Cortical representation learning
& Bayes-optimal cue integration
Deperrois, N., Petrovici, M.A., Senn, W., & Jordan, J. (2021). Learning cortical representations through perturbed and adversarial dreaming. arXiv preprint arXiv:2109.04261.
Jordan, J., Sacramento, J., Wybo, W. A., Petrovici, M. A., & Senn, W. (2021). Learning
Bayes-optimal dendritic opinion pooling. arXiv preprint arXiv:2104.13238.
Jakob Jordan
Department of Physiology, University of Bern, Switzerland
03.03.2022, SCN Retreat, Crans-Montana, Switzerland






- structured neuronal representations underlie our capacity to behave successfully, and our fast/flexible learning capabilities
- latent representations emerge from unsupervised learning principles
- here: we propose that sleep and in particular REM dreams implement adversarial learning
Motivation
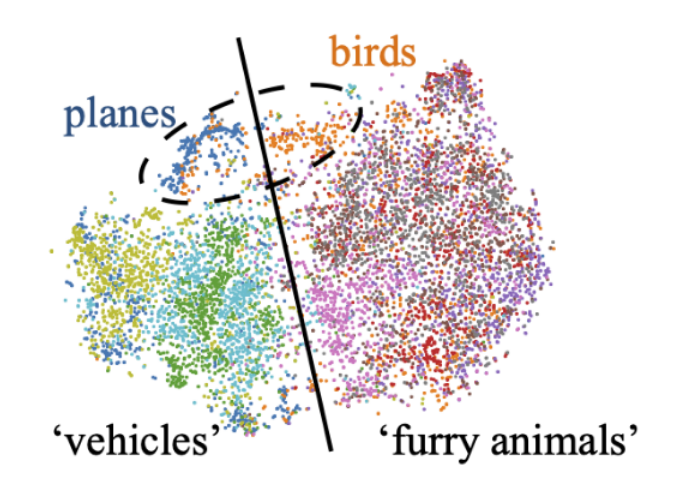
[Illing et al., 2021]
[Goetschalckx et al., 2021]
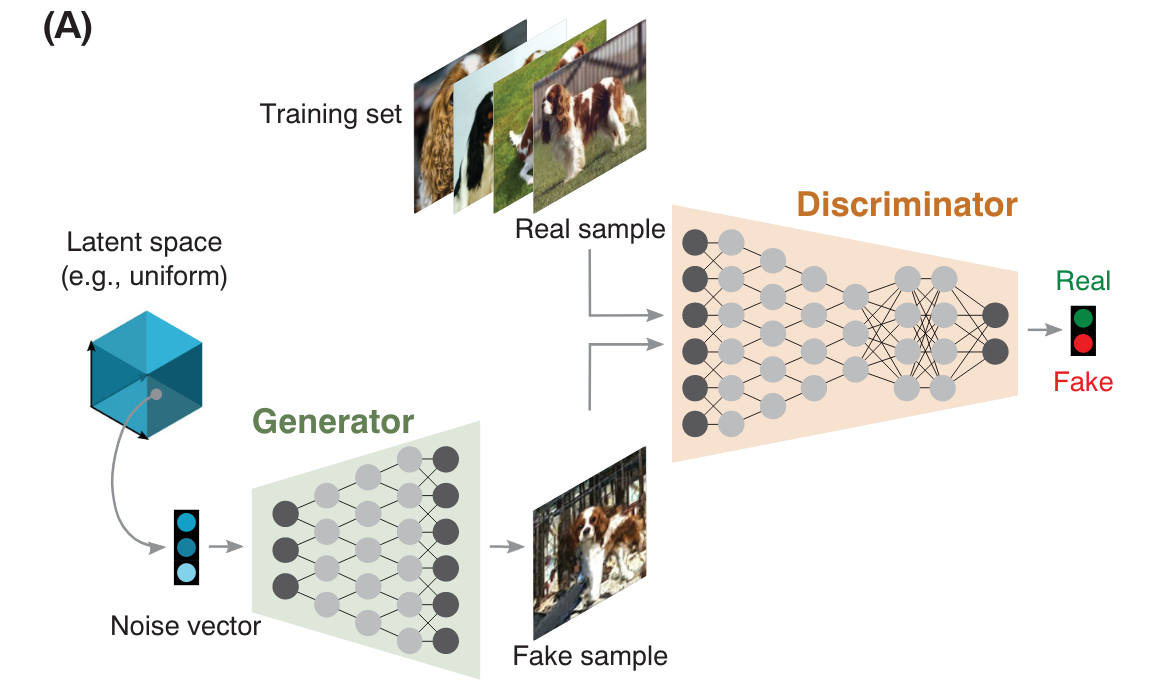
GenerativeAdversarial
Networks
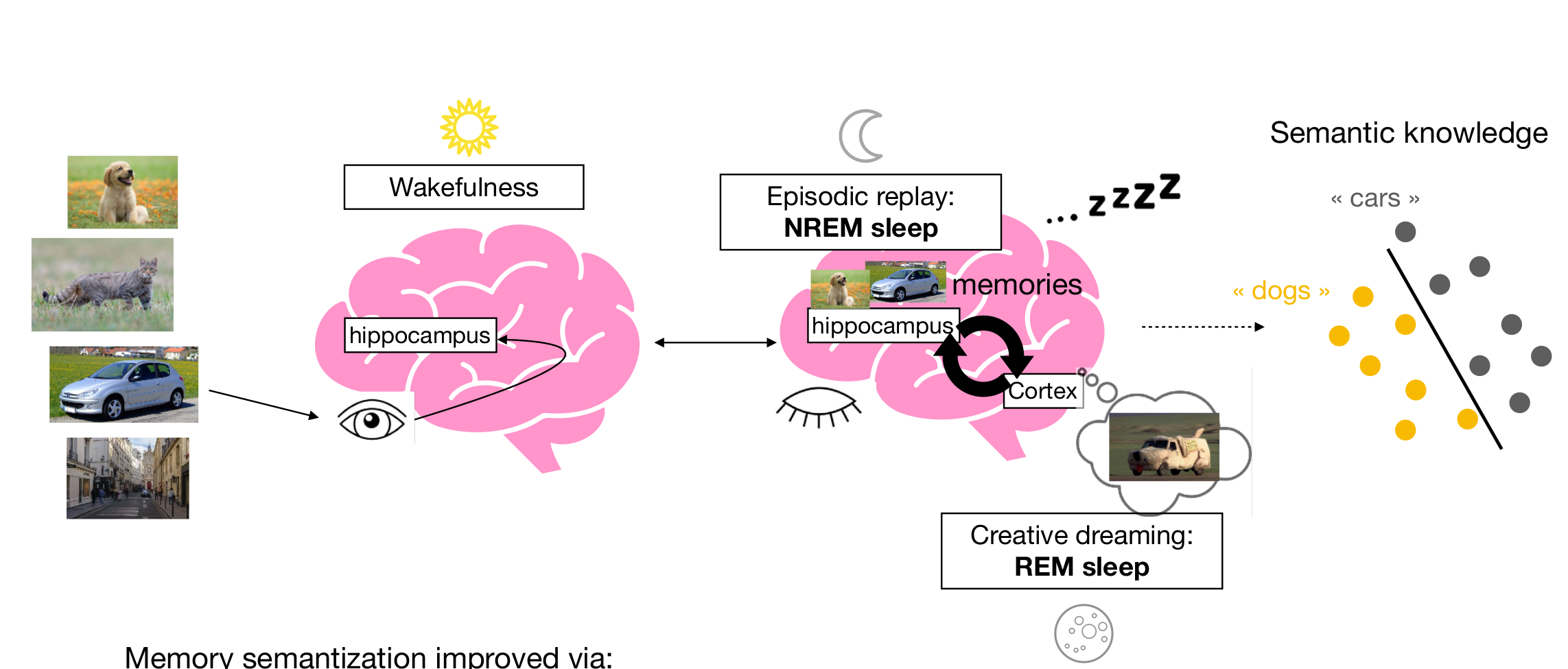
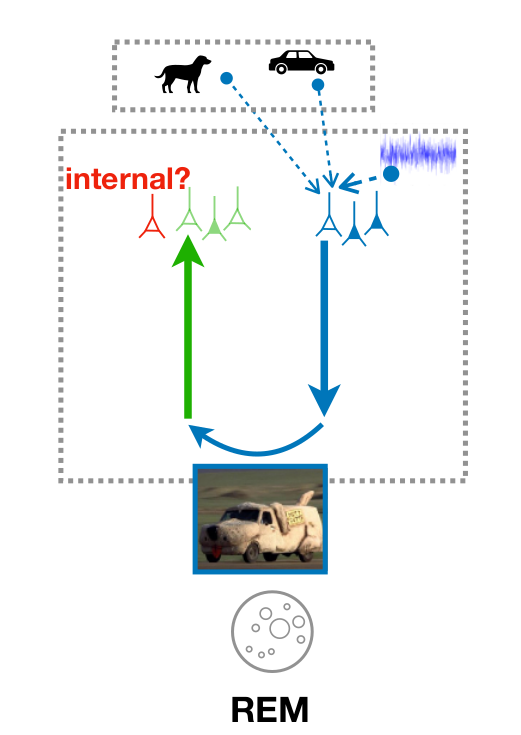
Learning during sleep (sketch)
- sensory experiences are encoded & stored during wakefulness
- preturbed replay during NREM robustifies encoding
- "creative" replay" during REM organizes encoding space

Nicolas Deperrois
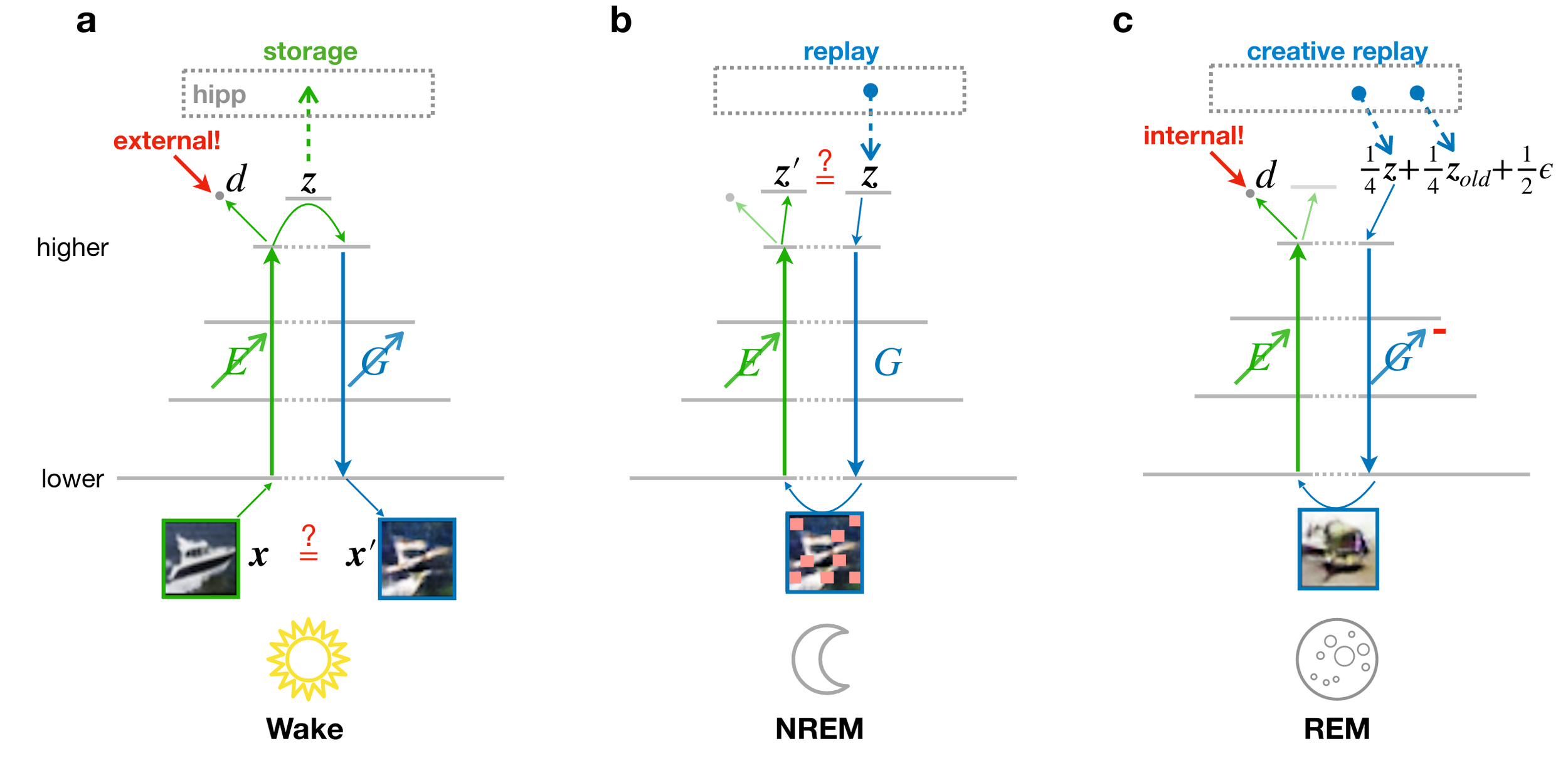
