Targeting Beyond the Tumor: Radiation's Evolving Role in Oncogene-Driven Lung Cancer
Jonathan Sackett
Prompt for Consistent Citations:
Please provide a citation for the following article in a compact format suitable for slides. Use the following structure:
- Include the first author's last name and initials (omit additional authors for brevity).
- Write the full title of the article, capitalizing only the first word and proper nouns.
- Italicize the journal name using
<i>tags.- Add year, volume (and issue if applicable), and page range.
- Include a clickable DOI or URL using
<a>tags withtarget="_blank".- Wrap the entire citation in a
<div>withcitation footnoteclasses for styling.Example:
html
Copy code
<div class="citation footnote"> AuthorLastName Initial(s). Title of the article. <i>Journal Name</i> Year;Volume(Issue):Page Range. <a href="DOI or URL" target="_blank">DOI or URL</a> </div>Use this format to provide the citation.
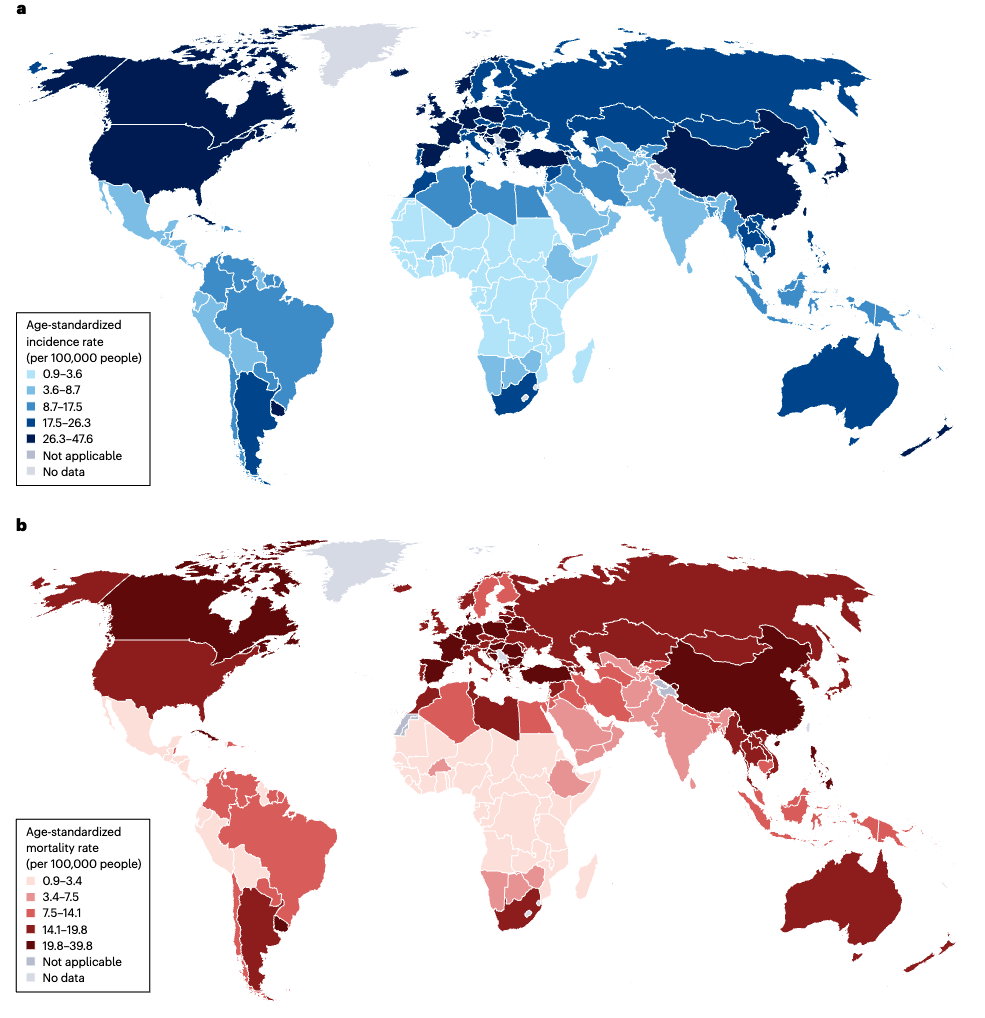
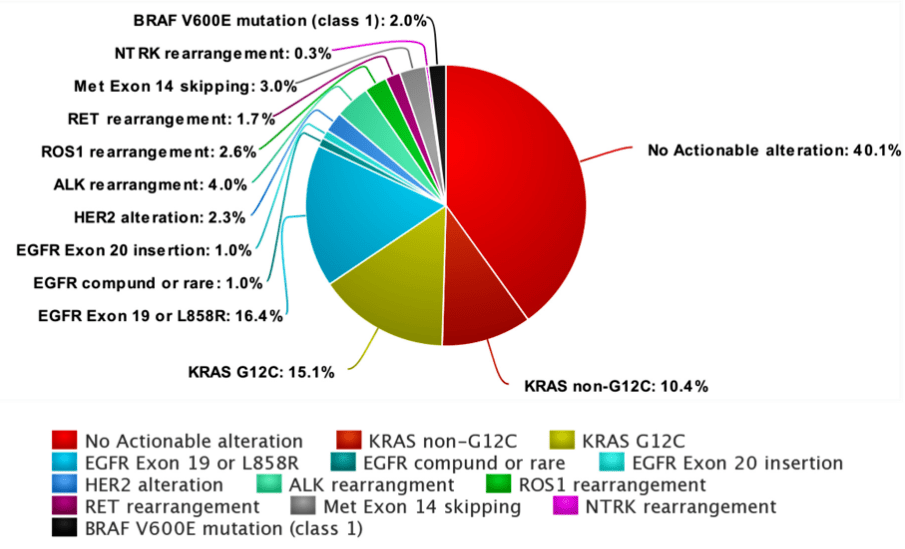
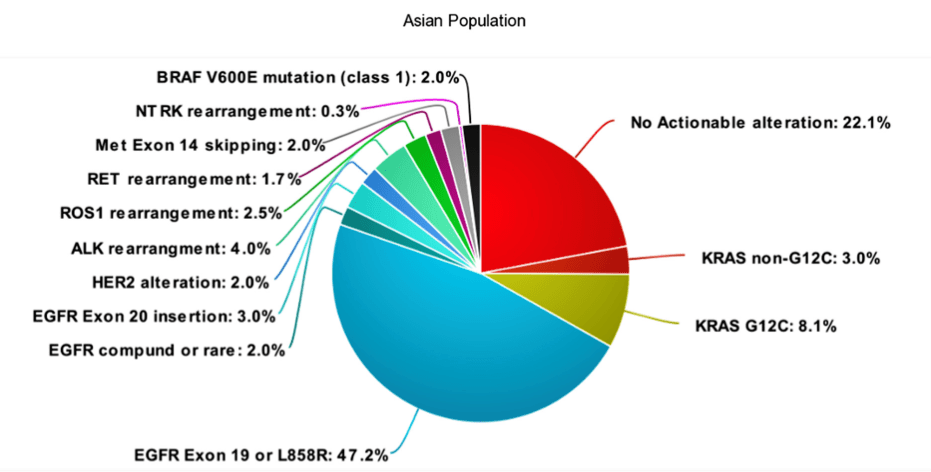
