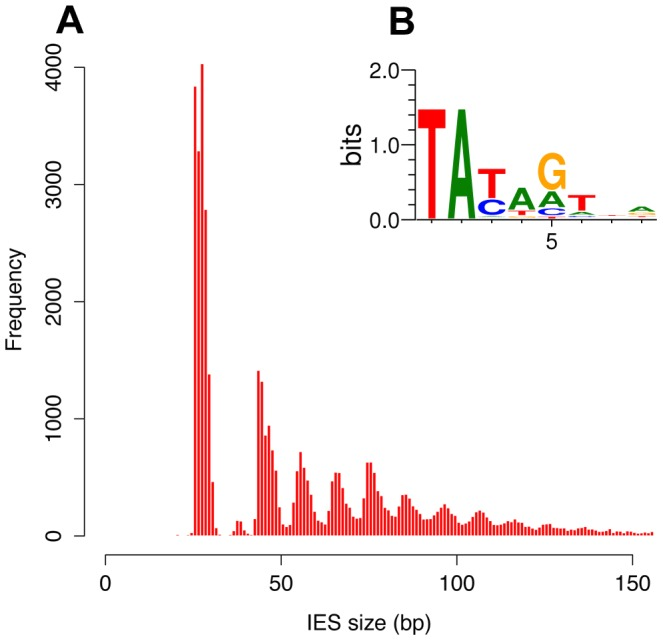
- 100% TA-Bounded
-
Weak consensus TAYAG
- Degenerated TC1-Mariner TE insertion site
- Not sufficient
- Periodic size distribution
30% of IESs only are small-ncRNA dependant (shown by DICER-like2-3 silencing)
- What about the majority remaining ?
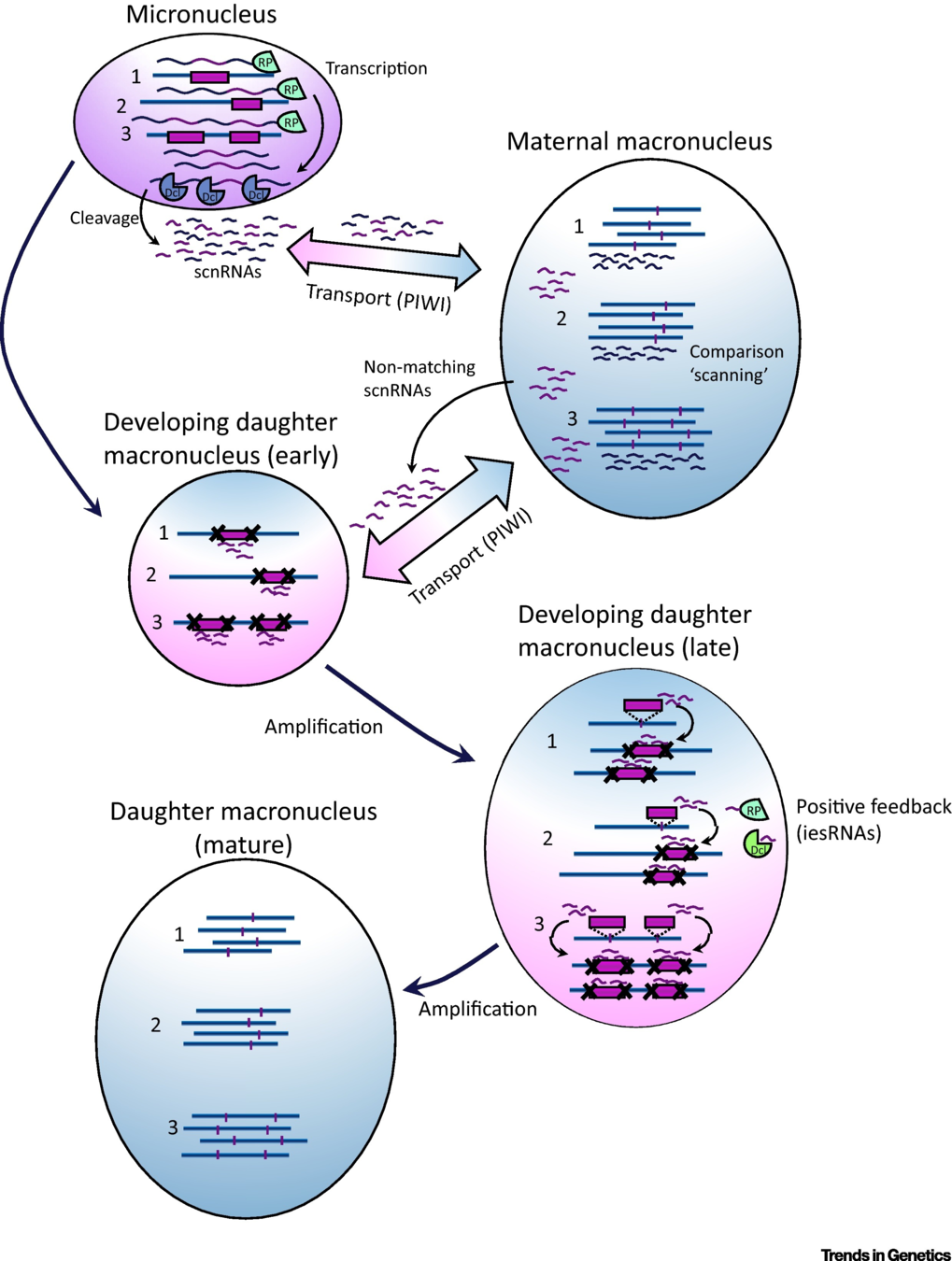
How are IES recognized ? (2/2)
The sc-RNA pathway
The DNA methylation hypothesis
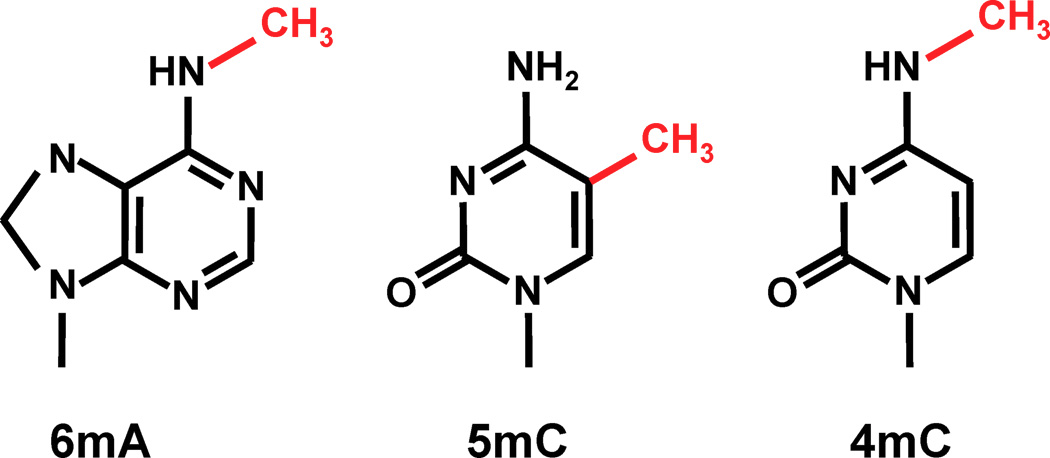
6mA likely to be abundant in Paramecium:
-
Suspected 2.5% in the MAC of P. aurelia by Cummings et Al (1975)
-
Also documented 6mA in the MIC
-
-
-
Detected methylation by SMRT in the MAC in our lab (unpublished data)
-
Detection by SMRT in the MAC by Sandra Duharcourt's lab (unpublished)
- Detection in Oxytrichia by L. Landweber et Al (2019)
- no 5mC, 4mC in the MAC a priori





