Mapping Your Review: Clarifying the Systematic Review Process and Searching
Systematic Reviews
Elizabeth Torres
What is Evidence Synthesis?
Evidence synthesis refers to the structured, transparent process of gathering, evaluating, and combining findings from multiple research studies to answer a specific question.
Rather than relying on a single study, evidence synthesis methods (such as systematic reviews, meta-analyses, and scoping reviews) bring together all available, high-quality research on a topic to identify overall patterns, strengths, and gaps in the evidence base.
It is a cornerstone of evidence-based practice (EBP)—helping clinicians, educators, and policy makers make decisions informed by the totality of existing research rather than isolated findings.
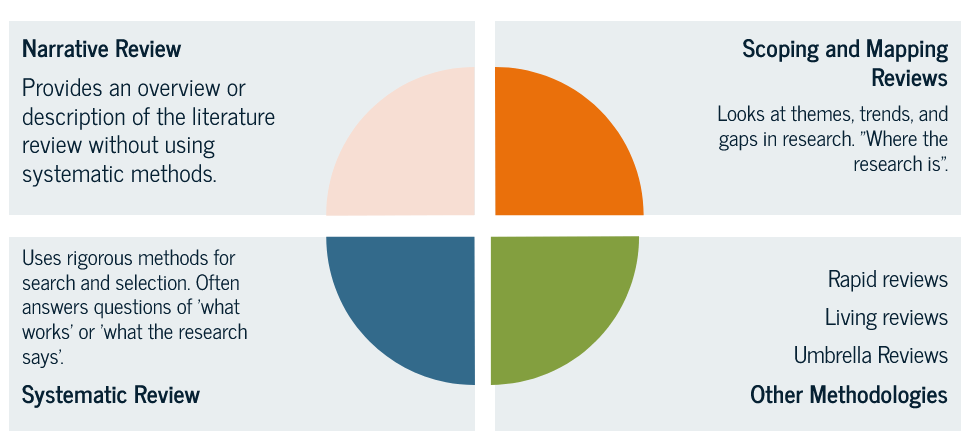
Types of Reviews
Grant, M. J., & Booth, A. (2009). A typology of reviews: An analysis of 14 review types and associated methodologies. Health Information & Libraries Journal, 26(2), 91-108. https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1471-1842.2009.00848.x




Does Your Project Qualify as a Systematic Review?
Text

-
Clearly defined question (often PICO)
-
Pre-registered protocol (PROSPERO, OSF)
-
Two-person screening and data extraction
-
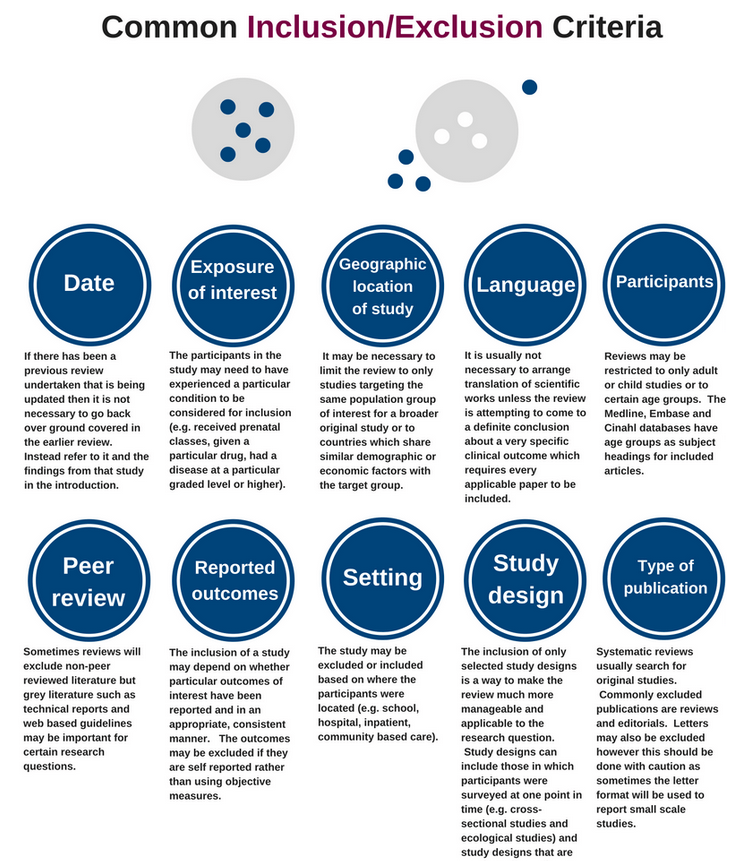
Transparent inclusion/exclusion criteria
-
Comprehensive search in multiple databases
-
PRISMA flow diagram
-
Critical appraisal and synthesis
Systematic Review Checklist

Typical Team Composition



PI, co-investigators, methodologist, subject experts, statistician, librarians.