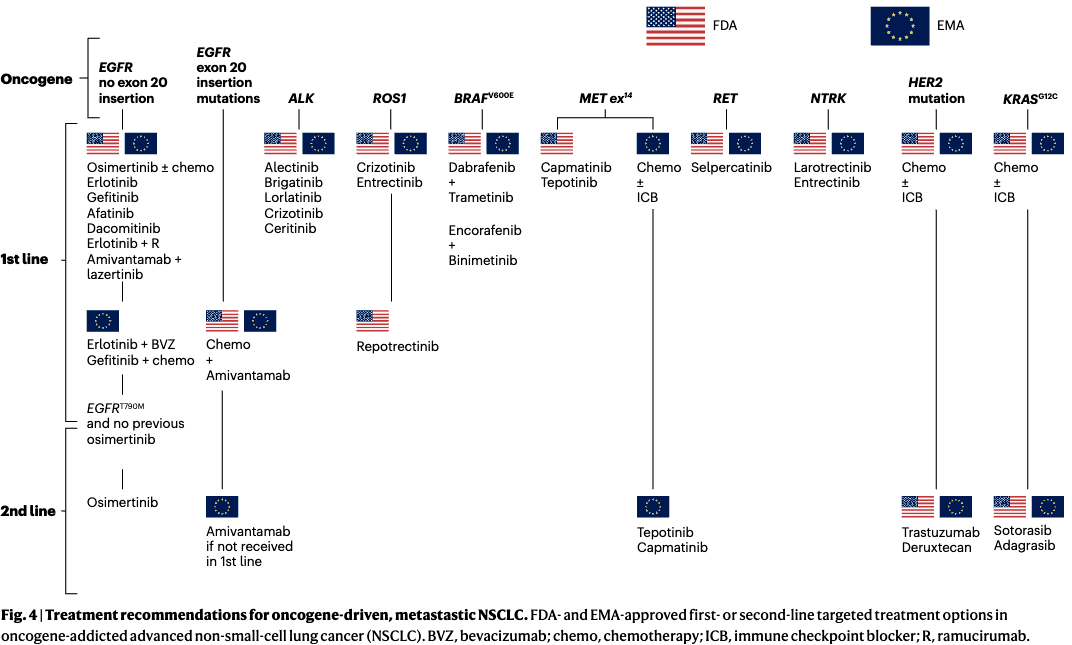
Evolution of Targeted Therapies in NSCLC



Meet the mutations
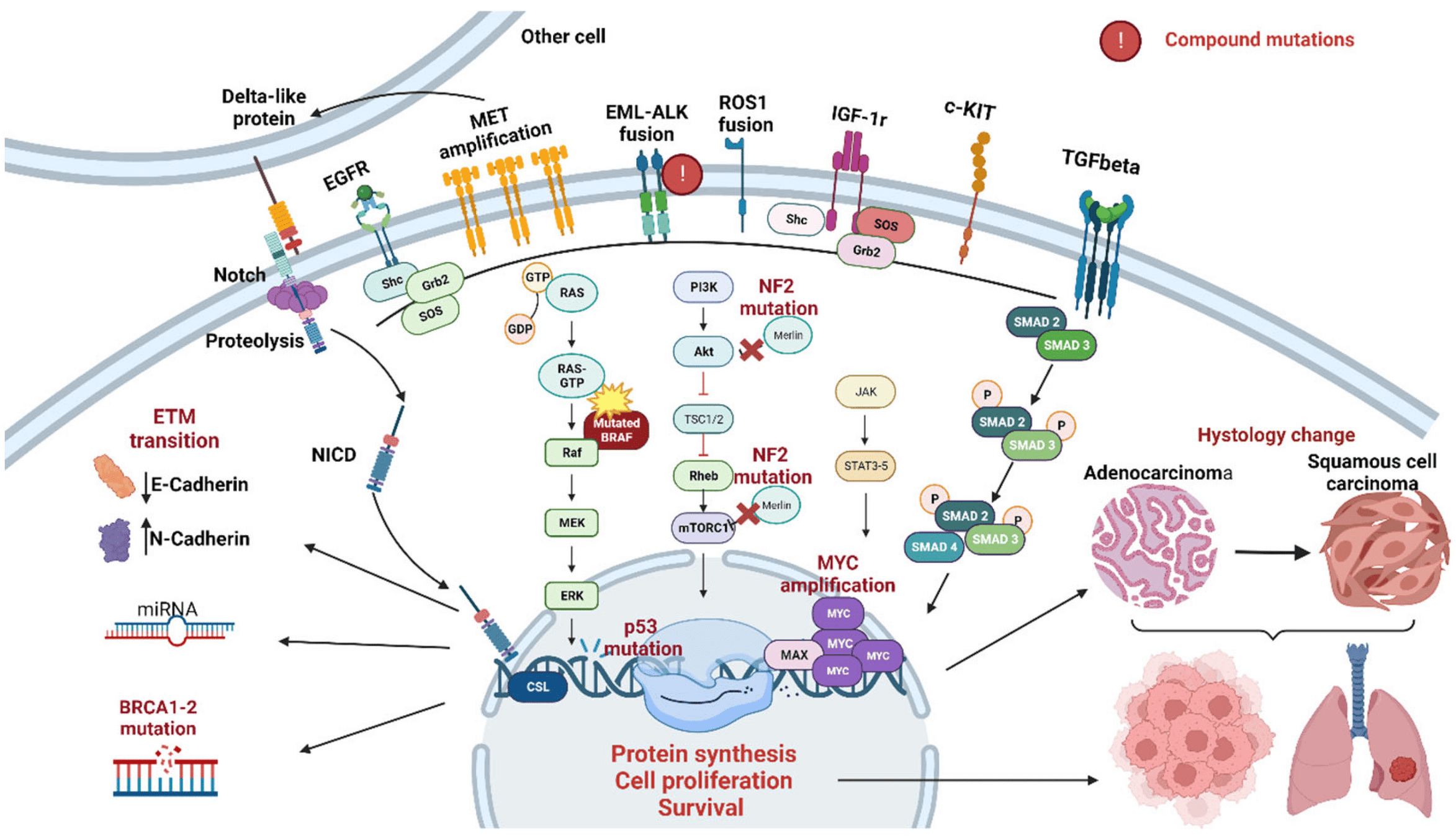
Exon 19 deletions, L858R mutations, and T790M
EGFR Mutations
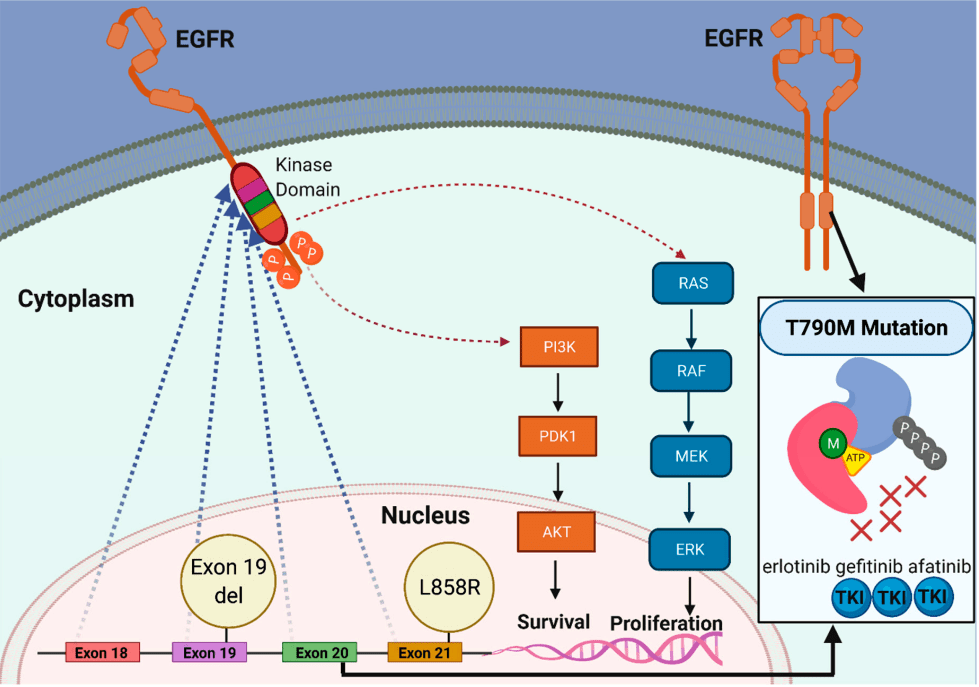
1st & 2nd Generations of EGFR TKIs
-
First-Generation
- Examples: Erlotinib, Gefitinib
- Mechanism: Reversible EGFR inhibitors
-
Key Points:
- Effective for activating mutations (e.g., exon 19, L858R)
- Limited against T790M mutation
- Poor CNS penetration
- Approval: Gefitinib (2003, IPASS), Erlotinib (2004, EURTAC)
-
Second-Generation
- Examples: Afatinib, Dacomitinib
- Mechanism: Irreversible EGFR/HER inhibitors
-
Key Points:
- Broader activity (e.g., G719X, S768I)
- Limited T790M activity
- Moderate CNS penetration
- Approval: Afatinib (2013, LUX-Lung 3/6), Dacomitinib (2018, ARCHER 1050)
EURTAC
Erlotinib
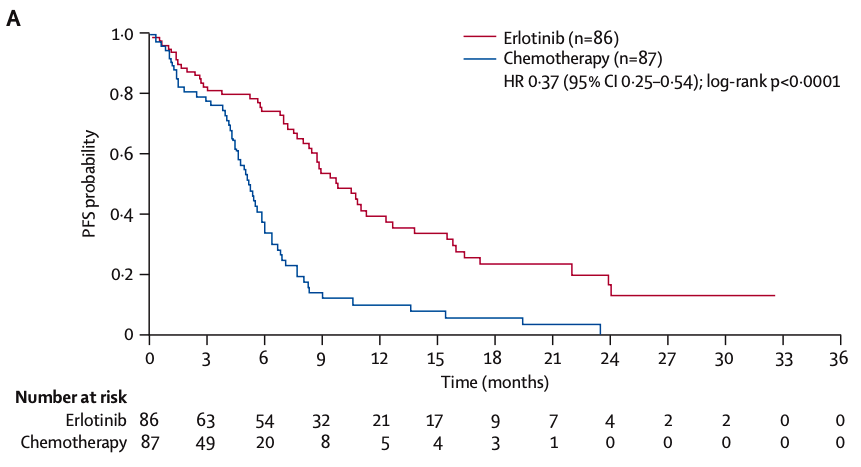
median PFS 9.7 mos vs 5.2 months
- Erlotinib: Continuous daily dosing (150 mg/day) until disease progression or intolerable toxicity.