2) Crosstalks in the new forming MAC
1) Constant pattern in the MIC
Transcient ?
Methylase candidates
Candidates:
- NM4, NM9, NM10
- Another family: MT1a, MT1b, MT2
2) PacBio sequencing with short inserts | 6mA ++
1) Grouped silencings by sequence homology
PacBio
TL;DR overview
PacBio SMRT principle


Reads up to 80 kbp

1) Sequencing (unique molecule)
2) High fidelity consensus
3) DNA methylation analysis
PacBio SMRT principle (2)

- Strategy 1: Long inserts (long reads)
- Ideal for assembly of long repeated sequences
-
Poor resolution for DNA methylation analysis
-
Strategy 2 : Short inserts (long reads)
- Much higher resolution for DNA methylation analysis
Step 1: Consensus

99% accuracy
Max
75% accuracy

Because our inserts are circular and shorts, we can make CCS of high accuracy despite a 15% error-rate
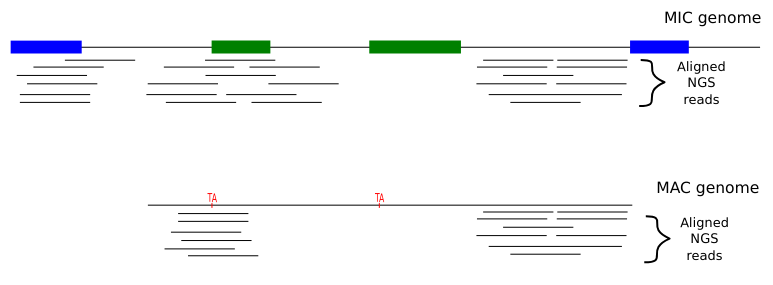
Step 2: Sorting
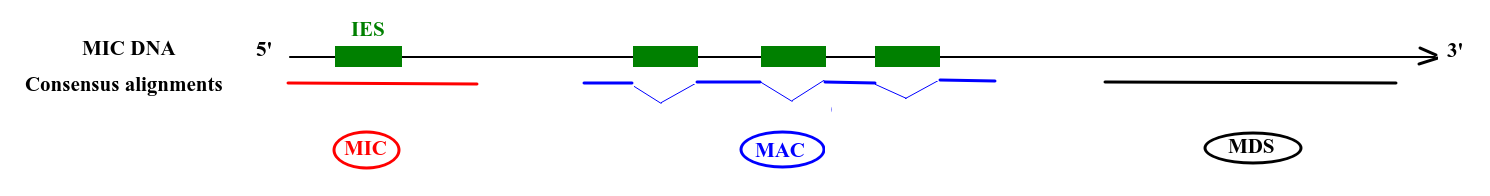
Deduced origin
MIC DNA
Alignment of consensus

TA
TA
TA
Step 3 : DNA methylation analysis
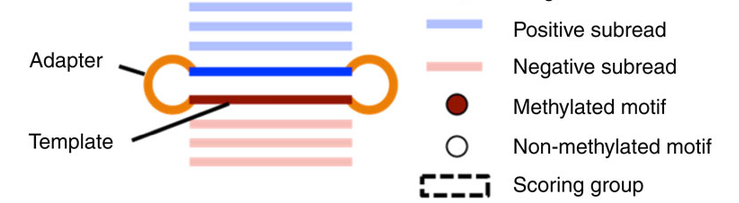
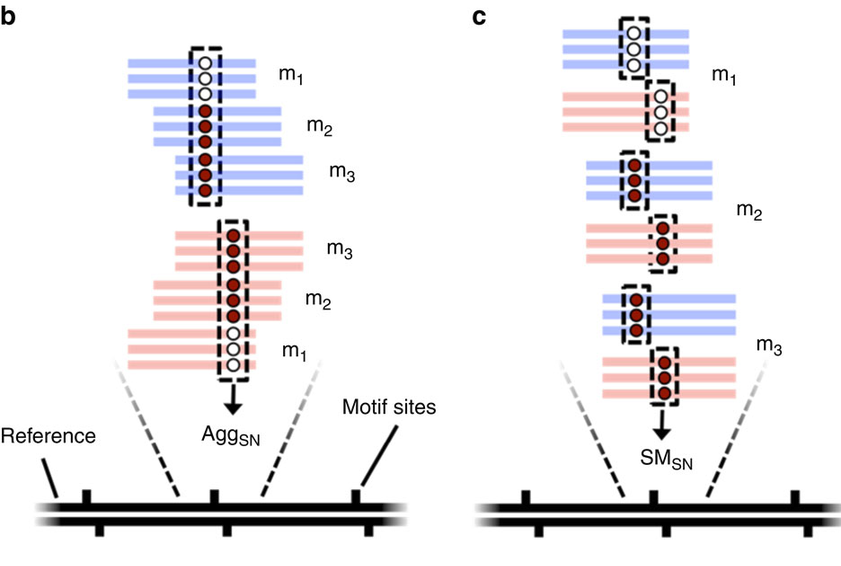
- Single-molecule
- Single-nucleotide resolution
- Independant yet pairable analysis on both strands
Some more details:
the random sampling approach