Different, but complementary objectives govern learning during wakefulness and sleep
- discriminator: external
- data reconstruction
- latent reconstruction
- discriminator: internal
- generator: external
Objectives:
Dreams become more realistic with training
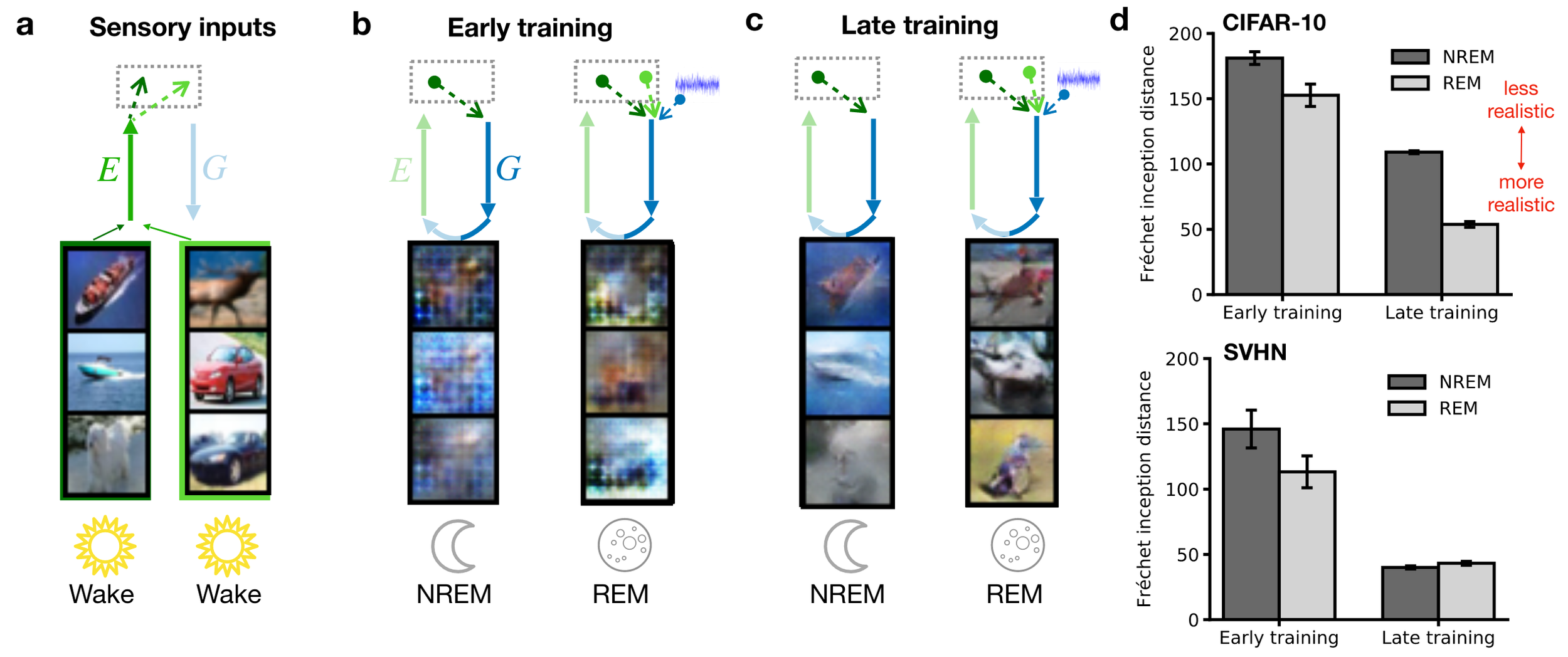
(remark: FID uses an Inception-v3 network, hence likely
focuses on local image statistics, e.g., Brendel & Bethge, 2019)
Adversarial dreaming during REM improves the structure of latent representations

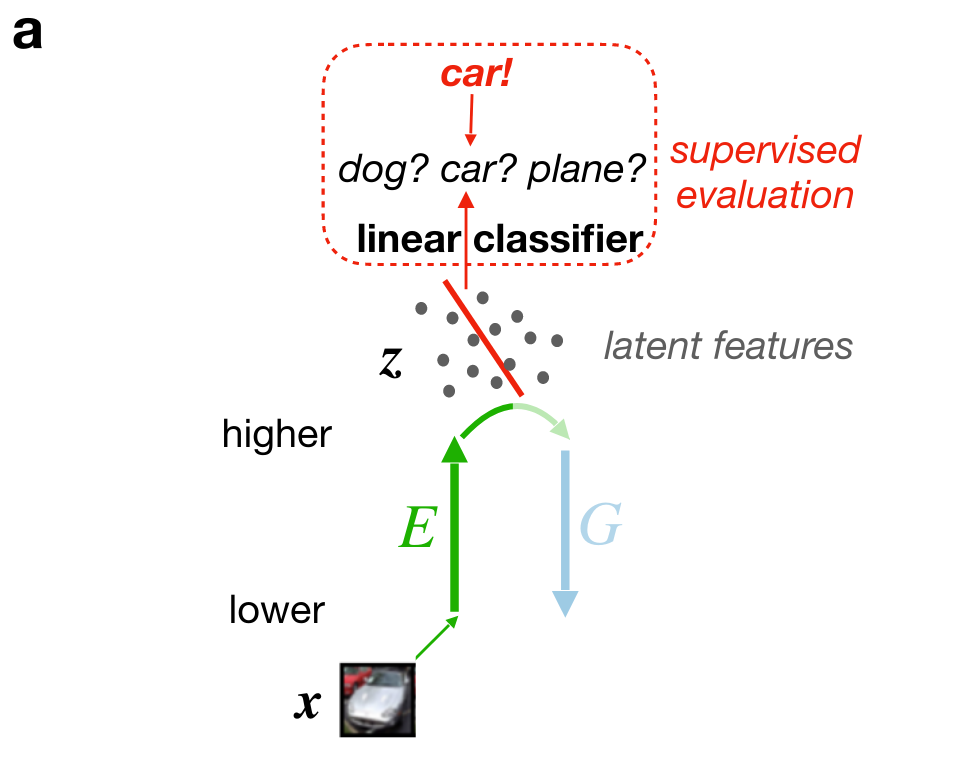
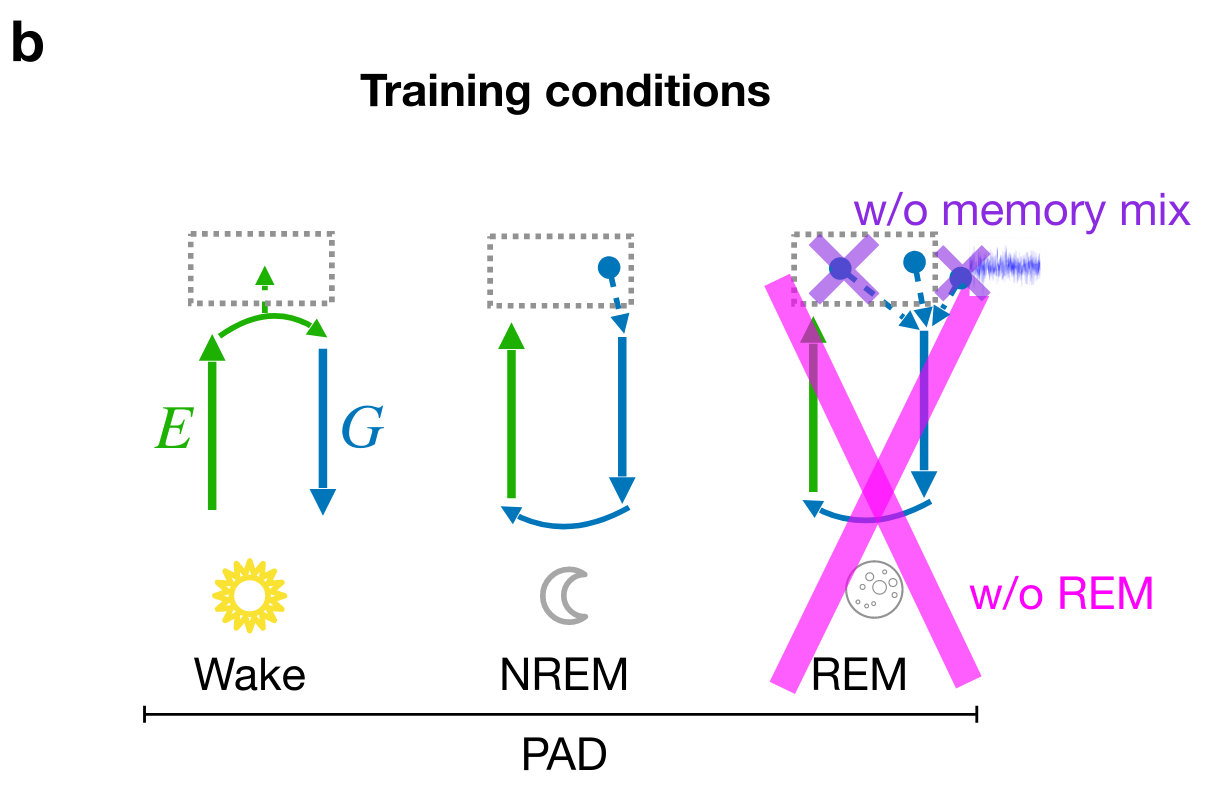
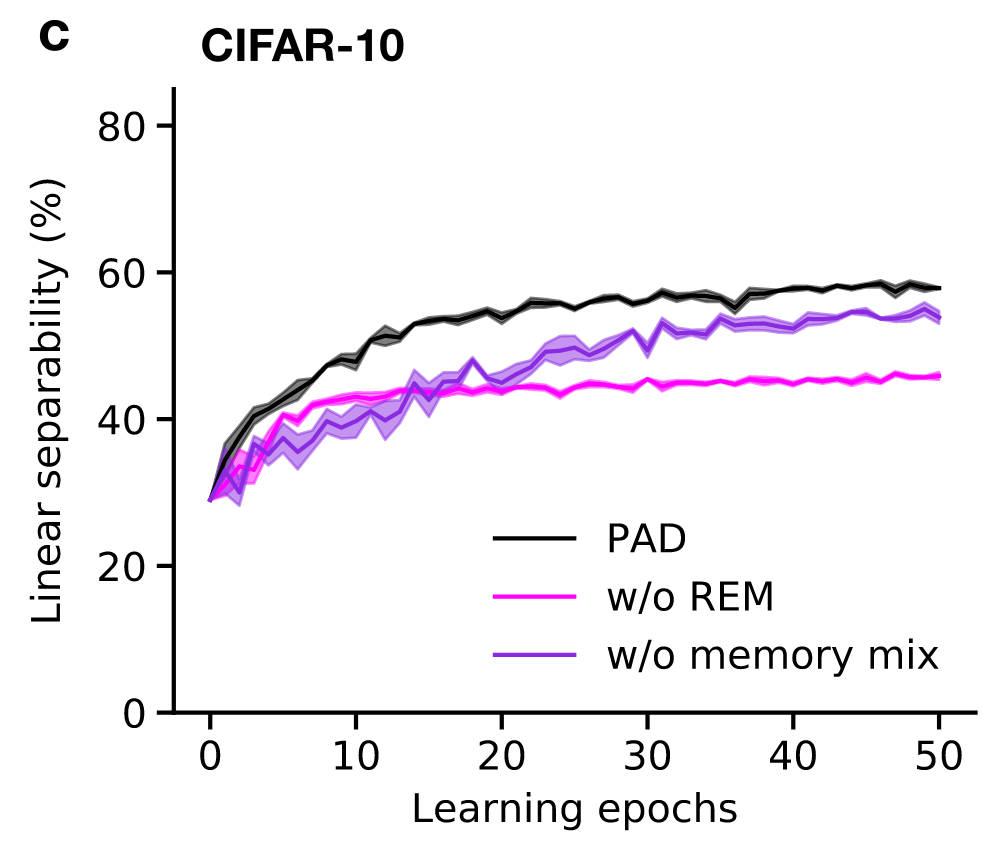
Perturbed dreaming during NREM improves the robustness of latent representations