1.
Preparation
Protocol Development
- Refine and finalize the research question PICO
- Determine inclusion/exclusion criteria
- Fill out protocol template for registration
2.
Retrieval
Perform Searches
- Translate master search strategy to syntax of all databases
- Hand search journals & databases
- Calls to listservs or know researchers
- Search grey literature
3.
Appraisal
Article screening and Risk of Bias Assessment
- Screen all studies from search against eligibility criteria
- Title & abstract screening
- Full text screening
- Perform risk of bias assessment
4.
Synthesis
Data Extraction & Synthesis
- Extract data from included studies
- Thematically characterize or quantitatively synthesis data from included studies
5.
Write Up
Writing the Article
- Write the various sections of the article
- Write up information retrieval methods section
- Prepare the search strategies for publication in appendix or supplement
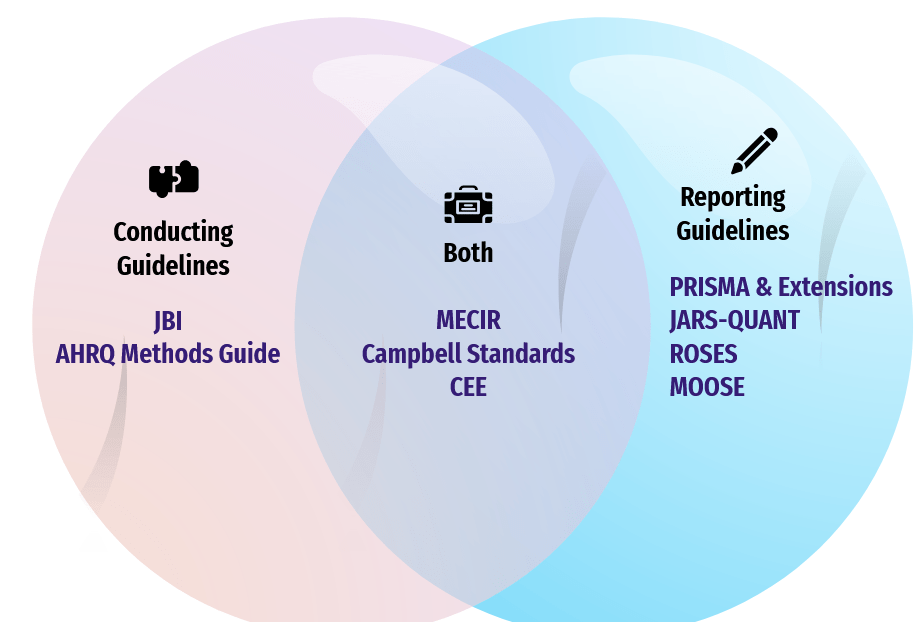
Conducting Guidelines
- Joanna Briggs Institute Manuals
- Agency for Healthcare Research Quality Methods Guide for Comparative Effectiveness Reviews
Reporting Guidelines
A concise playbook for systematic reviews: define eligibility, screen studies, and extract data.
List of reporting items for researchers to use when documenting study details in IMRAD (Intro, Methods, Results, & Discussion) and beyond (abstracts, appendix, supplemental).