Deduced origin
MIC DNA
Alignment of consensus
Only a few remaining: ~ 10 to ~200 sequences
100% should carry a methylation pattern
-
1 out of 200 comes from the MIC
-
1/6 of MIC inserts will carry an IES
-
For 50% of IESs, we cannot be sure whether it come from the MIC or from the MAC
- 30% of the remnants are scanRNA dependant
Final categories
- MDS
- = "MAC" for 99% of sequences
- MAC_IES
- "true" MAC_IES (never seen retained)
- other MAC_IES (sometimes retained)
- MIC
- Other MIC specific (TE, repeated sequences)
- MAC (TA Junction)
- Overlap a TA junction of excision
- rDNA
- mtDNA
-
Other:
- Contaminants
- Alternative excision boundaries ("LOWID")
- low identity consensus ("Trash")
"genome"
Total Paramecium
Total sequencing
Little bug to be corrected
30%
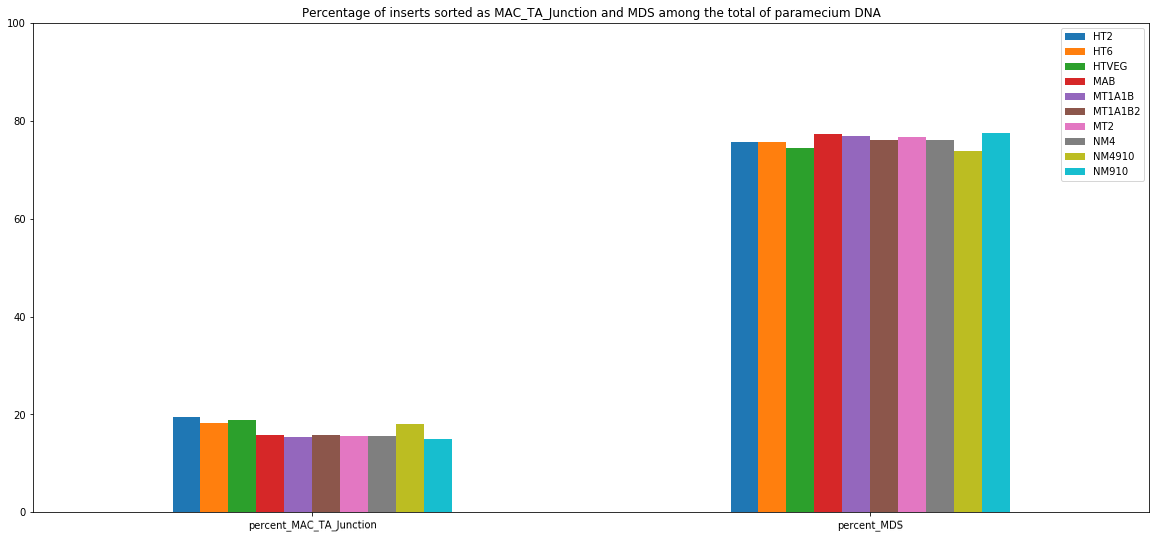
MDS and MAC_TA_Junction represent a vast majority
MIC specific and IES are as rare as expected
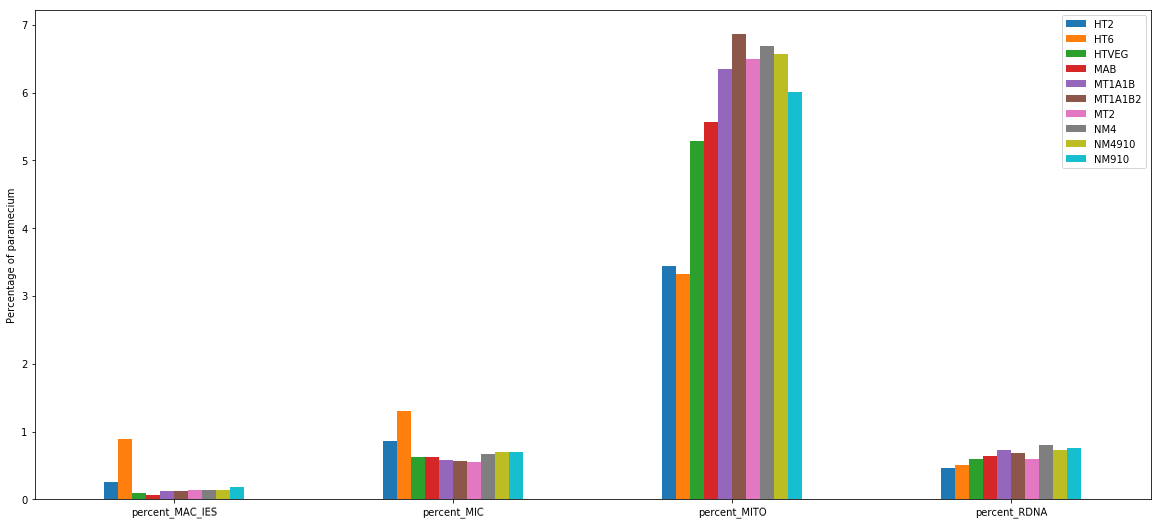
+ Applying the retention filter divides the number of "MAC_IES" approximately by 50%
Detecting m6A in details
Detecting m6A optimally
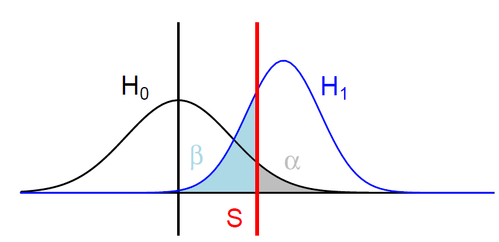
H0: ipdRatio of umethylated-Adenines
H1: ipdRatio of 6mA
S: Threshold on the pvalue
--> Specificity
--> Sensitivity
$$\alpha$$
$$1 - \beta $$
Adenine
6mA
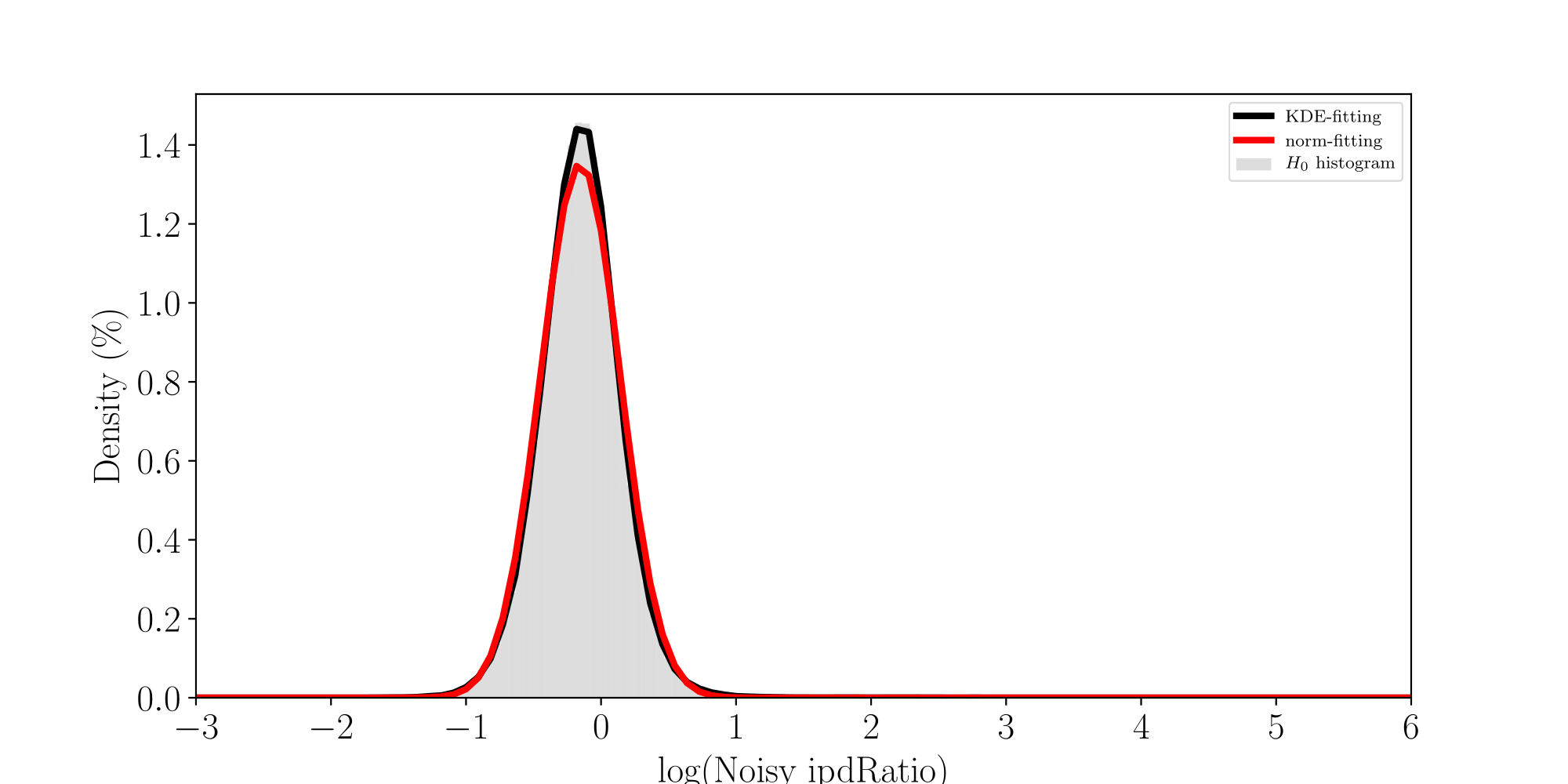
ln(ipdRatio) ~ N(0,1)
log(ipdRatio)
A pvalue is just the probability that log(ipdRatio) in the tail of H0
Using E.coli as ground truth
E.coli:
- Feeds paramecium: contaminants+++
- Nearly 100% symetrically-methylated with m6A
- GATC
- EcoK
- Few others
- Depends a lot of the strain
- Outside of GATC and EcoK: very low levels of m6A
I investigated the PacBio's output on it's GATC & EcoK VS other sites