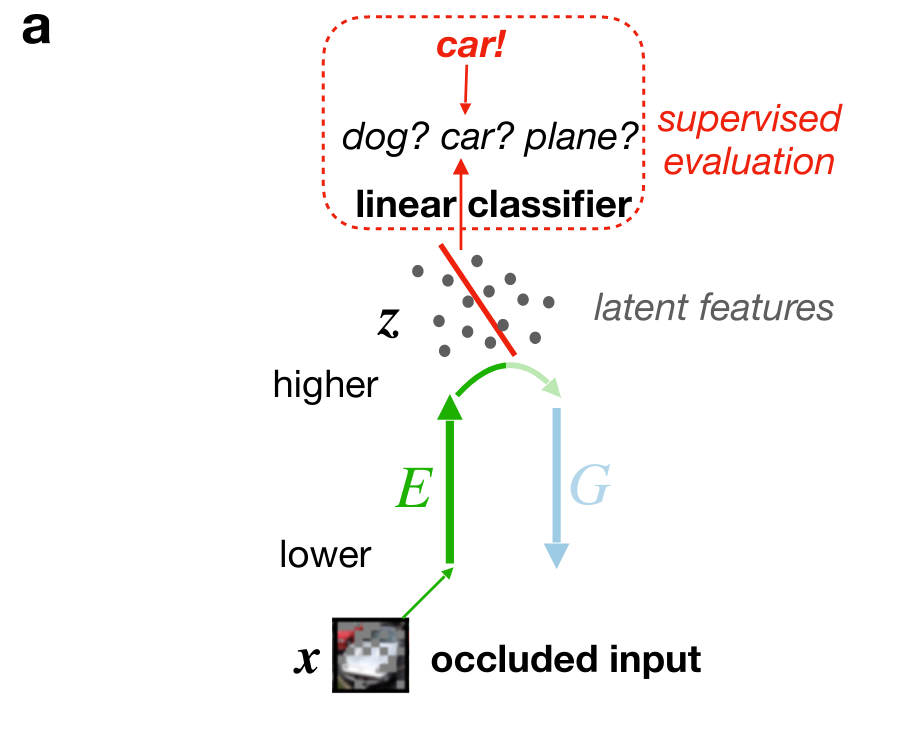
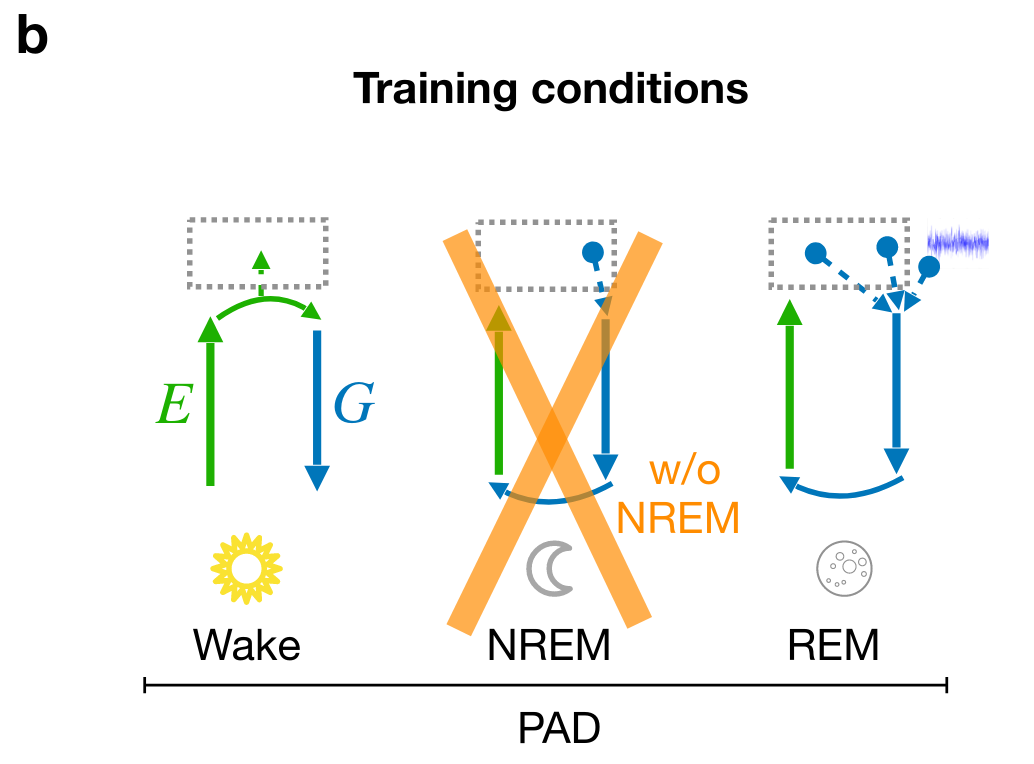
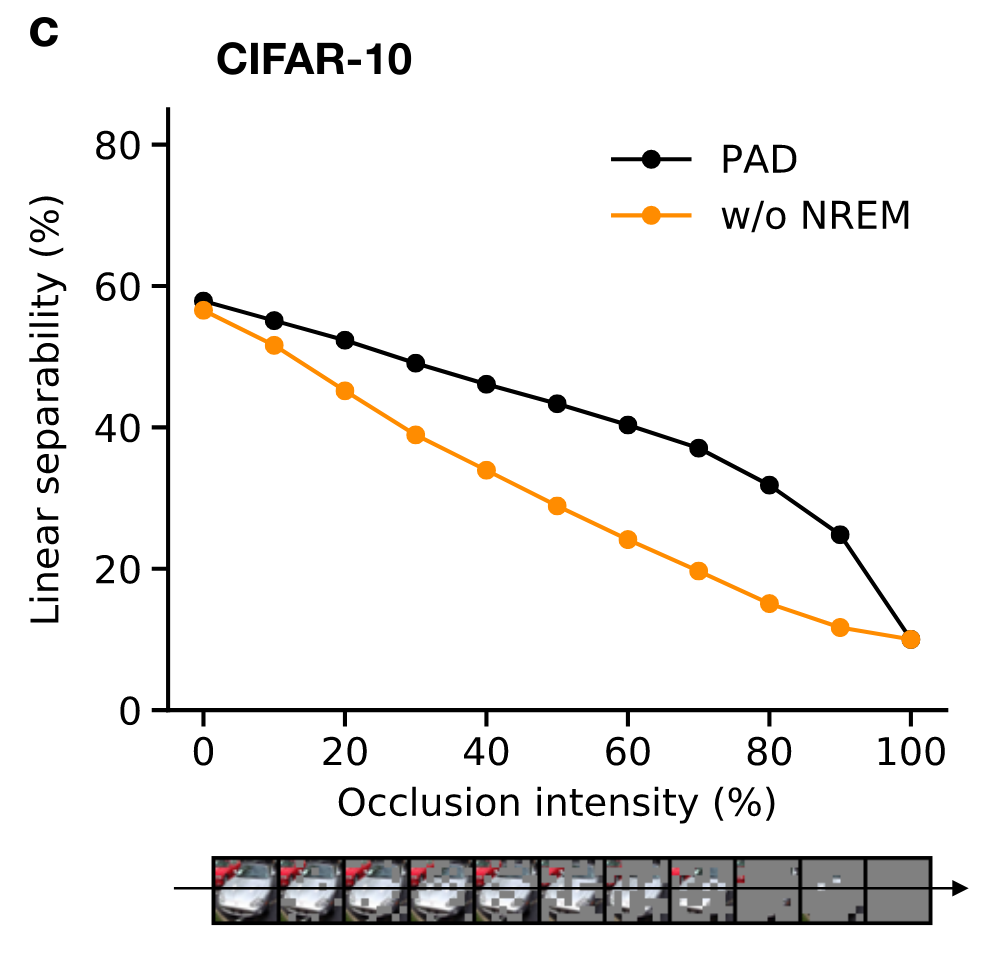
Cortical correlates of adversarial learning
- (trained) discriminator neurons are in different activity regimes during wakefulness and REM sleep; distinguish external from internal, hence may be involved in reality monitoring systems (ACC, mPFC?); impaired discriminator function could thus lead to the formation of delusions
- discriminator learning predicts opposite weight changes on feedforward synapses during wakefulness and REM sleep for identical low-level activity
- generator learning predicts opposite plasticity rule on feedback synapses during wakefulness and REM sleep