- Chemotherapy: Four cycles of platinum-based chemotherapy unless progression or toxicity occurred earlier.
3rd Generations of EGFR TKIs
-
Third-Generation
- Examples: Osimertinib
- Mechanism: Irreversible, T790M-specific
-
Key Points:
- Effective for T790M and CNS metastases
- First-line for EGFR-mutated NSCLC
- Approval: Osimertinib (2017, FLAURA/AURA3)
-
Fourth-Generation
- Examples: BLU-945, Lazertinib
FLAURA trial
Osimertinib
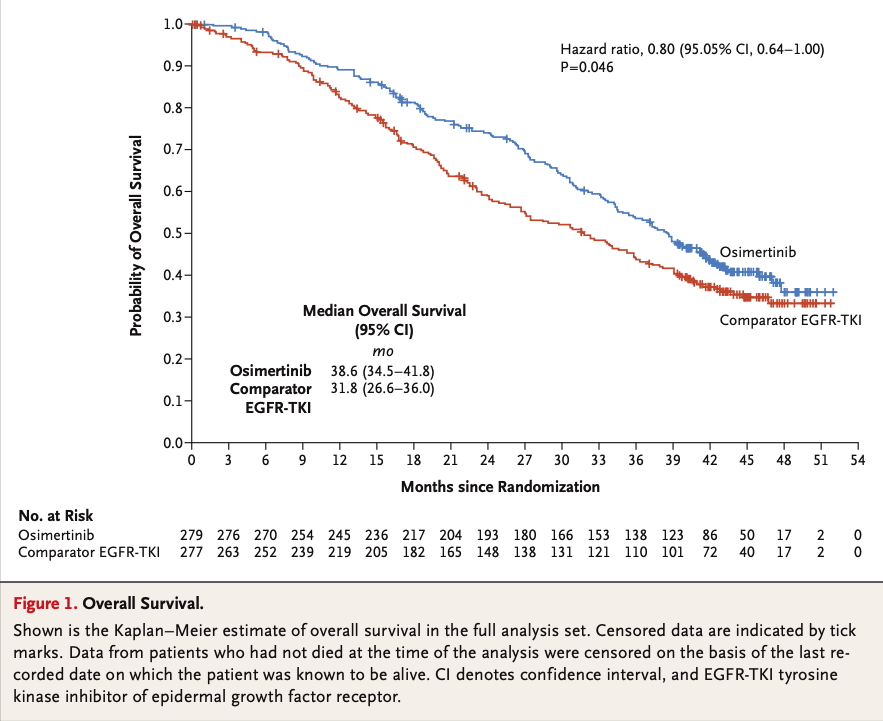
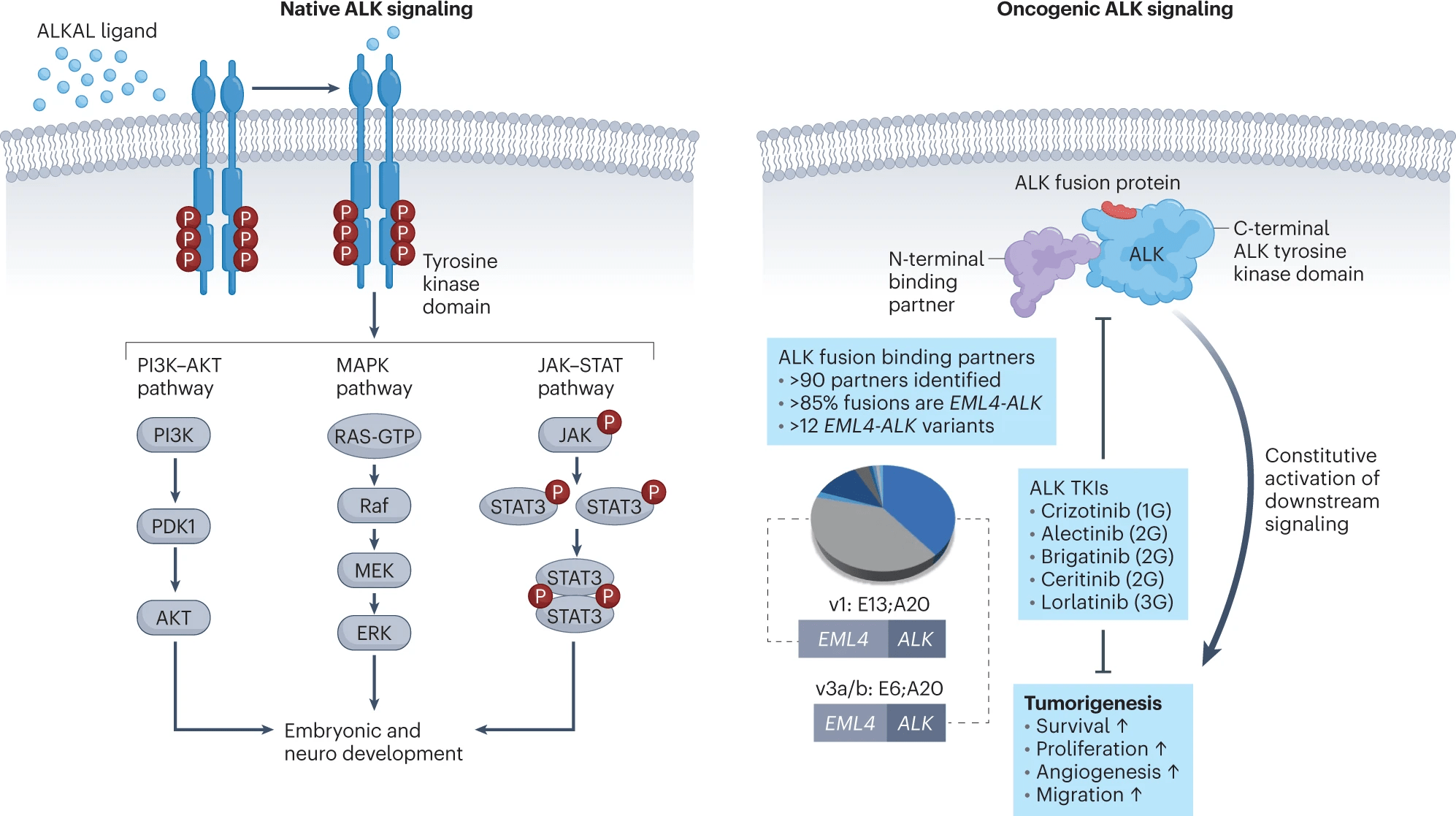

First-Generation ALK Inhibitors
- Example: Crizotinib
- Mechanism: Inhibits ALK, ROS1, and MET.