How log(ipdRatios) look in E.coli
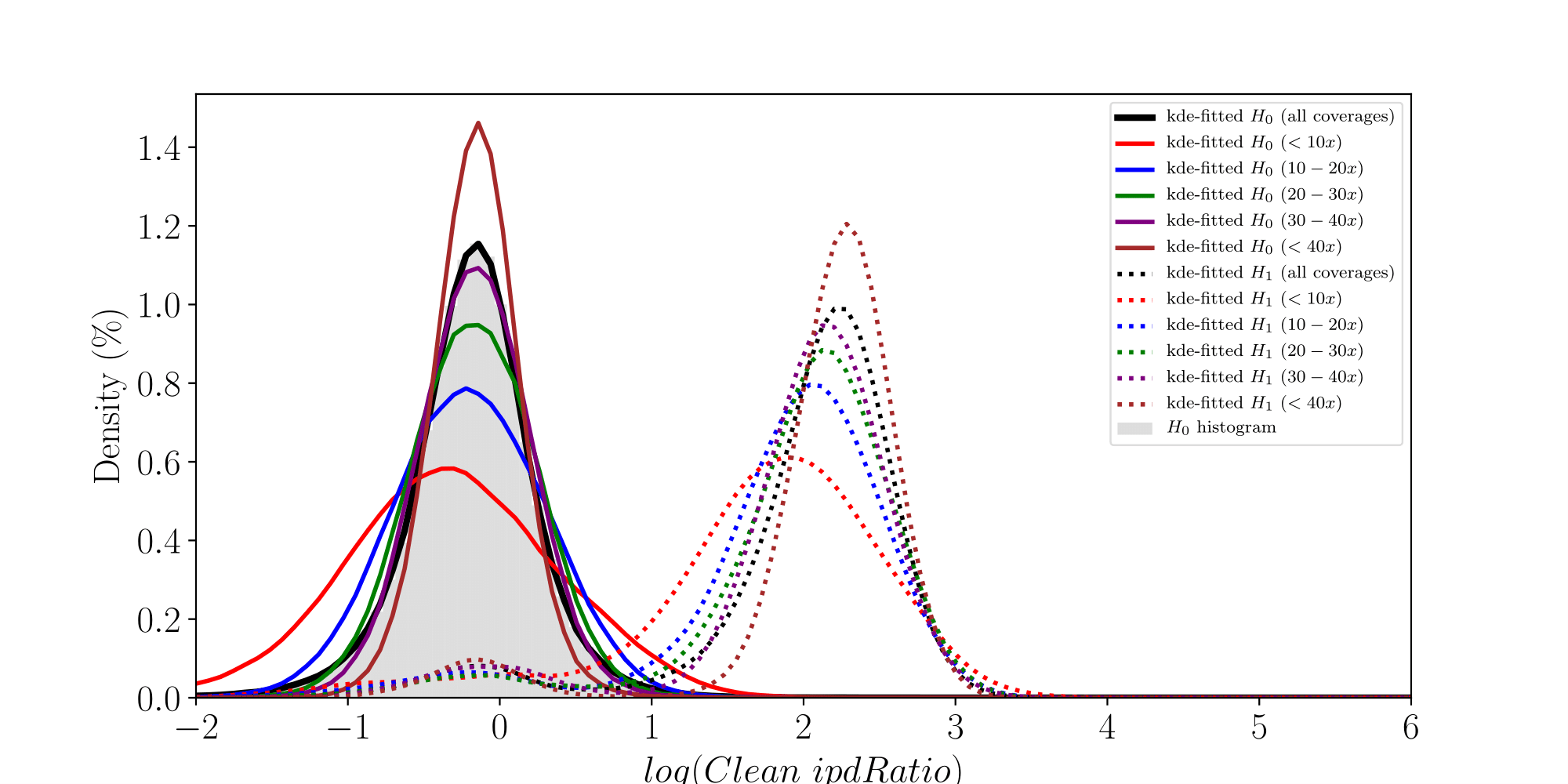
Separability raises with coverage, which is expected
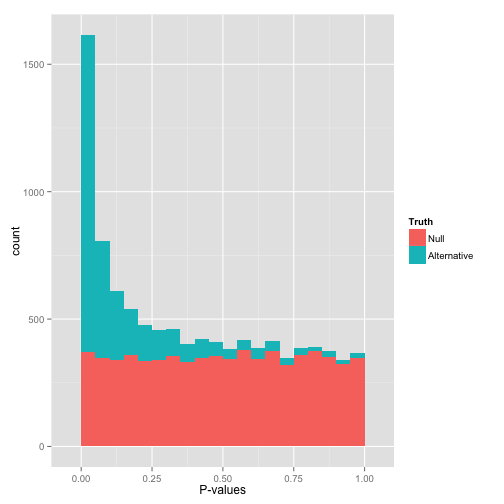
PacBio pvalues do have some biological meaning
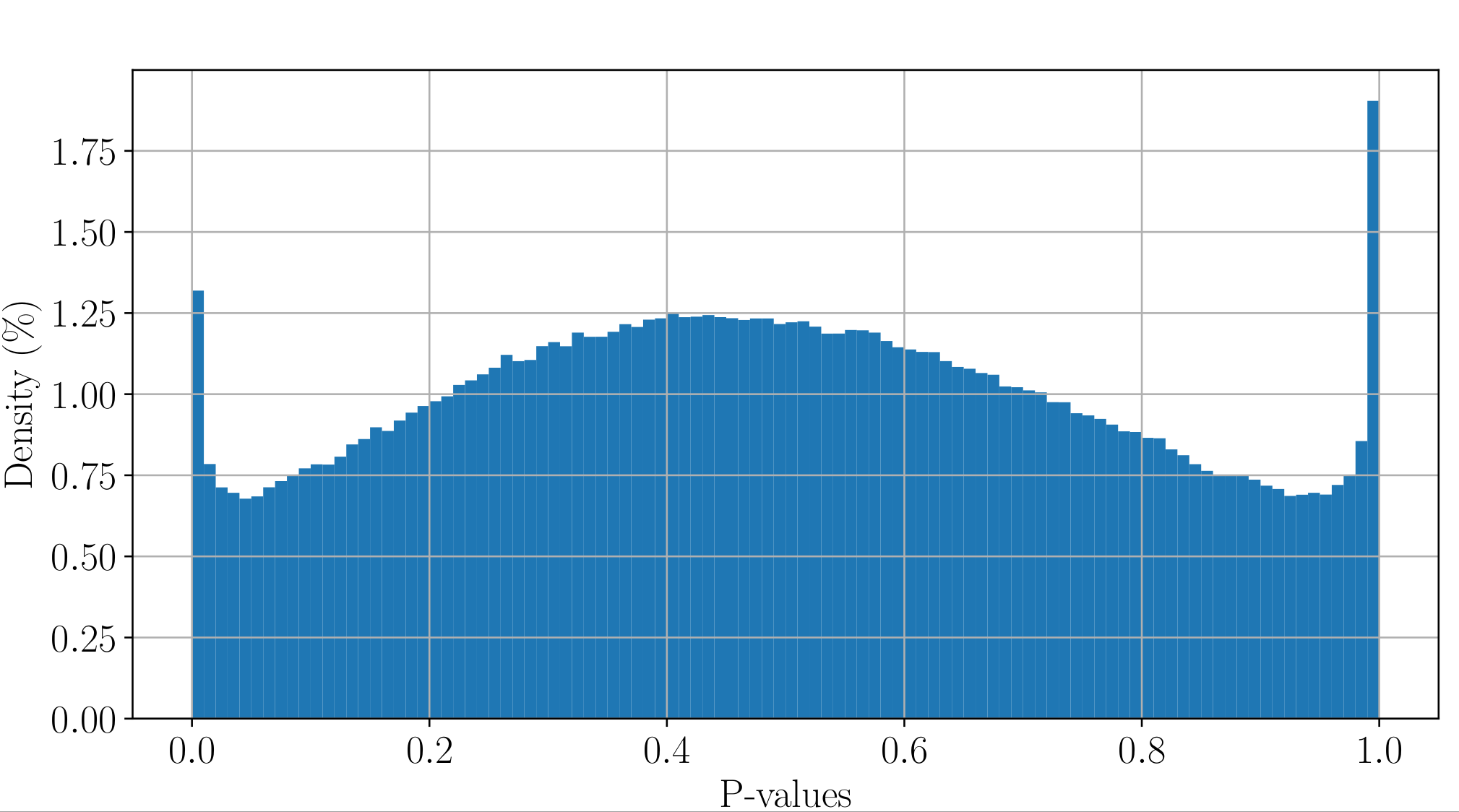
PacBio pvalues do have a meaning...
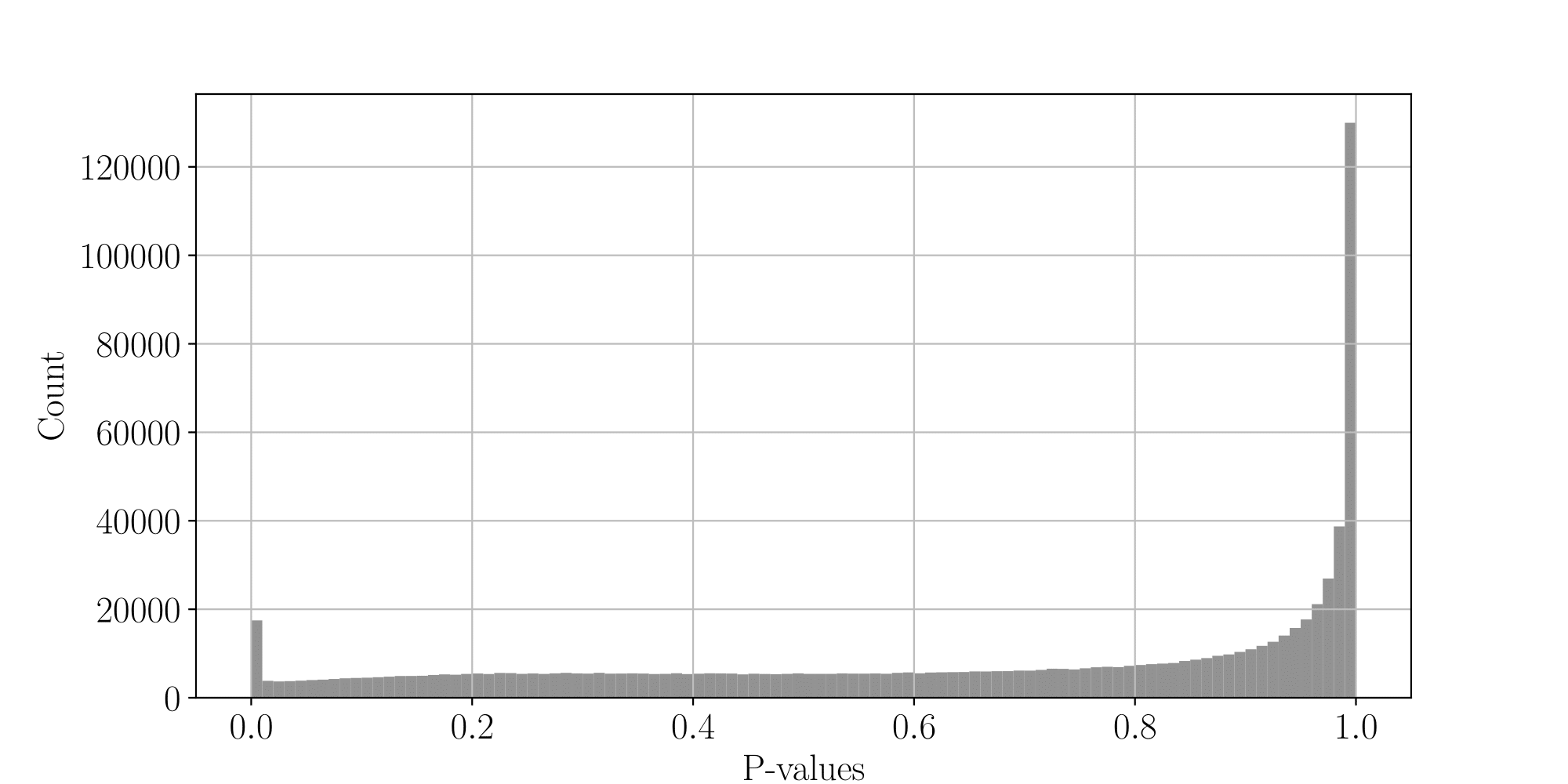
...but are not ideally distributed
Ideal pvalues
--> Allows magic !
PacBio's
- Allow estimation of
- Allows optimal, adaptative FDR control
$$\pi_{0}, \pi_{1}$$
- Just don't
-
Obvious point n°1:
- log(ipdRatio < 1) -> -inf
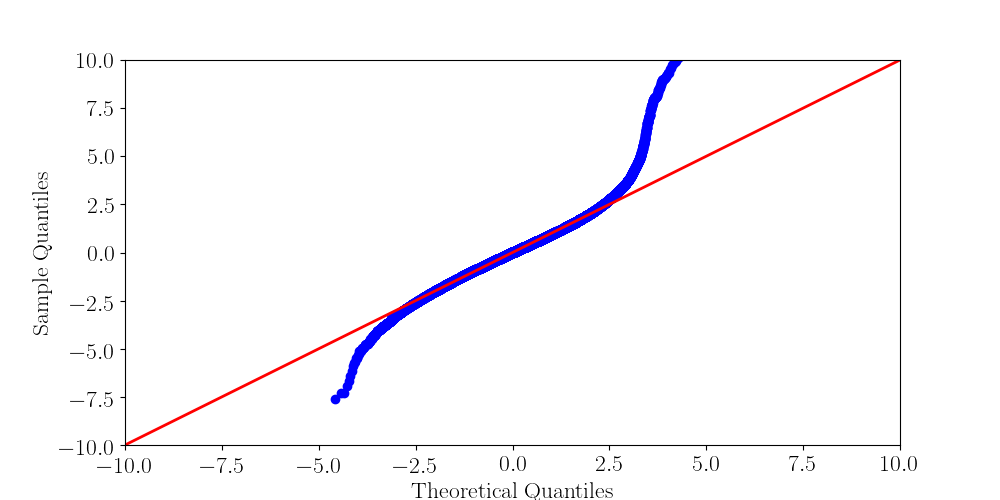
Normality assumptions under H0 are broken under high coverages
- The higher the coverage, the worse (here, >40x)
-
Will cause bell-shaped pvalues under null
- Hidden phenomena on the previous curve
Normality assumption matters
All Adenines' pvalues [E.coli] coverage > 40X
Long story short
PacBio's pvalues:
- Are biologically relevant
- We can build a reliable ad-hoc system with them
- Are produced by a linear combinations of > 150 different coverages
- Which forbids the usual statistical treatments
- Are somehow broken on a coverage-dependant manner
- Which forbids a simple fix for point 2
- We can't use the classical statistical treatments directly on pvalues
Other PBio's scores for m6A
For n6mA, PacBio produces:
-
A modification score --> Slowing of the polymerase
- pvalue against H0 only (the one we presented earlier)
-
An identification score --> Kinetic signature of a modification
-
loglikelihood between H0 and H1 (H1 = Other modifications or secondary peaks)
-
loglikelihood between H0 and H1 (H1 = Other modifications or secondary peaks)