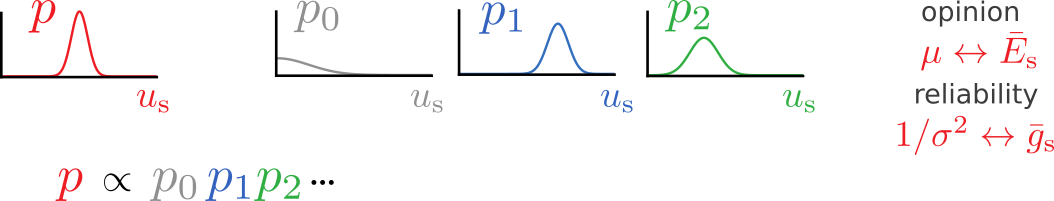
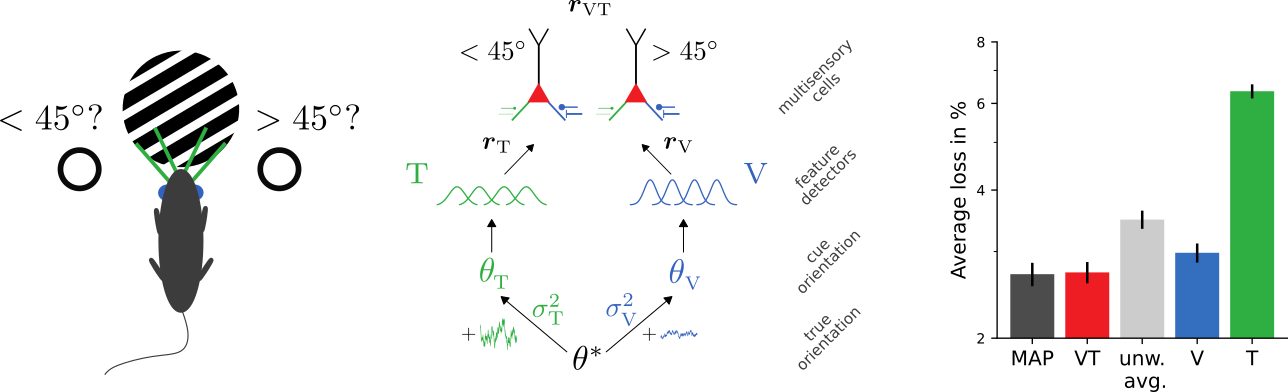
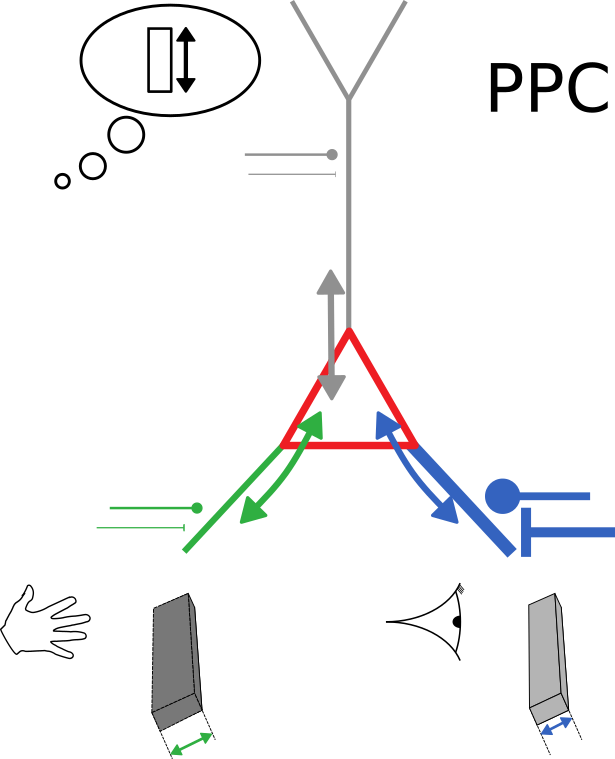
(High-level) neuronal representations reflect contributions from multiple modalities
How to (optimally) combine uncertain information from different sources?



visual
auditory
olfactory
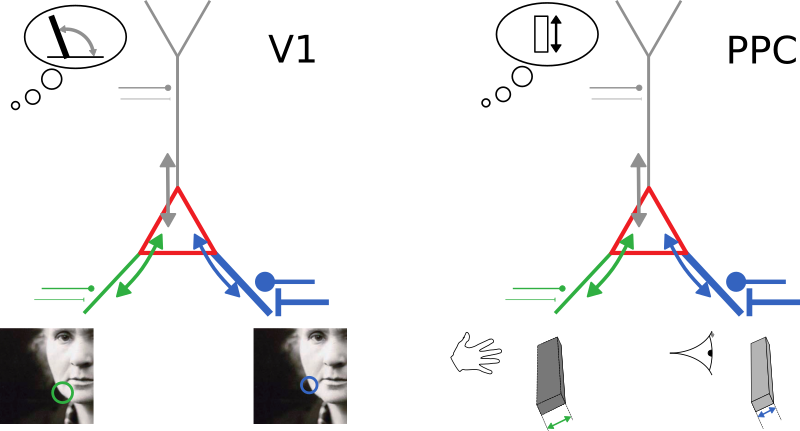
Neurons with conductance-based synapses
naturally implement probabilistic cue integration
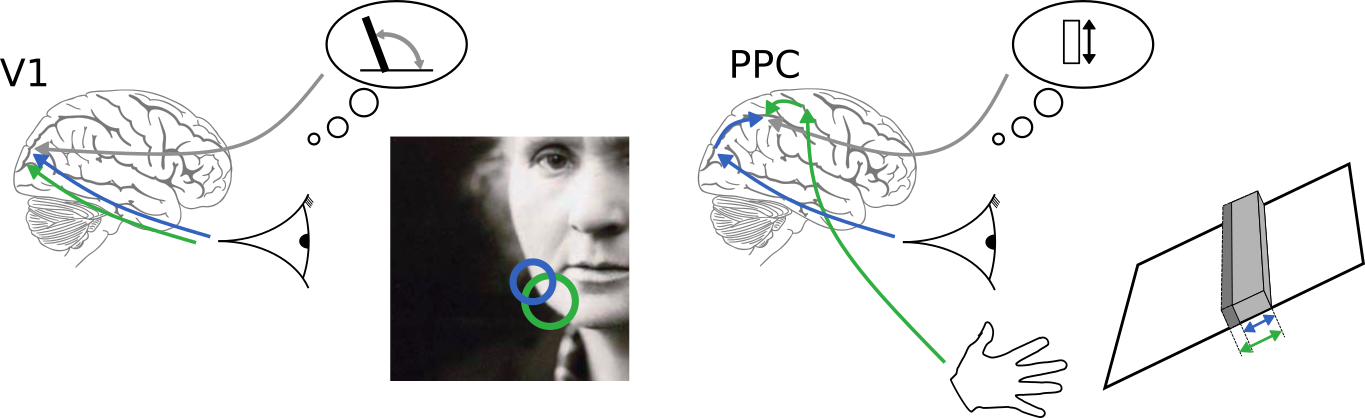
An observation
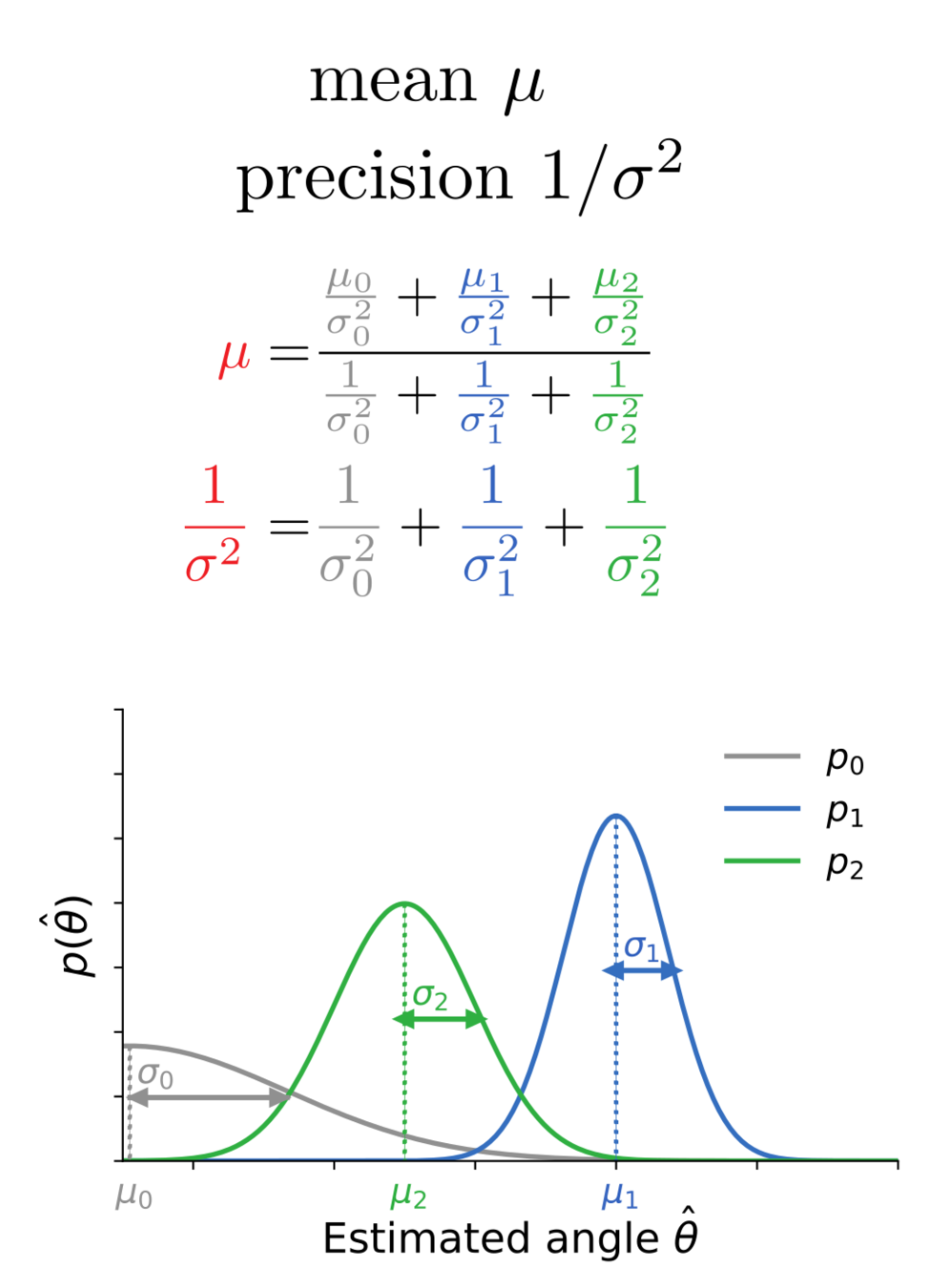
Bayes-optimal inference
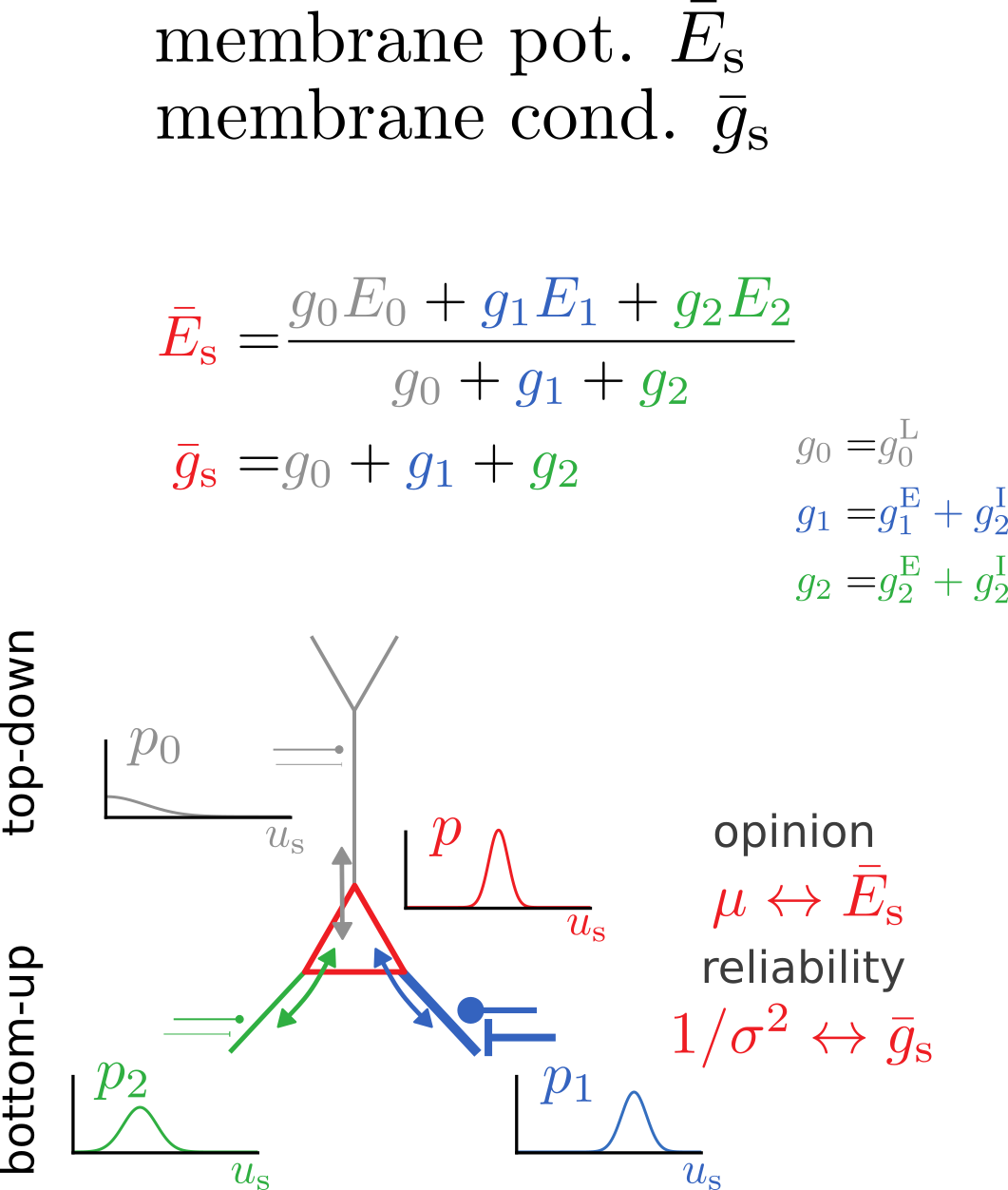
Bidirectional voltage dynamics
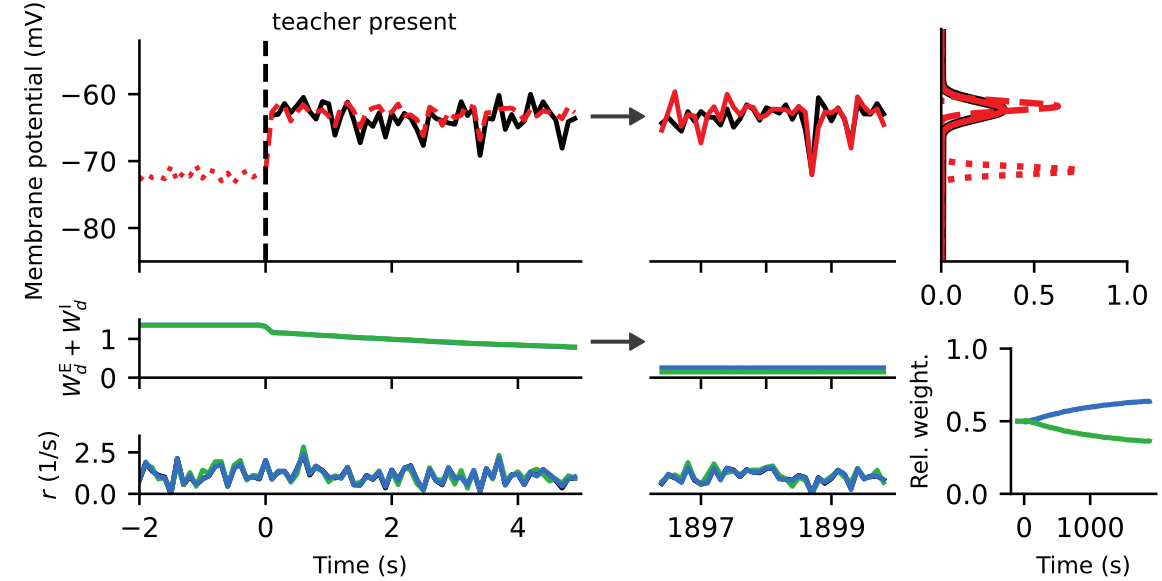