Conducting & Reporting Guidelines Examples
- Methodological Expectations of Cochrane Intervention Reviews (MECIR)
Standards for the conduct and reporting of new Cochrane Intervention Reviews
- Campbell Standards for Conduct and Reporting of Systematic Reviews
Standards for the conduct and reporting of Campbell systematic reviews
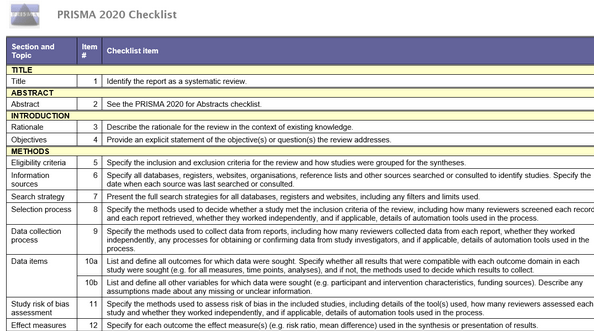
PRISMA checklist + flow diagram
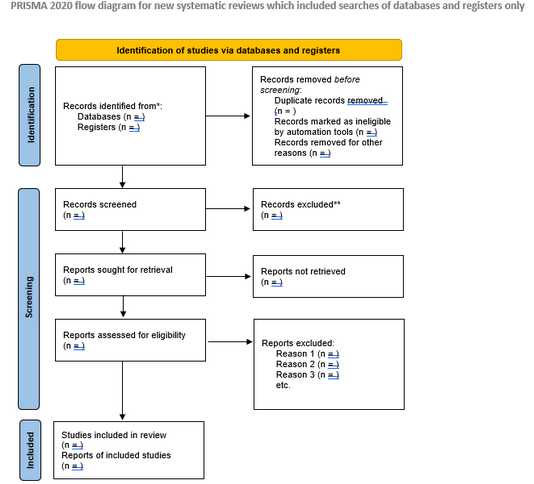
PRISMA, PRISMA-S, and PRISMA-P What’s the difference?
| PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) | Reporting the review | The main guideline for how to write and report a completed systematic review or meta-analysis transparently. | When you’re writing up your finished review. |
| PRISMA-S (PRISMA extension for Searching) | Reporting the search process | An extension of PRISMA that focuses specifically on how to describe your literature search—databases, search strategies, updates, peer review of search, etc. | When you want to document or publish your search strategy in detail. |
| PRISMA-P (PRISMA for Protocols) | Planning the review | A guideline for how to write and register your systematic review protocol, including your objectives, eligibility criteria, and planned methods. | When you’re developing or registering your protocol (e.g., in PROSPERO). |
-
A blueprint for your entire project
-
States your rationale, hypothesis, and planned methodology
-
Created a priori ensures decision are made objectively
-
Registered/shared for transparency
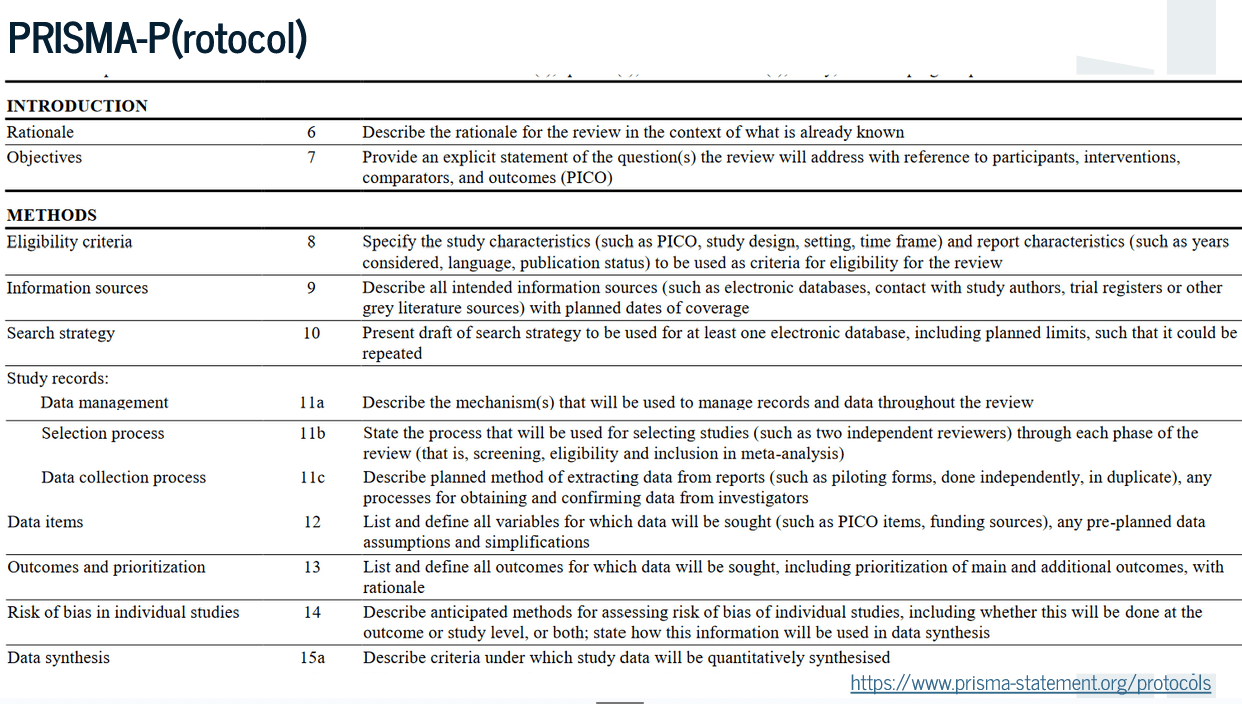
What is a Protocol?