-
Key Points:
- Effective against ALK rearrangements (e.g., EML4-ALK).
- CNS Penetration: Limited, leading to high rates of CNS progression (PROFILE 1014 trial).
- Radiation Sensitivity: Enhances radiosensitivity by suppressing DNA damage repair mechanisms.
- Resistance: Mutations like L1196M limit long-term efficacy.
- Approval: 2011 (PROFILE trials).
Second-Generation ALK Inhibitors
- Examples: Ceritinib, Alectinib, Brigatinib
- Mechanism: Overcomes crizotinib resistance and achieves superior CNS penetration.
-
Key Points:
- Effective against ALK resistance mutations (e.g., L1196M, G1269A).
- CNS Penetration: Significant improvements over crizotinib. Alectinib and Brigatinib achieve prolonged CNS control (ALEX and ALTA trials).
-
Approval:
- Ceritinib: 2014 (ASCEND trials).
- Alectinib: 2015 (ALEX trial).
- Brigatinib: 2017 (ALTA trial).
Third-Generation ALK Inhibitors
- Example: Lorlatinib
- Mechanism: Targets ALK resistance mutations (e.g., G1202R) and CNS metastases.
-
Key Points:
- CNS Penetration: High blood-brain barrier permeability; intracranial response rate of ~82% (CROWN trial).
-
Radiation Considerations:
- Often delays need for SRS or WBRT.
- Recommended after failure of second-generation inhibitors.
- Approval: 2018 (CROWN trial).
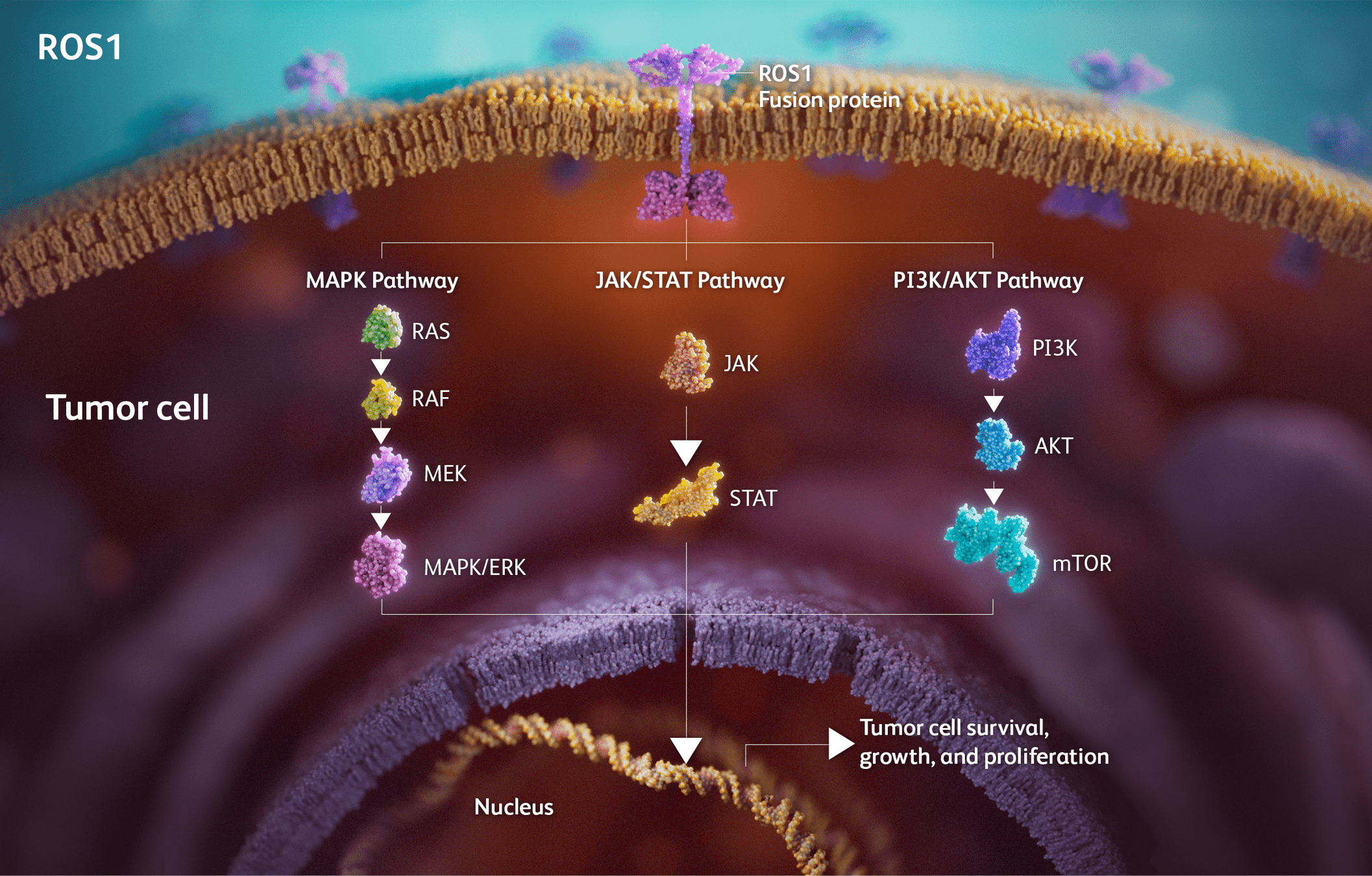
Drugs Targeting ROS1
- Examples: Crizotinib, Entrectinib, Lorlatinib, Repotrectinib.
- Mechanism: Inhibit ROS1 fusion-driven signaling to block tumor growth and progression.
-
Key Points:
- Crizotinib: First ROS1 inhibitor approved; limited CNS activity.
- Entrectinib: Significant CNS penetration, durable intracranial responses (STARTRK trials).
- Lorlatinib: Effective against crizotinib-resistant ROS1 mutations.
- Repotrectinib: Promising in both ROS1 TKI-naïve and pretreated patients (TRIDENT-1 trial).
-
Approval:
- Crizotinib: 2016 (PROFILE trials).
- Entrectinib: 2019 (STARTRK trials).

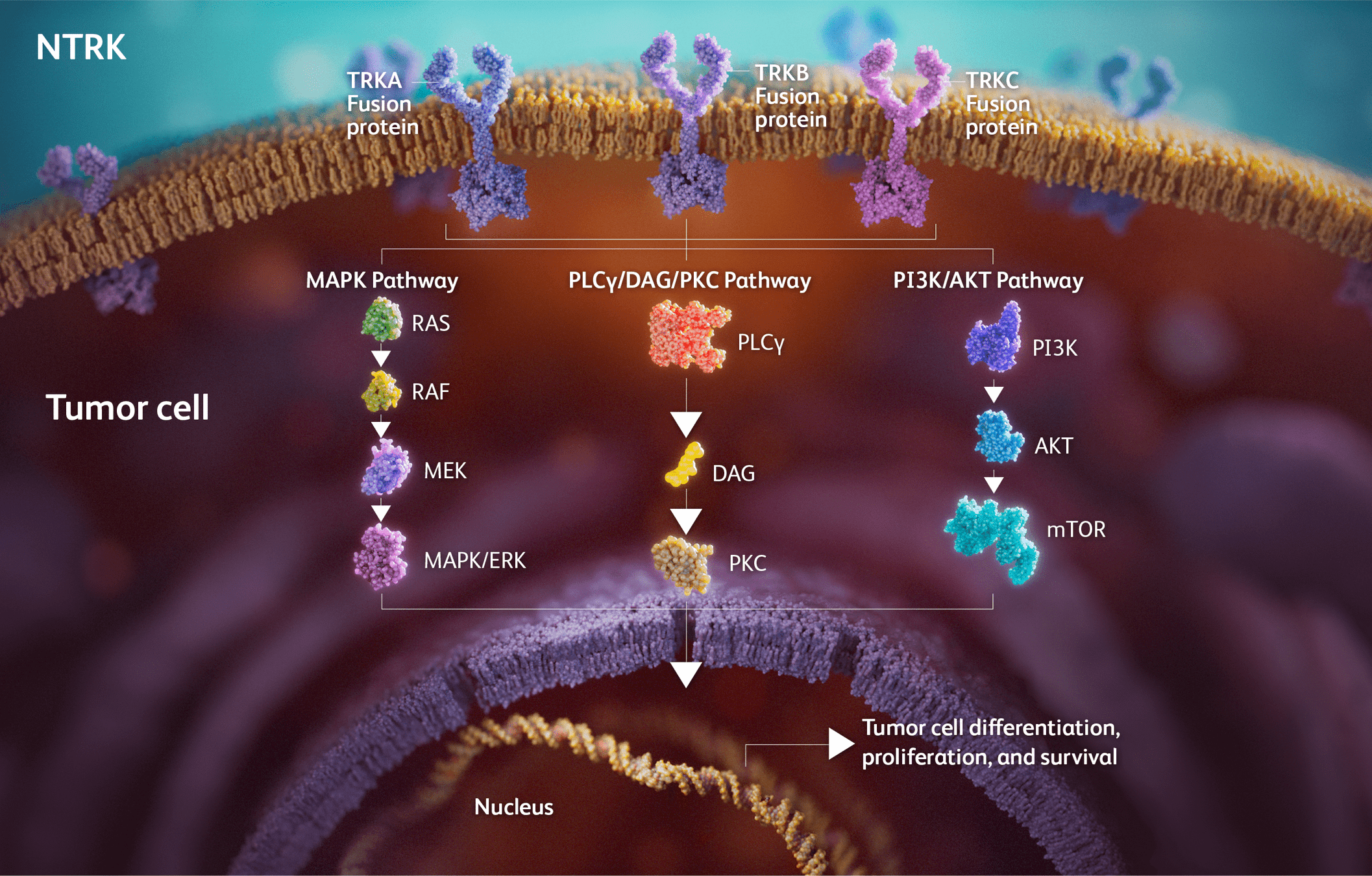
Drugs Targeting NTRK
- Examples: Larotrectinib, Entrectinib.