Membrane potential dynamics from noisy gradient ascent
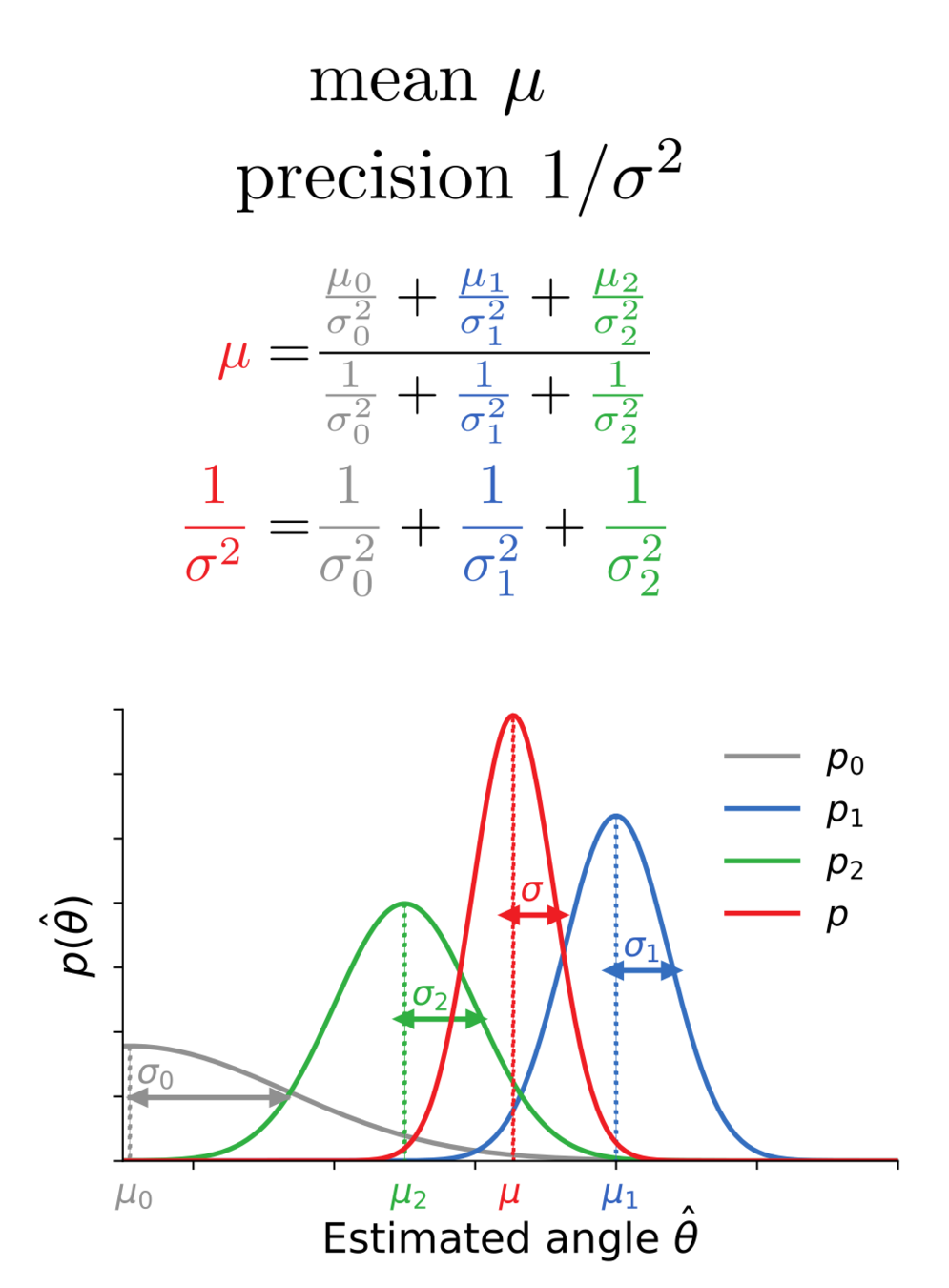
Average membrane potentials
= reliability-weighted opinions
Membrane potential variance
= 1/total reliability
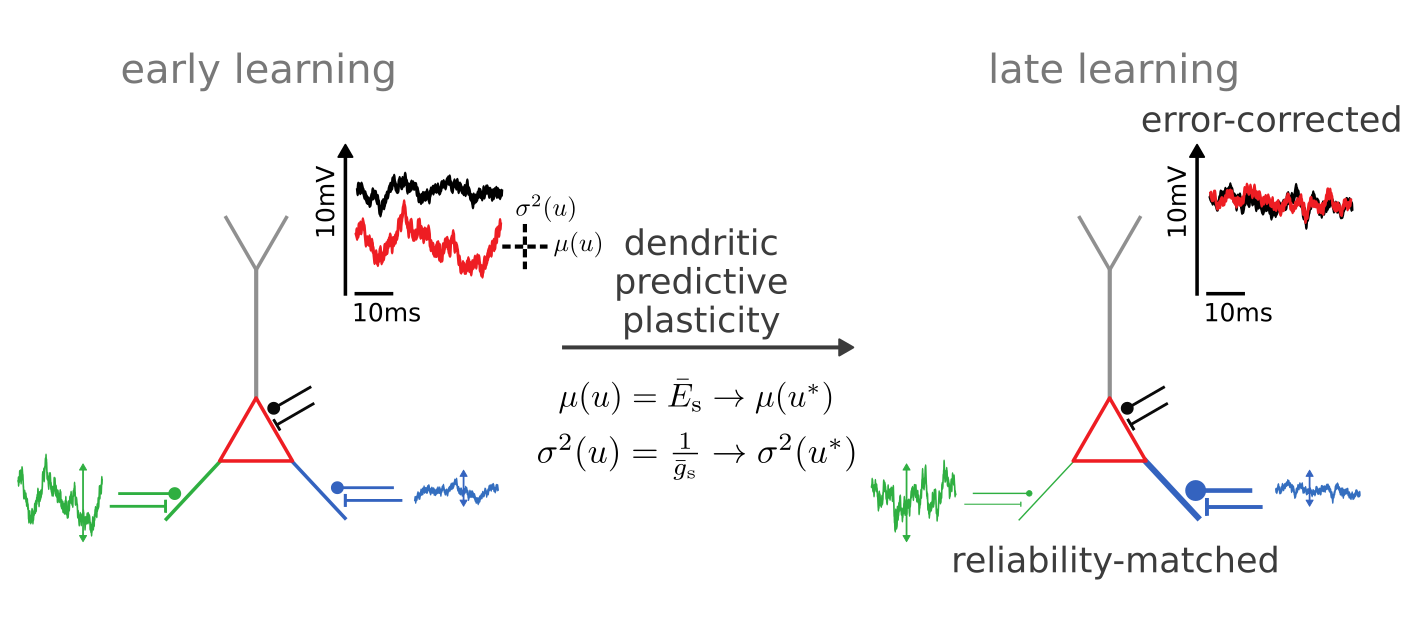
Synaptic plasticity from stochastic gradient ascent
Synaptic plasticity modifies excitatory/inhibitory synapses
- in approx. opposite directions to match the mean
- in identical directions to match the variance
\(u_\text{s}^*\): sample from target distribution \(p^*(u_\text{s})\)
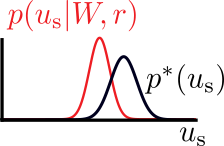
target
actual
Synaptic plasticity performs
error-correction and reliability matching