They are PHRED-transformed p-values of two different statistical tests, that rely on the mean of the IPDs
PHRED scores
The scores (Qv) are PHRED-transformed p-values
Typical covscore plot
Modification score / coverage
Using flat threshold on modification score = Hudge lack of power
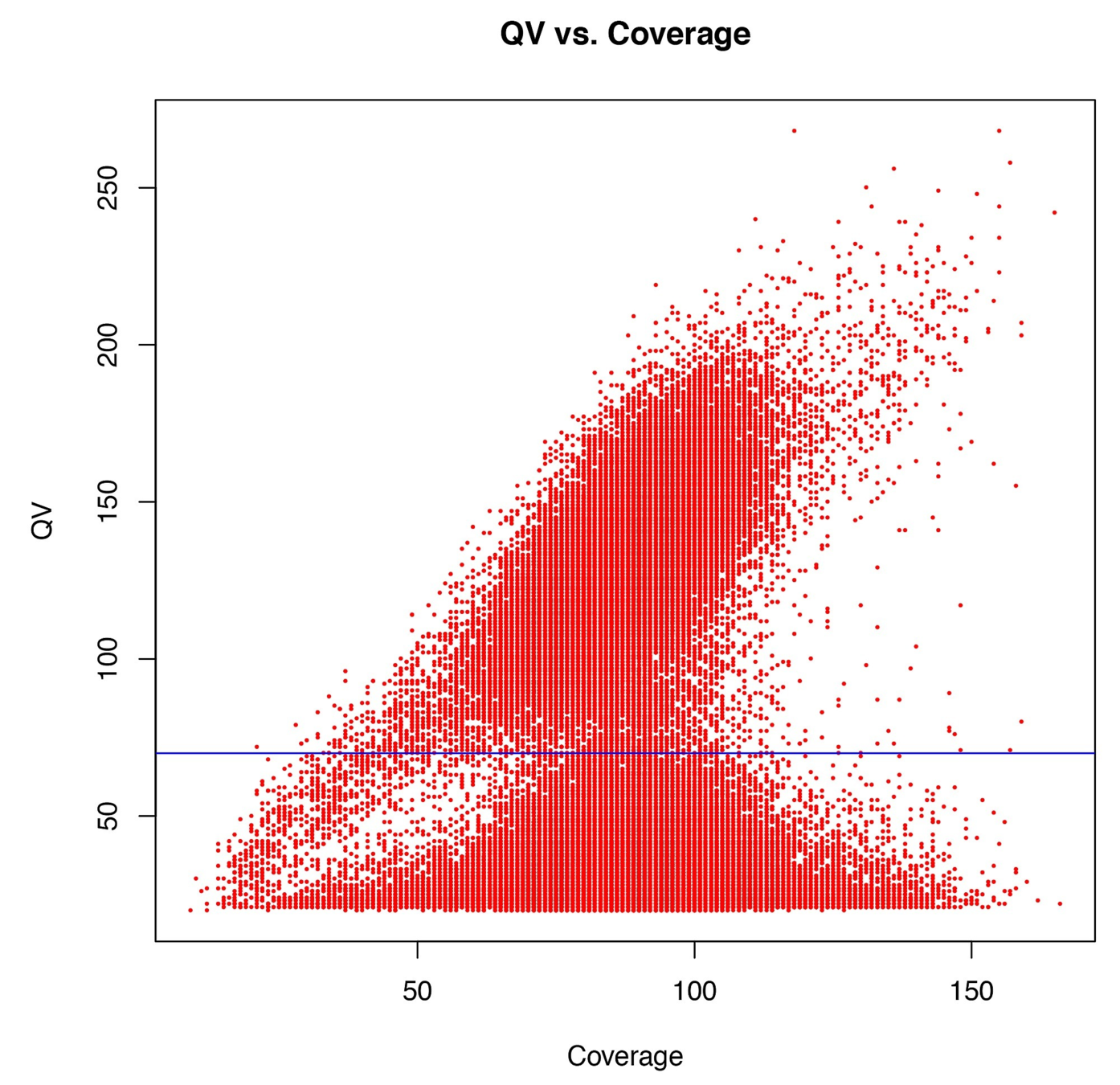
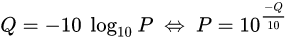
Solution : A coverage-dependant threshold on the scores
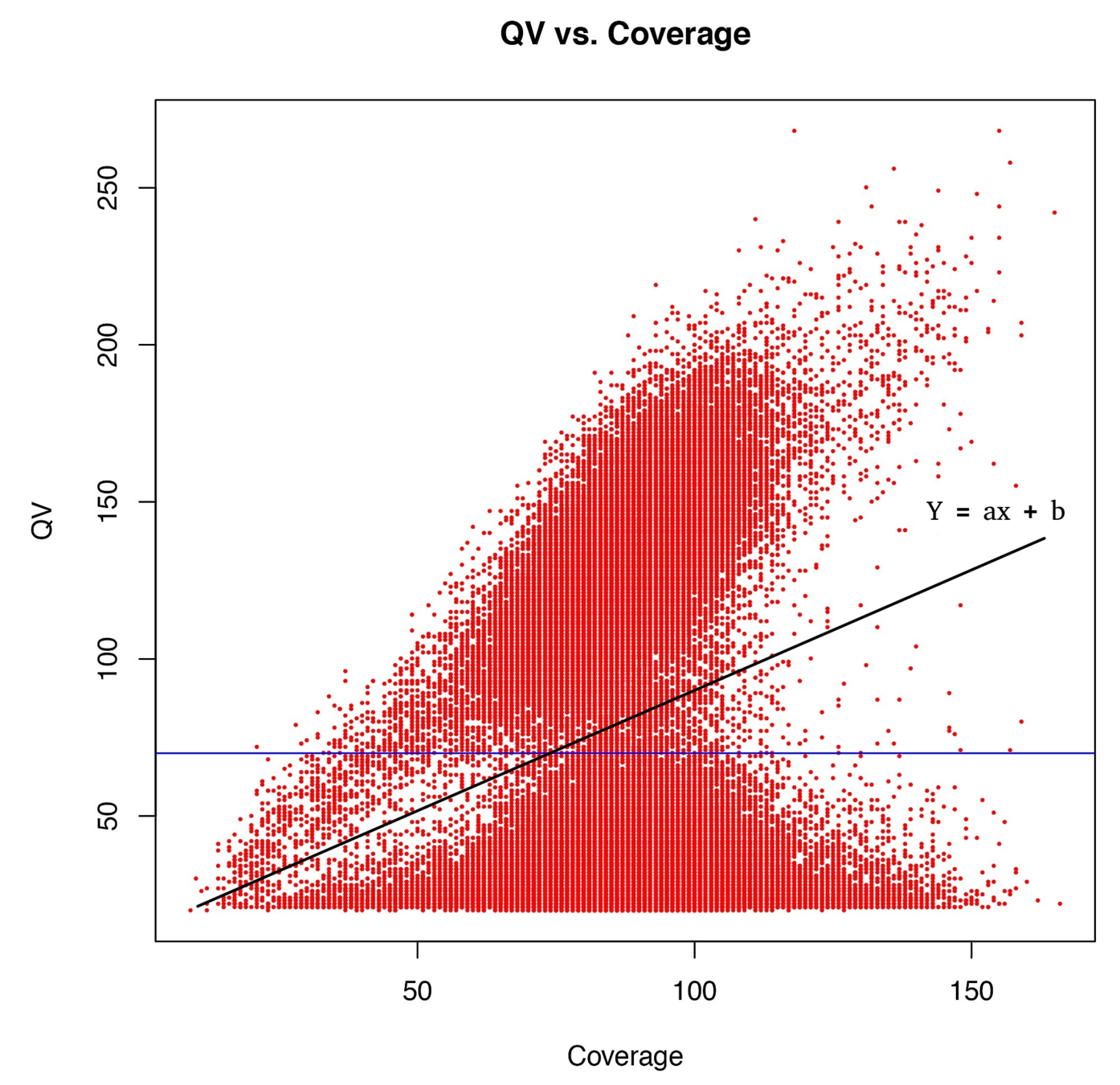
From now on
"positive detection"
=
score > linear thershold
(only >25X considered)
Benchmarking
How good (or bad) is our method ?
$$Se = P(D^+|M) ~ 92\%$$
$$Sp = P(D^-|NM) ~ 99.8\%$$
Starting from sufficient coverages (~20X to ~30X), Se and Sp don't depend on the coverage anymore
The 4 scenarii for Se and Sp
- We don't care about Se and Sp
- We care about the fact that eventual mis-estimations of them doesn't really change anything