How much detail do I need?
- PRISMA-P - Preferred Reporting Items for Systematic Reviews & Meta-analysis - for Protocols (https://www.prisma-statement.org/protocols)
- Chapter 5 (Protocols) in Campbell Systematic Reviews: Policies & guidelines
- Guidance for scoping reviews: Peters et al., (2022). Best practice guidance and reporting items for the development of scoping review protocols. JBI Evidence Synthesis, 20(4), 953-968. https://doi.org/10.11124/jbies-21-00242
Protocol Registries for Evidence Syntheses with Health Related Outcomes


Inappropriate or inadequate search strategies may fail to identify records that are included in bibliographic databases.
Research Strategy & Planning
Research Strategy & Planning
Research Strategy & Planning
Research Strategy & Planning
Evidence synthesis requires not only top notch search results, but reproducibility.
- Platform capabilities: Which databases or interfaces are you using (e.g., PubMed, CINAHL, Medline via EBSCO or ProQuest)?
- Saving searches & histories: Does the platform allow saving, rerunning, and exporting your search strategies for PRISMA-S documentation?
- Reproducibility: Can another researcher repeat your search exactly (same filters, syntax, date limits)?
- Working with results: Are there tools for citation management, tagging, or exporting to Covidence/Rayyan/Zotero?
- Downloading results: How many results can you export at once? (some limit to 200–500 per batch)
Document everything - decisions, what worked, what didn’t.
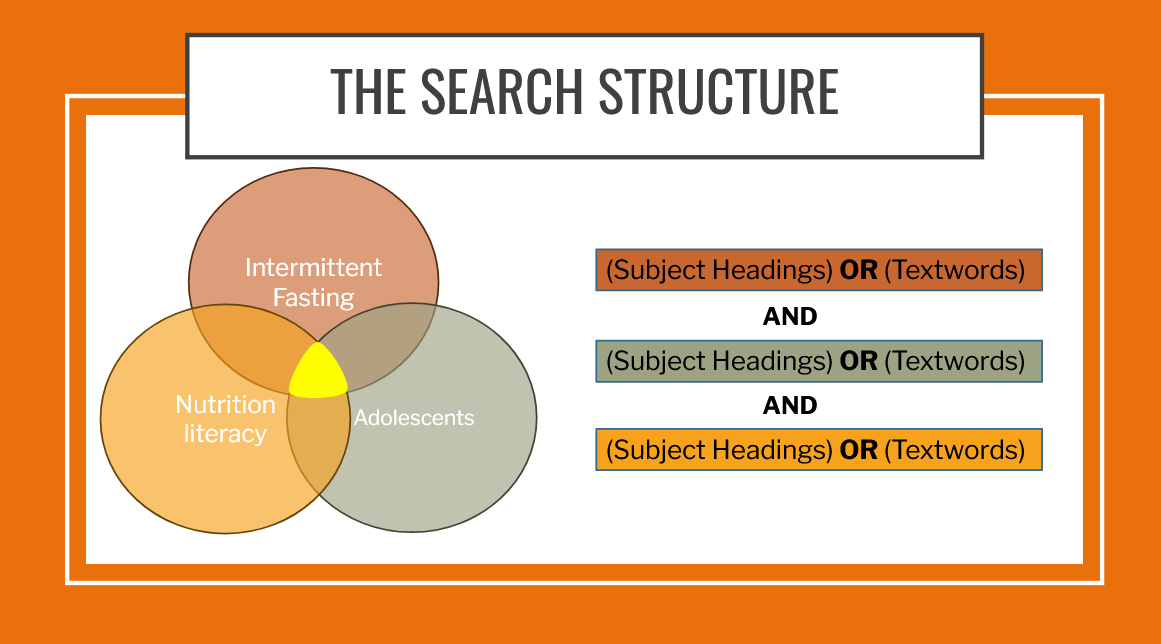
The Search
Research Strategy & Planning
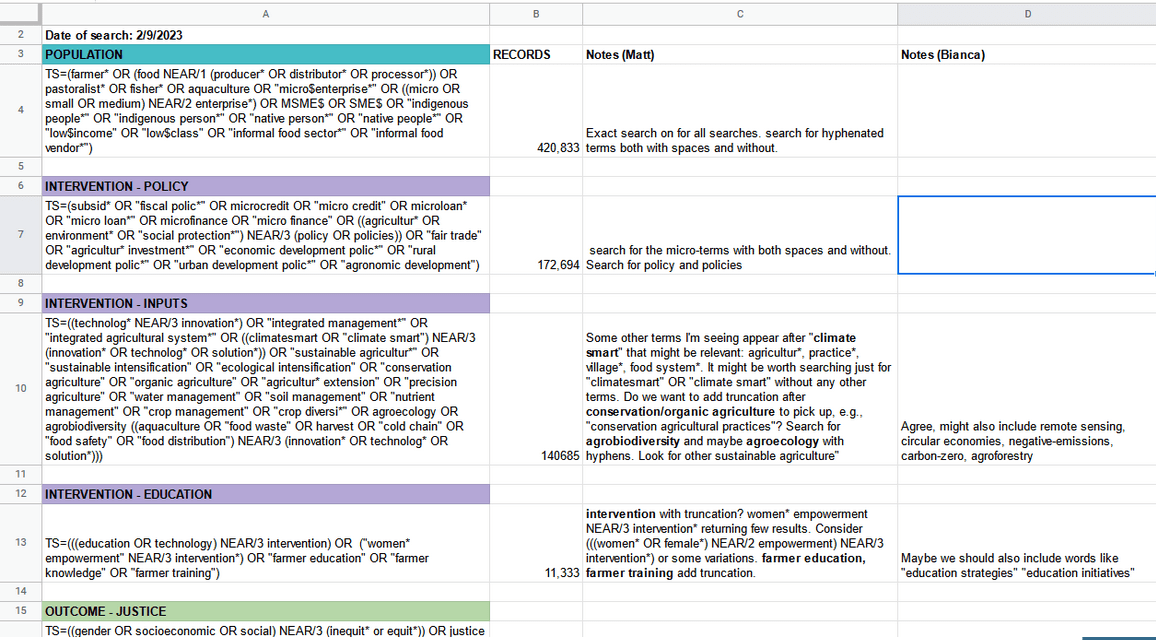
Search strategies need to be customized for each database
-
Not all criteria need to be included in the search strategy and are better incorporated during screening process
-
This may vary between databases
-
Think about the concepts that are likely to appear in titles/abstracts vs. full text