Learning Bayes-optimal inference of orientations from multimodal stimuli
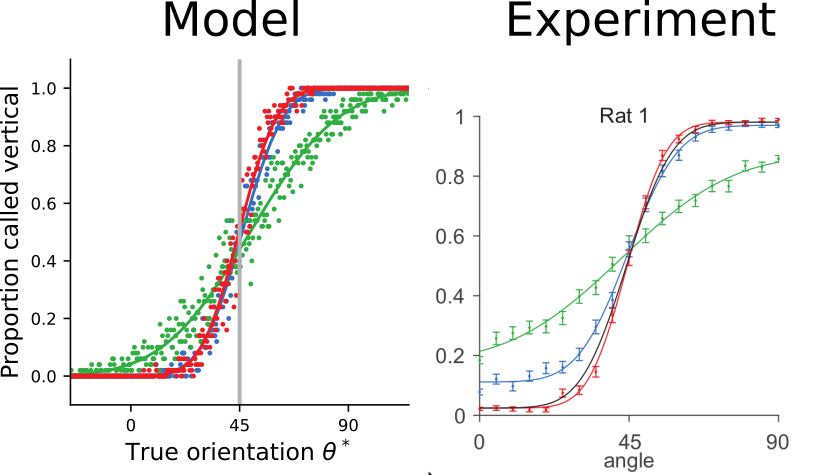
The trained model approximates ideal observers
and reproduces psychophysical signatures of experimental data
[Nikbakht et al., 2018]
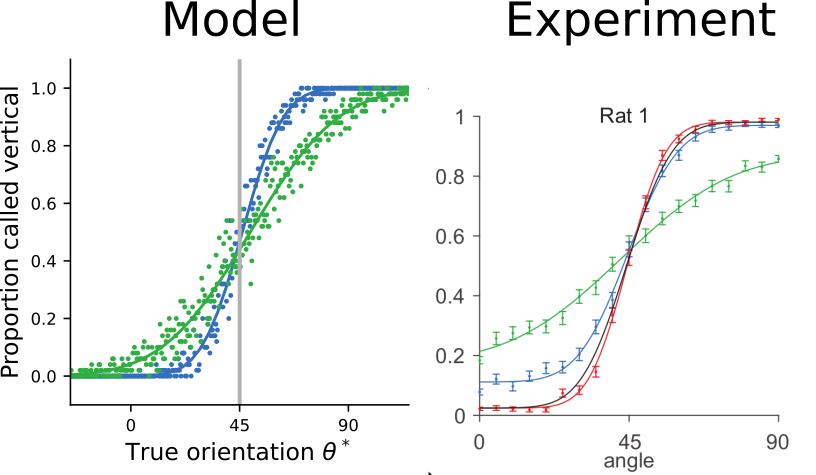
Cross-modal suppression as
reliability-weighted opinion pooling
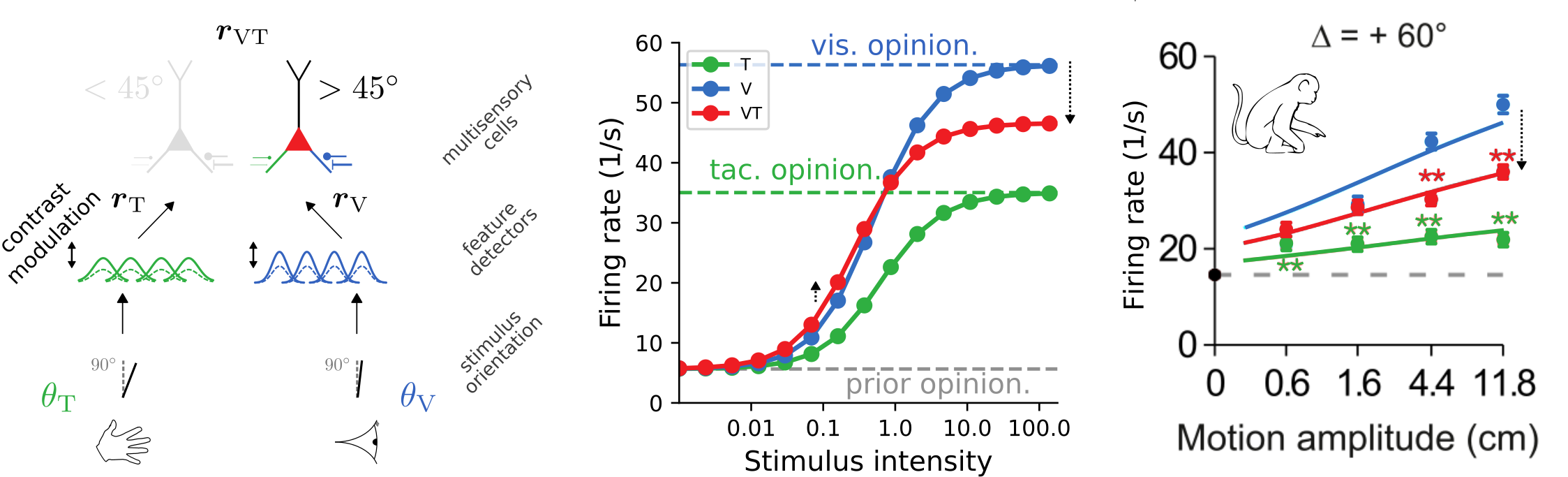
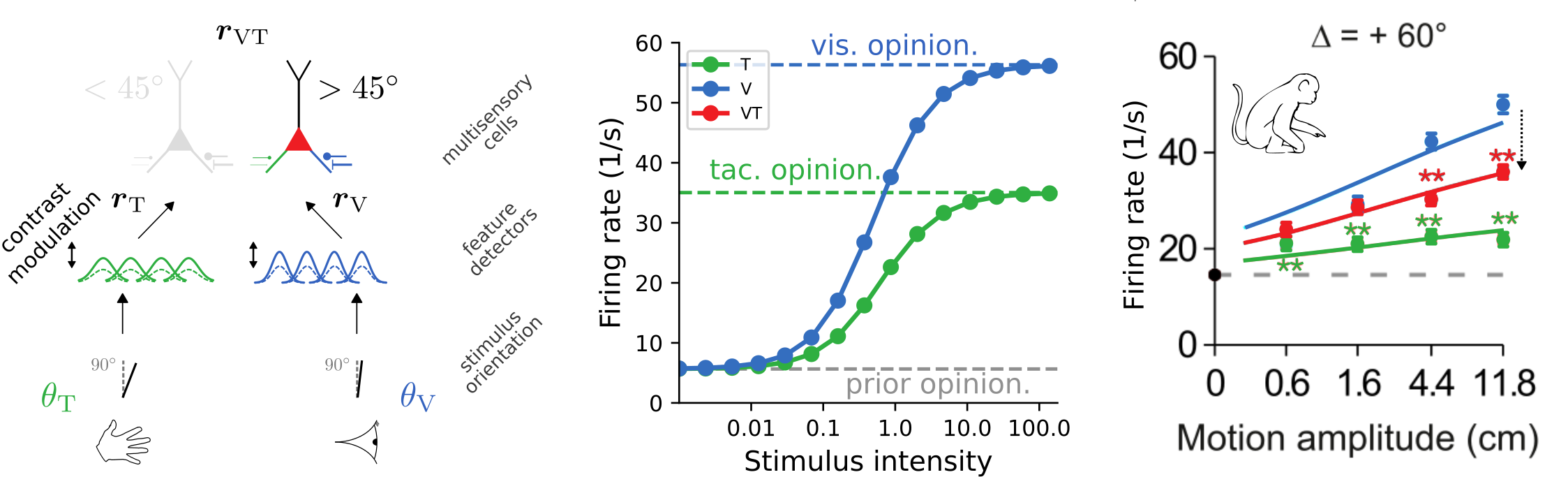
The trained model exhibits cross-modal suppression:
- at low stimulus intensities, firing rate is larger bimodal condition
- at high stimulus intensities, firing rate is smaller in bimodal condition
- example prediction for experiments: strength of suppression depends on relative reliabilities of the two modalities
[Ohshiro et al., 2017]
Summary
Adversarial learning during wakefulness and sleep allows the emergence of organized cortical representations.
Single neurons with conductance-based synapses learn to be optimal cue integrators.
Deperrois, N., Petrovici, M.A., Senn, W., & Jordan, J. (2021). Learning cortical representations through perturbed and adversarial dreaming. arXiv preprint arXiv:2109.04261.
Jordan, J., Sacramento, J., Wybo, W. A., Petrovici, M. A., & Senn, W. (2021). Learning
Bayes-optimal dendritic opinion pooling. arXiv preprint arXiv:2104.13238.
Representations & cue integration
By jakobj
Representations & cue integration
- 232