Finding unbiased estimators for and FDR
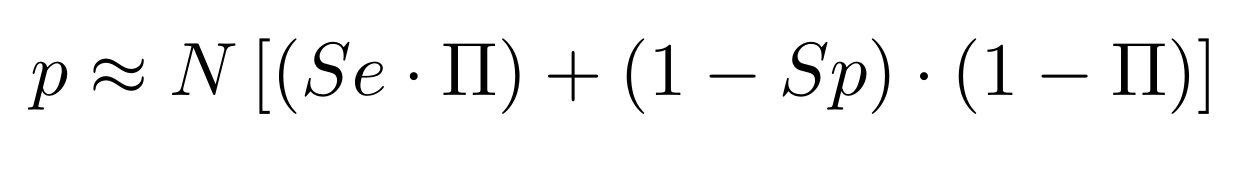
If p number of positive detections among N tests:
p = FP + TP
$$\pi$$
So,
Which means
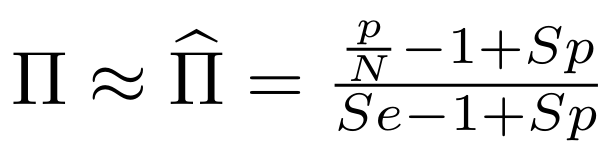
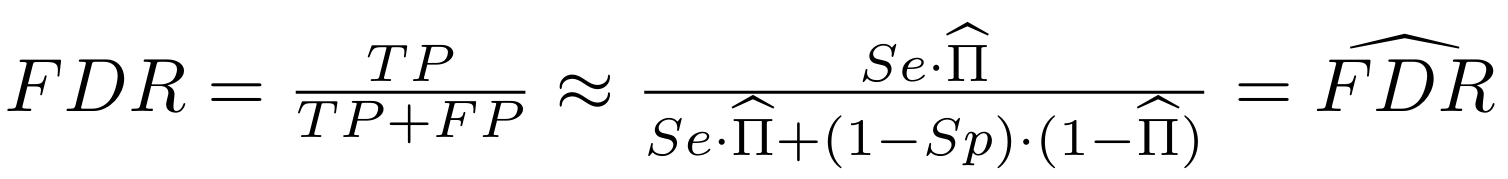
And:
What it gives in Paramecium