- Mechanism: Inhibit TRK fusions (NTRK1/2/3) to block tumor growth.
-
Key Points:
- Larotrectinib: High ORR (~75%) across multiple tumor types (NAVIGATE trial).
- Entrectinib: Durable responses in CNS-positive disease (STARTRK trials).
- Resistance: Secondary mutations (e.g., solvent front mutations) drive resistance to first-line TRK inhibitors.
-
Approval:
- Larotrectinib: 2018 (NAVIGATE, SCOUT trials).
- Entrectinib: 2019 (STARTRK trials).
STARTRK Trials: Design and Patient Population
Study Overview:
- Combined analysis of three trials:
- STARTRK-1 and ALKA-372-001: Phase I trials.
- STARTRK-2: Phase II global basket trial.
- Target Population: Adults with ROS1 fusion-positive, locally advanced or metastatic NSCLC.
Inclusion Criteria:
- ROS1 fusion–positive NSCLC (confirmed by NGS or local testing).
- Measurable disease (RECIST v1.1).
- ECOG performance status 0–2.
- Asymptomatic or pretreated CNS metastases allowed.
Exclusion Criteria:
- Extracranial progression after prior ROS1 TKI (except CNS-only progression).
- Significant comorbidities or unresolved toxicities.
STARTRK Trials: Methods
- Intervention: Oral entrectinib 600 mg/day until progression, unacceptable toxicity, or withdrawal.
-
Endpoints:
- Primary: Objective response rate (ORR) and duration of response (DoR) (assessed by blinded independent central review [BICR]).
- Secondary: Progression-free survival (PFS), overall survival (OS), and intracranial efficacy.
- Assessments: Imaging every 8 weeks and CNS-specific scans for patients with baseline CNS metastases.
- Safety Monitoring: Adverse events assessed per CTCAE v4.03.
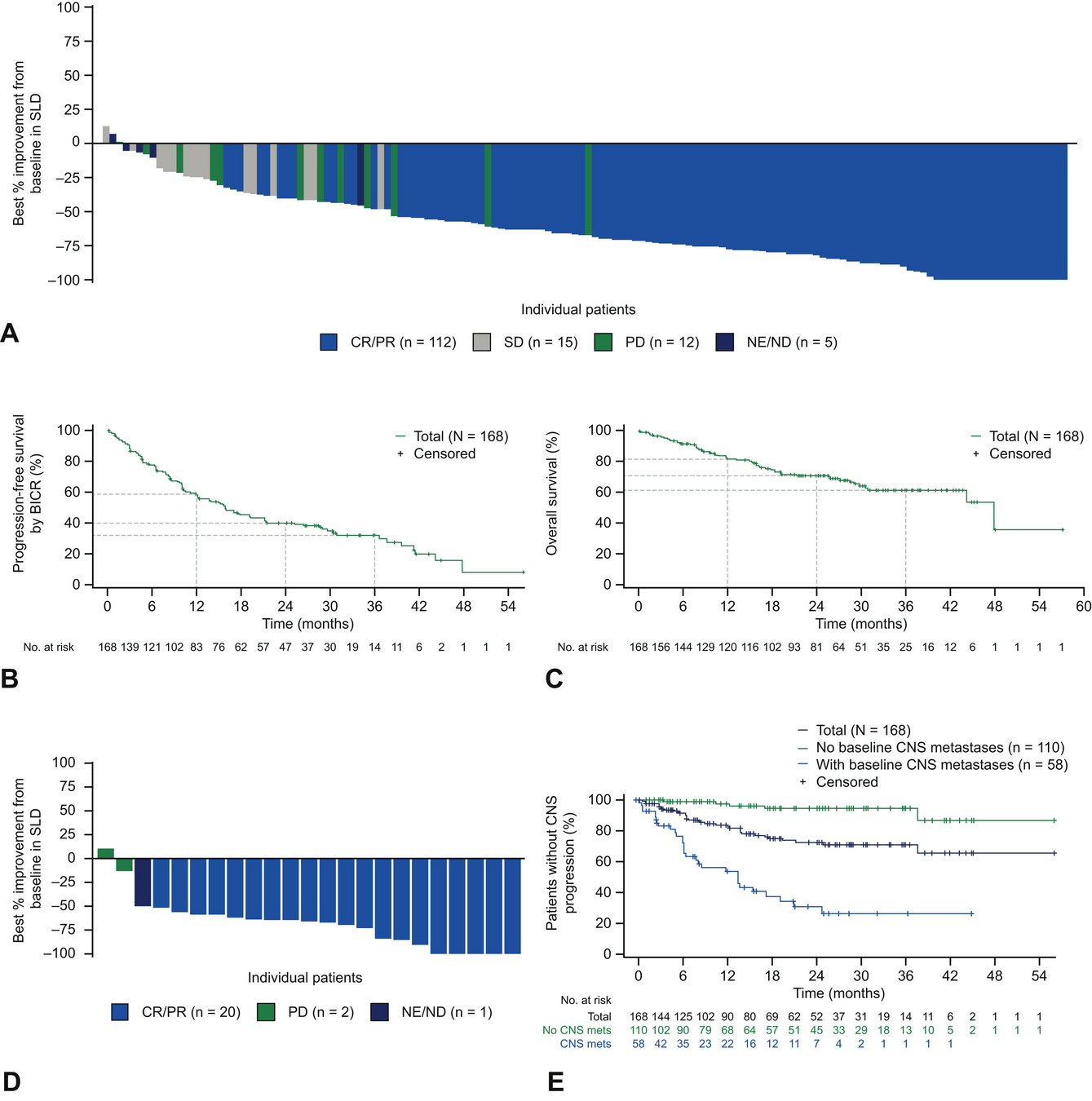
STARTRK Trials: Key Results
-
Overall Population (ROS1 TKI–Naïve):
- ORR: 68% (95% CI: 60.2–74.8).
- Median DoR: 20.5 months.
- Median PFS: 15.7 months; median OS: 47.8 months.
-
Patients with Baseline CNS Metastases:
- Intracranial ORR: 80% (95% CI: 59.3–93.2).
- Median intracranial DoR: 12.9 months.
- Median intracranial PFS: 8.8 months.
-
Post-Crizotinib Cohort:
- ORR: 11%; Median PFS: 4.7 months.