-
Finding a balance between too narrow (few) and too broad (many)
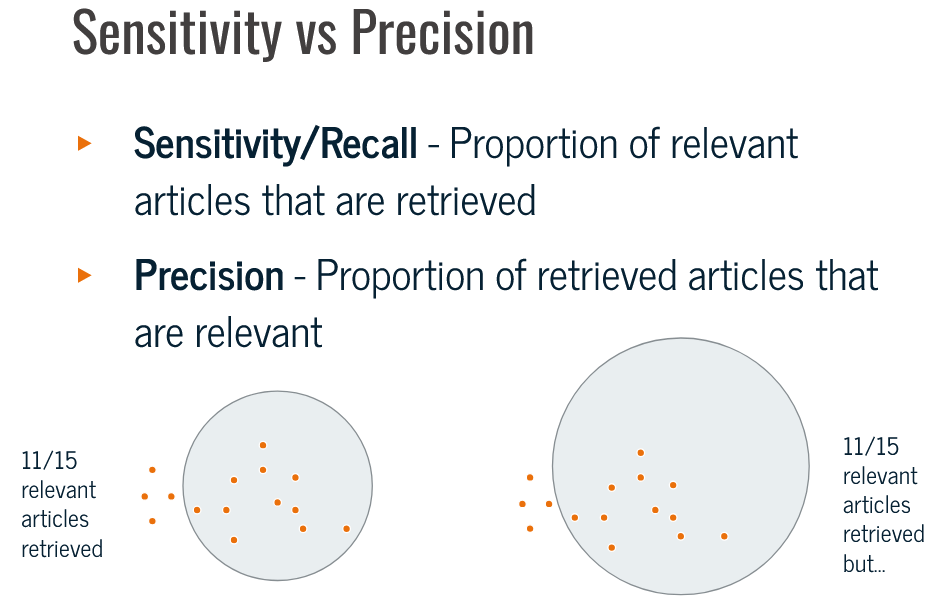
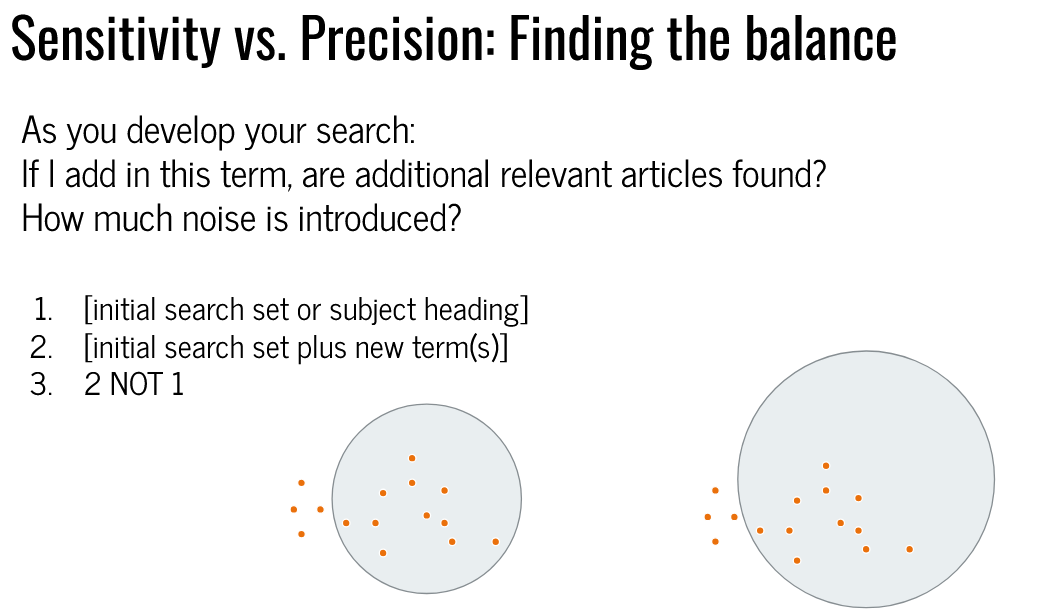
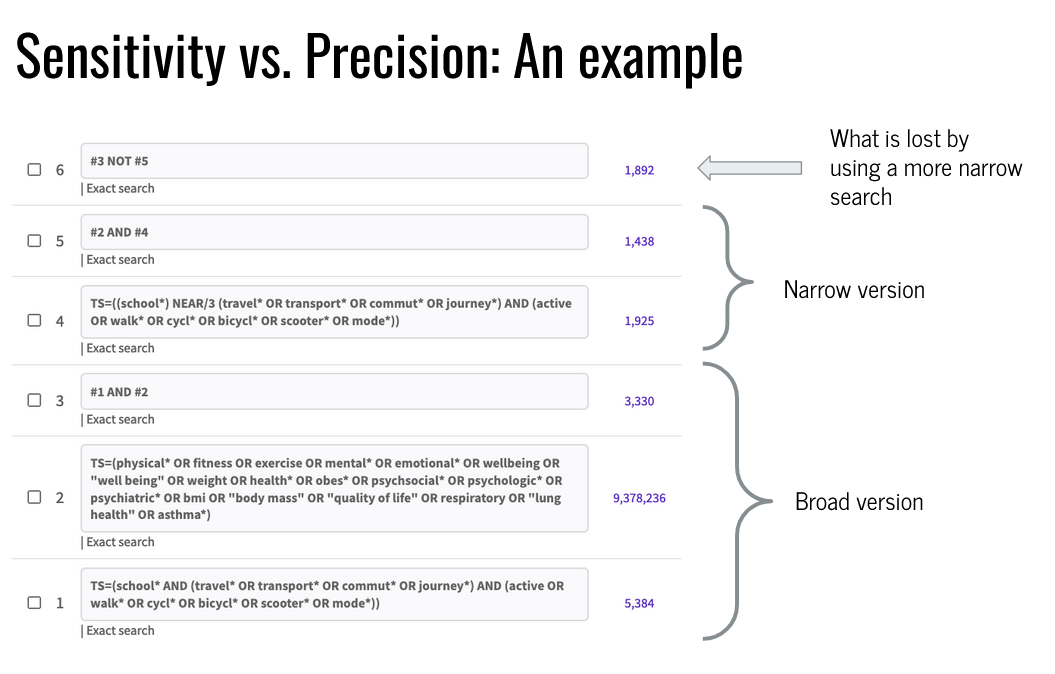
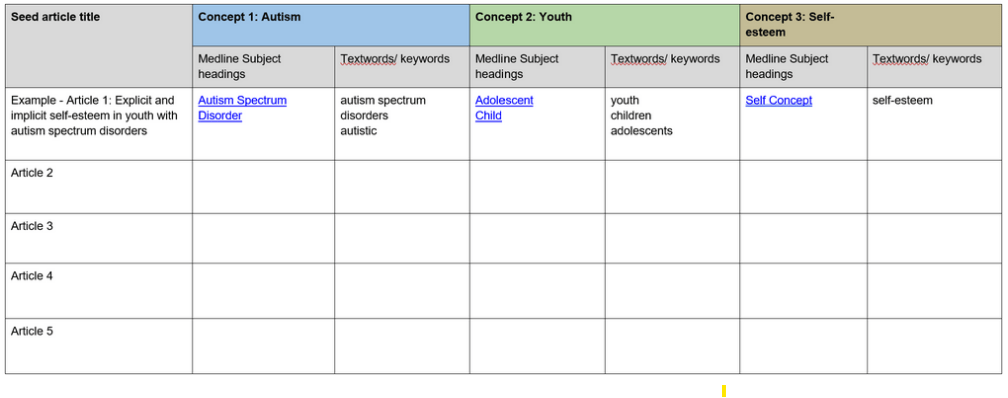
Gold standard’ or Seed articles,
Document everything - decisions, what worked, what didn’t.
For databases with a good thesaurus, look up these articles and explore the assigned subject headings.
-
Look at other protocols and/or evidence syntheses on your topic. Did they include details on grey lit sources searched?
-
GreyNet International has some guidance http://www.greynet.org/
-
Grey literature search checklists (but mostly in the health sciences),
Gray Literature
PRISMA-S item 3,4,5,6
- Was grey lit included? If so, what efforts were made to identify? Where did you search and how?
- Was hand-searching / TOC searching included? By whom, and what sources?
- Were experts in the field contacted? If so who, and how?
- Were citing/cited references searched? If so, how?
☆ At least one of these things should have happened
Gray Literature

-
Screen all studies retrieved from the search against eligibility criteria
-
Perform risk of bias assessment for included studies
Appraisal
Article Screening Software: Tools & Library Support
- We don’t license Covidence or DistillerSR.
- Use free options via the SR Toolbox (tool directory by review stage).
- Practical stack we support: Zotero, Excel/Sheets.
- We can help handle deduplication and PDF harvesting/organizing.
- We don’t license Covidence or DistillerSR.
Bias Assessment Tools
-
Consider the populations that your research team works with and how research quality may be defined differently in different knowledge systems
-
Risk of Bias and Critical appraisal tools must be designed and fit for purpose
Get In Touch
etorres@hpu.edu
library@hpu.edu
Systematic Reviews
By Elizabeth Torres
Systematic Reviews
- 113