Debiased levels of m6A in the MAC_TA inserts
Details in MAC HTVEG
-
~95% of the methylation locates in AT dinucleotides in the MAC
-
True in any condition
-
-
75% of the methylation in an AT dinucleotide is actually symetrically modified, independantly from being in the MAC or the MIC

Kept in MIC and MAC
All conditions
MAC_TA outside of AT sites
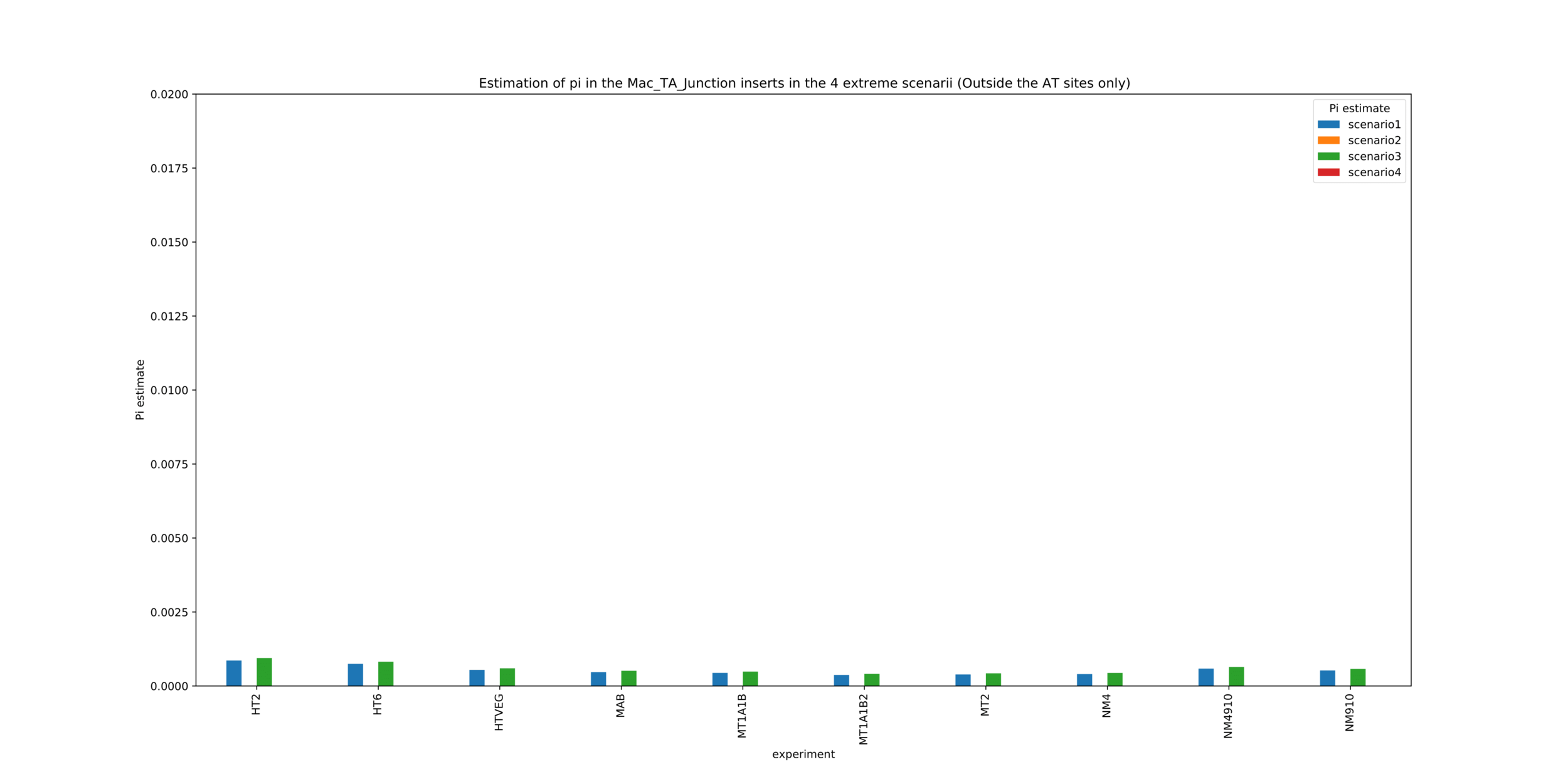
Detections outside AT sites are likely to be at least partly true positives