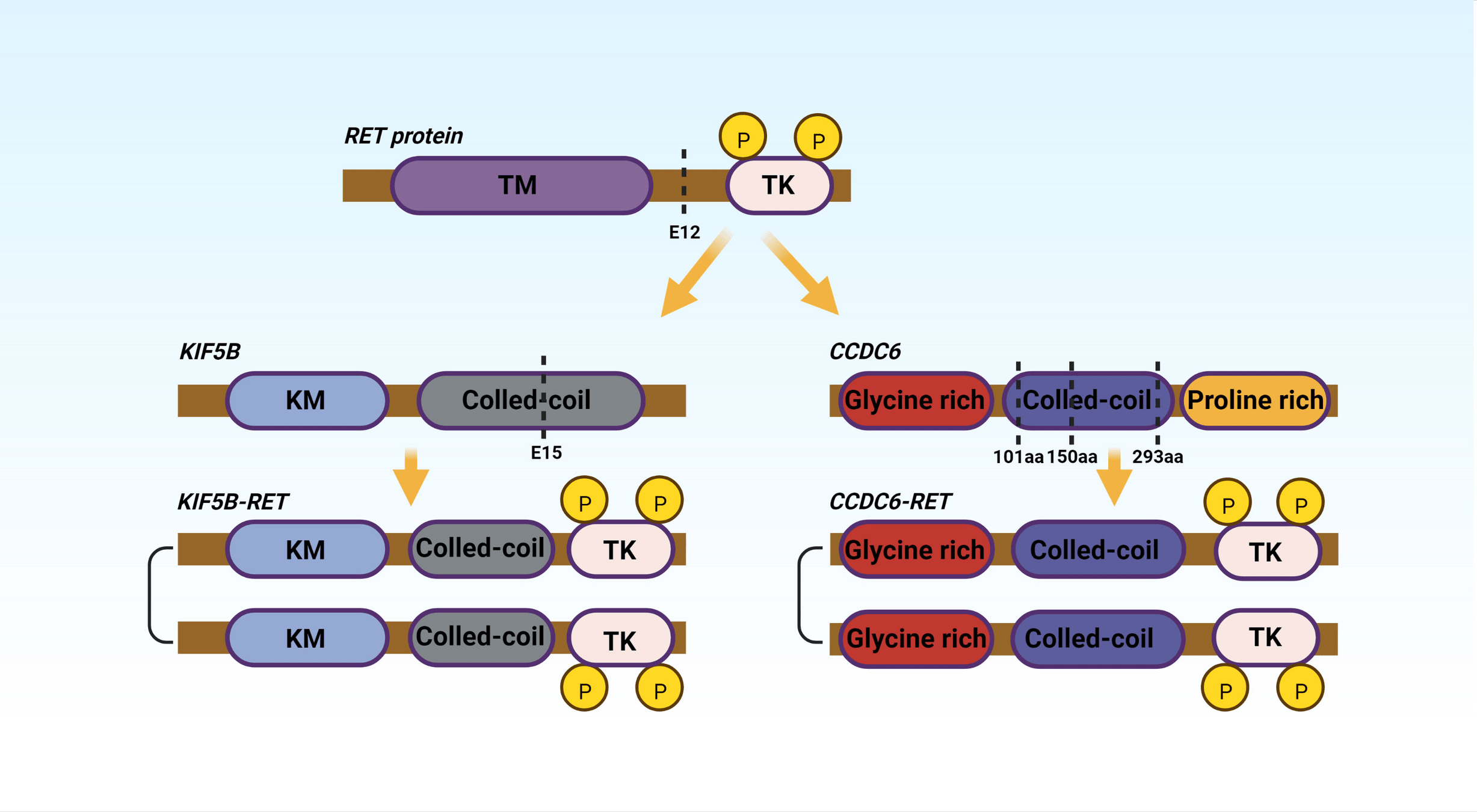
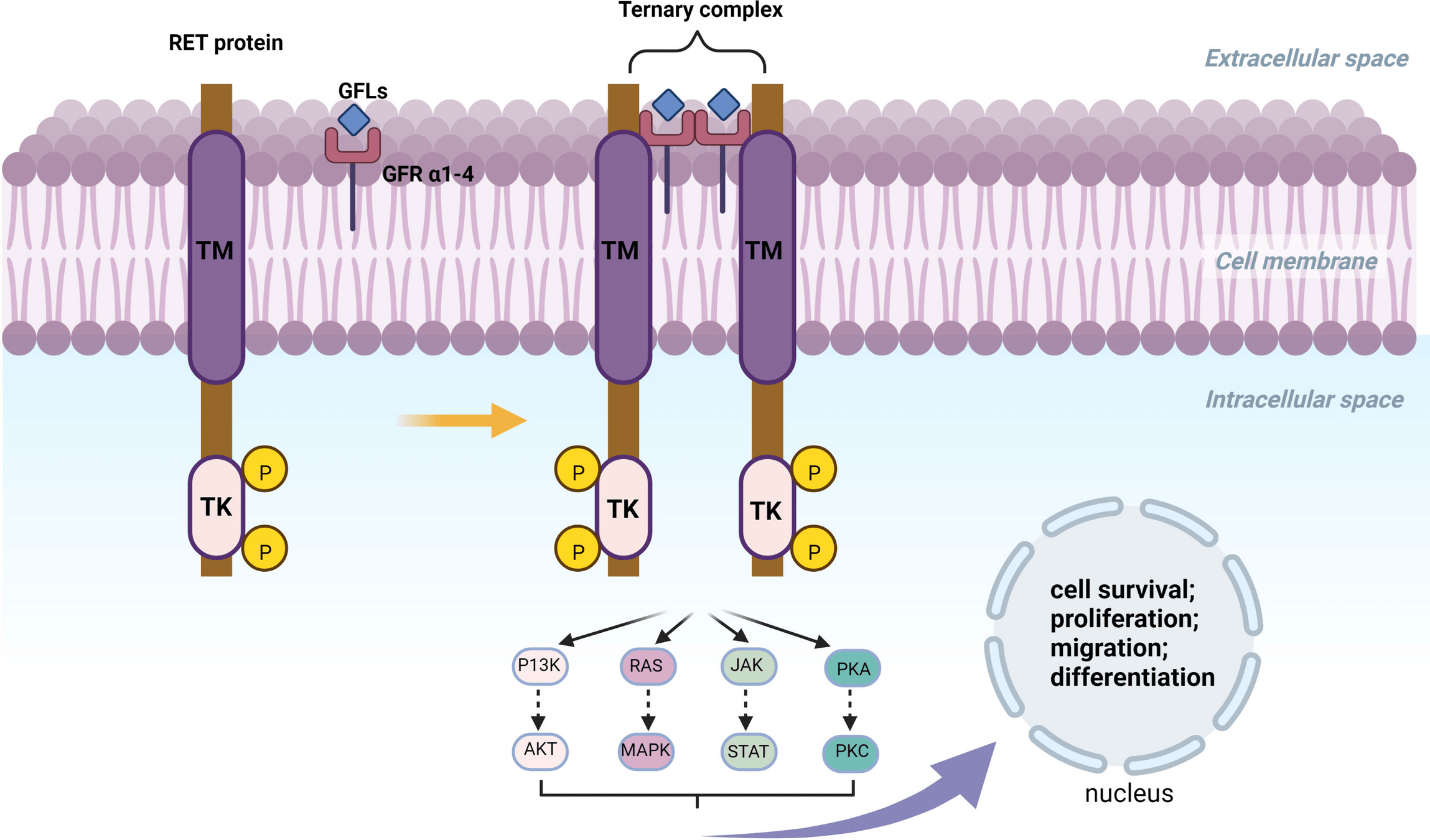
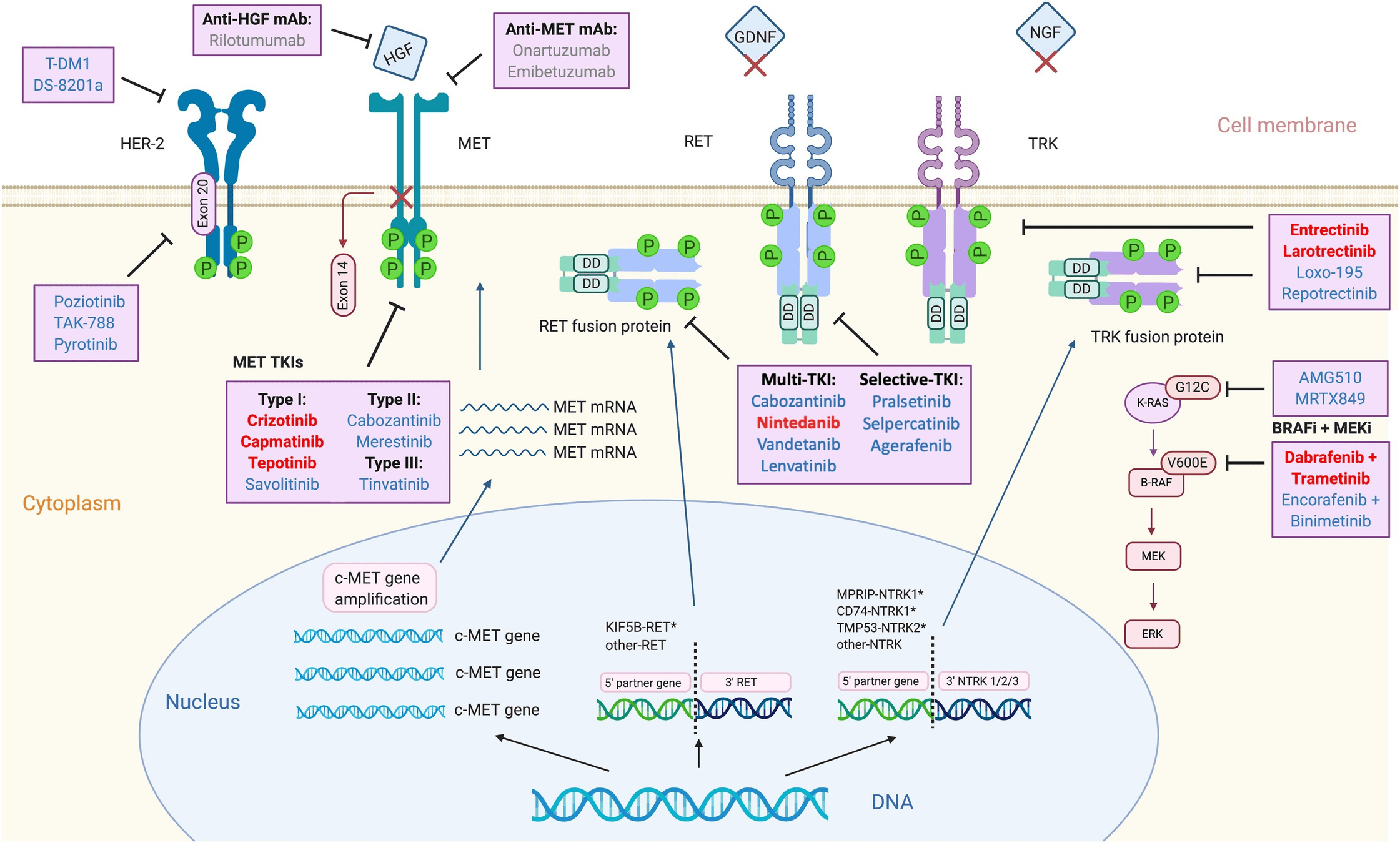
Drugs Targeting RET Fusions
- Examples: Selpercatinib, Pralsetinib.
- Mechanism: Inhibit RET fusion-driven signaling to block tumor growth and progression.
-
Key Points:
- Selpercatinib: High ORR (~85%) with durable responses (LIBRETTO trials).
- Pralsetinib: Efficacious in both RET fusion-positive NSCLC and thyroid cancers (ARROW trial).
- Well-tolerated with manageable toxicities (e.g., hypertension).
-
Approval:
- Selpercatinib: 2020 (LIBRETTO trials).
- Pralsetinib: 2020 (ARROW trial).
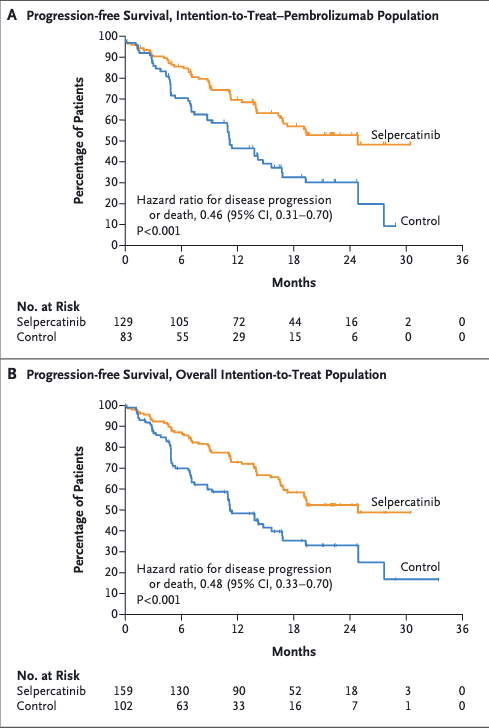
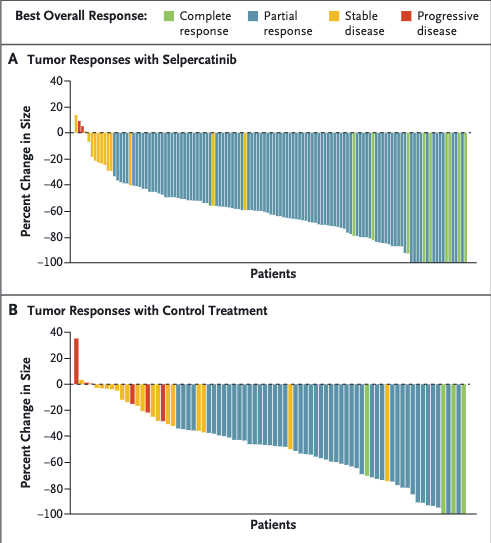
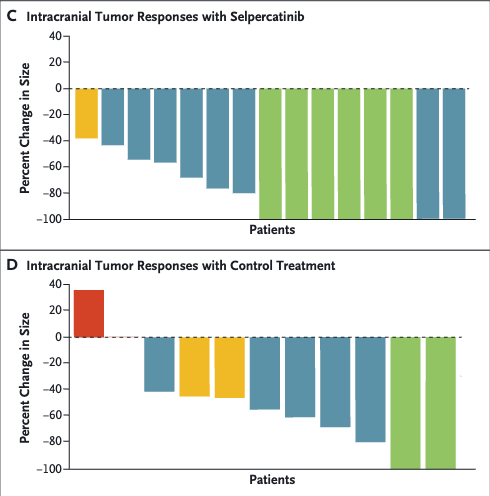
LIBRETTO trials
Selpercatinib

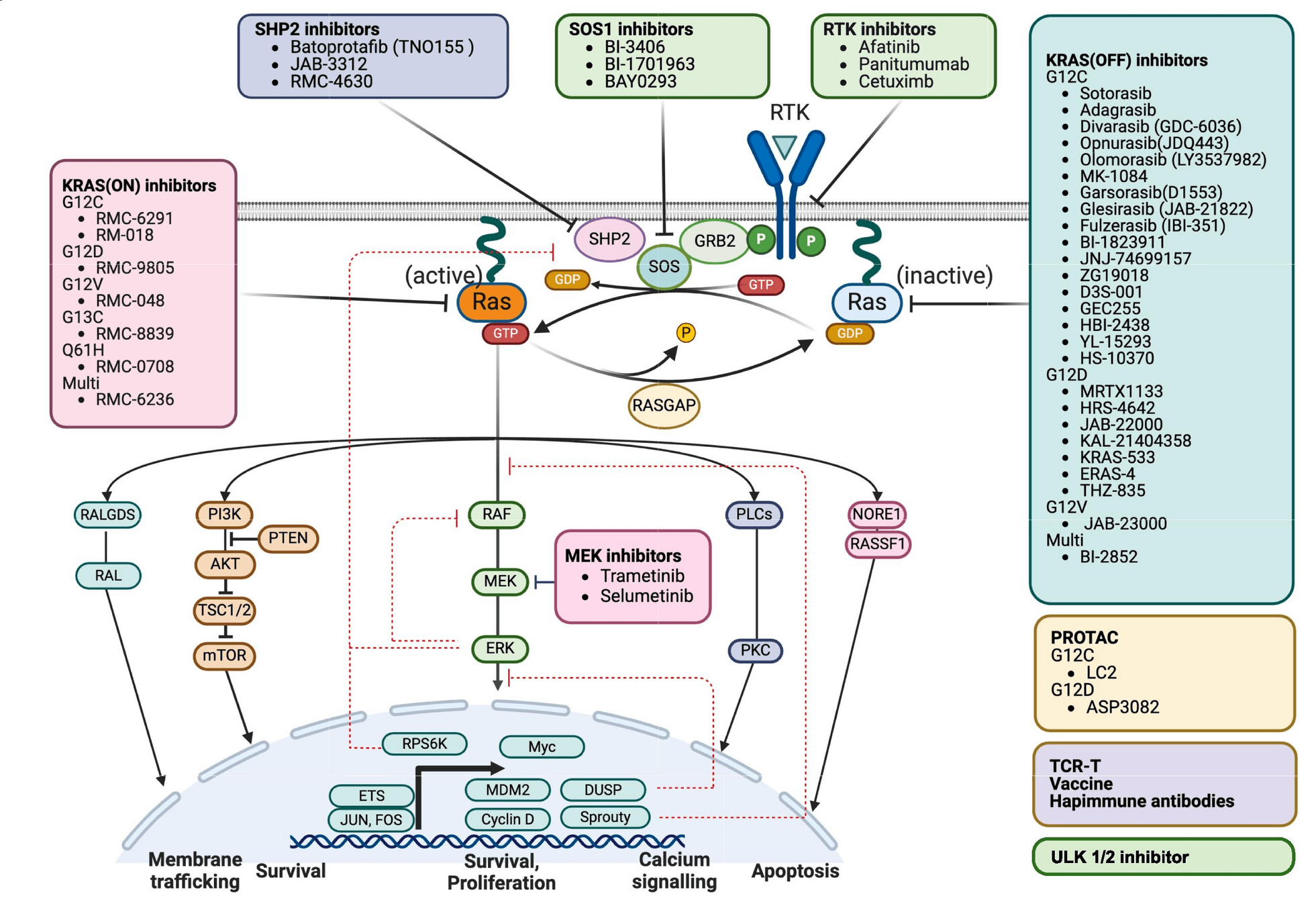
Drugs Targeting KRAS Mutations
- Examples: Sotorasib, Adagrasib.
- Mechanism: Inhibit KRAS G12C mutant protein, blocking downstream MAPK signaling.
-
Key Points:
- Sotorasib: First FDA-approved KRAS G12C inhibitor (CodeBreaK trials).
- Adagrasib: High CNS activity; promising in pretreated patients (KRYSTAL-1 trial).
-
Approval:
- Sotorasib: 2021 (CodeBreaK trials).
- Adagrasib: 2022 (KRYSTAL-1 trial).

Drugs Targeting MET Exon 14 Skipping
- Examples: Capmatinib, Tepotinib.
- Mechanism: Inhibit MET signaling to block tumor growth in MET exon 14 skipping mutations.
-
Key Points:
- Capmatinib: ORR ~40%, durable intracranial responses (GEOMETRY mono-1 trial).
- Tepotinib: High CNS activity and sustained efficacy (VISION trial).
-
Approval:
- Capmatinib: 2020 (GEOMETRY mono-1 trial).
- Tepotinib: 2021 (VISION trial).
The Role of TKIs in Early-Stage Disease
- Adjuvant Therapy: ADAURA trial demonstrated improved disease-free survival (DFS) with osimertinib in EGFR-mutated, resected NSCLC.
- Neoadjuvant Potential: Trials such as Blakely et al. suggest possible tumor reduction prior to surgery or radiation.
ADAURA Trial:
Inclusion Criteria:
- Stage IB-IIIA NSCLC with EGFR mutations (Exon 19 deletions or Exon 21 L858R).
- Complete tumor resection with or without adjuvant chemotherapy.
- Age ≥18 years with ECOG performance status ≤1.
Exclusion Criteria:
- Presence of unresectable or metastatic disease.