Present in all samples
Never erased
Conclusion: We are either in scenario 1 or 3
(Sp largely underestimated)
Was expected but confirmed
Methodological development to correct hemi-methylation detection (1/2)
Let FD1 and FD2 be resp:
- Fraction of AT sites detected hemi-methylated
-
Fraction of AT sites detected symmetrically methylated
PZ0, PZ1, PZ2: unbiased estimators of non, hémi, symetrically methylated AT sites
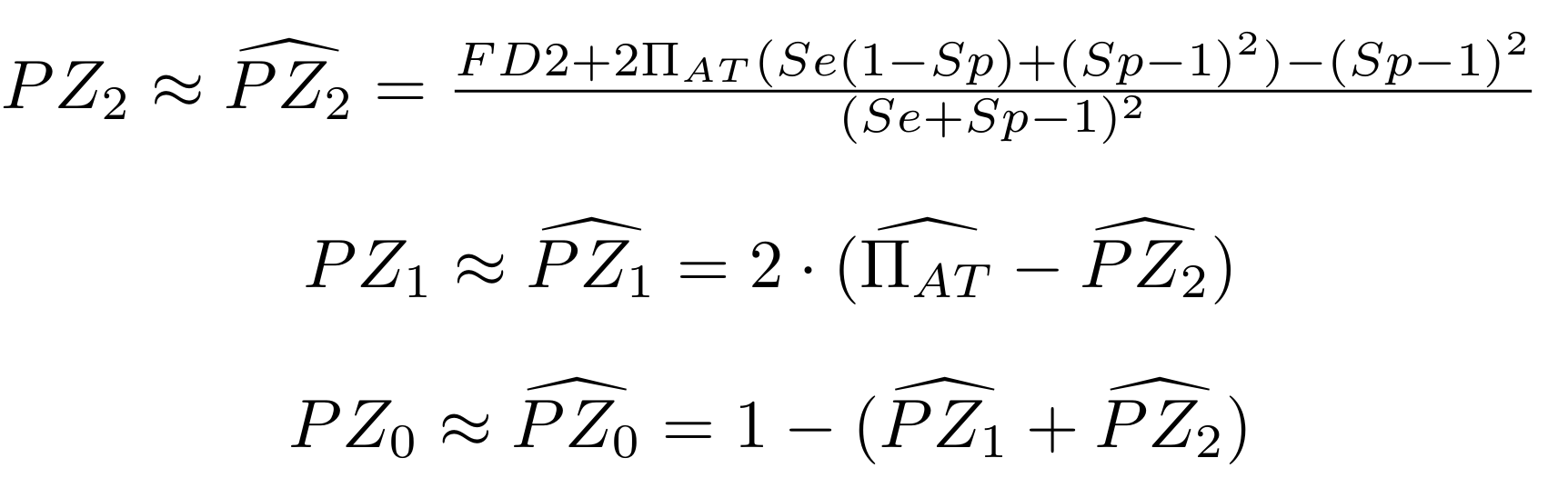
Then:
With
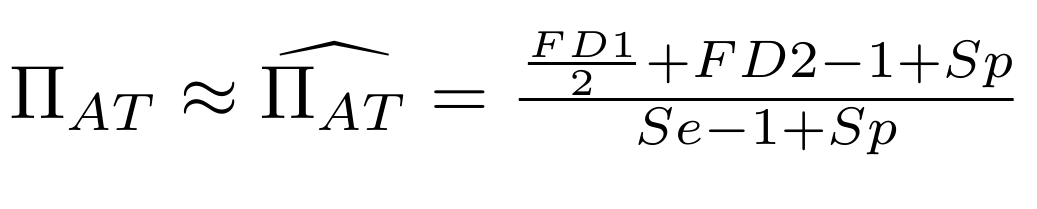
What it gives in Paramecium
Debiased Hemi-methylation in MAC_TA

n6mA in the MIC (preliminary)
Pure MIC sequences: Cannot yet be trusted (error in the pipeline)


Coverage >= 40X made us lose too many materials, should restart with >=20X
n6mA in the MAC_IES rarely retained ("TRUE MAC IES")

HTVEG

MT2
--> Some molecules carry all the detections, in sym-A*T
Very likely to be sequences comming from the MAC to my opinion
At first look, seems like the same in all samples
Conclusion
- Lots of sweat spent on methods : Now we start the cool things
-
In the MAC
- Quantification in the MAC is well-characterized in all the samples
- Our 6 genes are implied in the bulk of the MAC m6A
- MT and NM families: Functional analogs of DNMT1 ?
- Lots of questions raised by the MAC methylation: TSS, IES Junctions, nucleosome positionning...
-
In the MAC IES
- Very preliminary analysis between TRUE mac IES and other MAC IES tends to show that all detections could come from accidental retention in the MAC, and the MIC could actually carry 0% m6A or very lower levels than 1%. Really to premature to be sure
- HT2 and HT6 are still potentially full of surprises
- TE in the MIC: No idea yet
- It's just a question of time now ! Which we asked (prolongation LABEX memolife)

Sorry for the headaches !! Thanks for your time :)
Ciliates are great model organisms

1862 - Pasteur: Refutation of the spontaneous generation theory with infusoria


1937 - Sonneborn: Non-mendelian inheritance of sexual type in paramecium


Elizabeth blackburn & Carol Greider: 1985 - Telomeres and telomerases in Tetrahymena (Nobel Prize 2009)

Eric Meyer, Sandra Duharcourt
(IBENS, I. Jacques Monod 2014)
Sexual type in paramecium are transmitted by maternal RNAs, not by DNA
2.1 Retroingineering
The capping of IPDs
-
modelPrediction is the predicted IPD value by the model in a given context of nucleotides at this position
-
globalIPD is the mean of all the IPD values of the read.
-
localIPD represents all IPDs that have been mapped at a given position in the genome, including those from other sequences
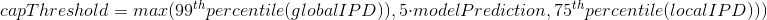
Conclusion on the capping
- Isn't coded as advertized by PacBio
- The way it's implemented for AggSN is problematic and doesn't really make sense
- Paradoxally, it should be more relevant for our approach than for the default one
- We expect no methylation to be undetected due to the capping
Laura landwebehr 2020
Oxytrichia trifallax
p values (A)
p-values (A score >20)
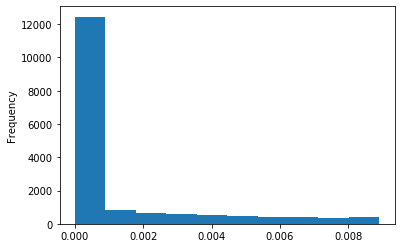
Out GATC score < 20
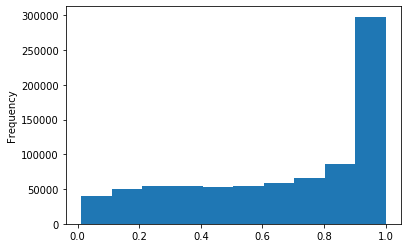
Out GATC
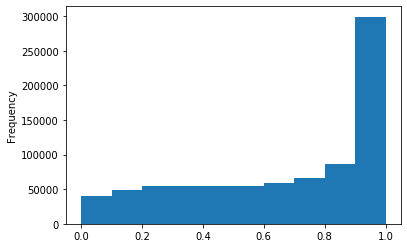
ipdRatio score 20
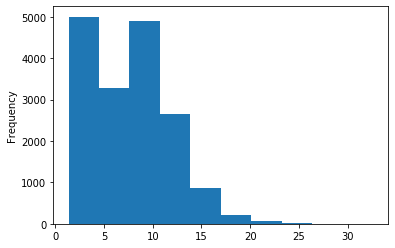
ipdRatio idv20/score20


A outAT score 20 isQv20 (812 seq)
A outAT score20 idQv20 + Strong BH correction (176 seq)
ipdRatio out GATC before filtering BH vs after (qv20/idqv20)
In GATC
Copy of CCG presentation - D
By biocompibens
Copy of CCG presentation - D
28/02/19
- 93