- Patients with prior EGFR TKI therapy or incomplete recovery post-surgery.
- Active, uncontrolled systemic illnesses.
Key Results:
- Disease-Free Survival (DFS): Median DFS not reached in osimertinib group vs. 19.6 months in placebo.
- Risk Reduction: 79% reduction in recurrence or death (HR = 0.21).
- Significant CNS Benefits: Reduced CNS recurrence in osimertinib group.
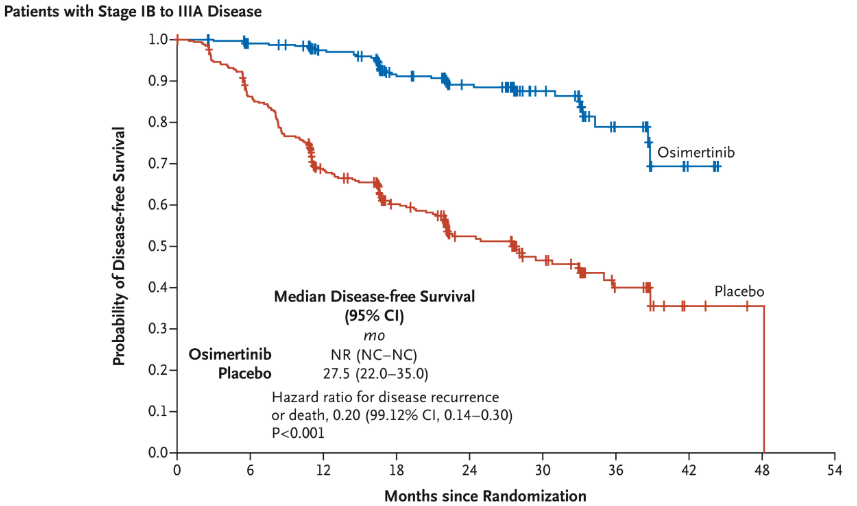
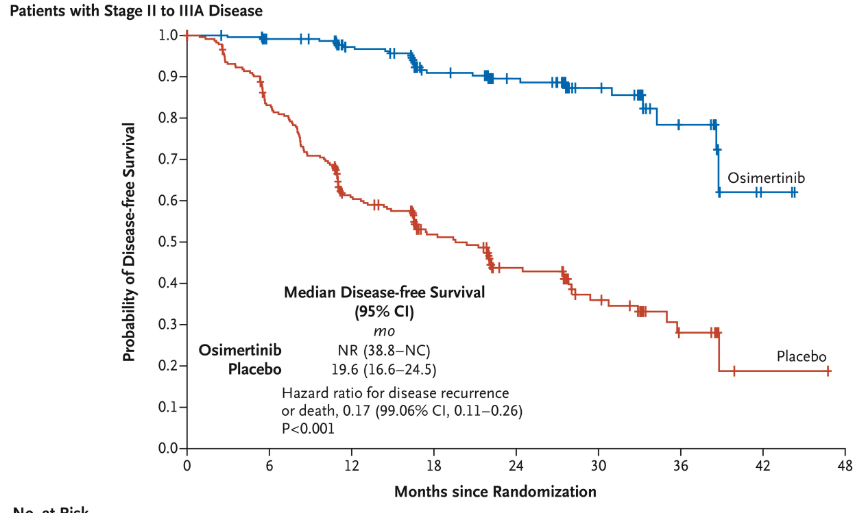
ADAURA Trial: Methods and Patient Flow
Methods:
- Study Design: Randomized, double-blind, placebo-controlled, phase III trial.
- Population: Stage IB-IIIA EGFR-mutated NSCLC (Exon 19 deletions or L858R mutations).
-
Local Therapy Requirements:
- Patients underwent **complete surgical resection** of their primary tumor prior to enrollment.
- Adjuvant chemotherapy was allowed but not required, with regimens guided by institutional standards.
- Radiation therapy was not mandated but could be utilized as part of the institutional post-surgical care plan before trial enrollment.
- Intervention: Adjuvant osimertinib (80 mg daily) vs. placebo for up to 3 years
- Monitoring: Imaging every 12 weeks for 2 years, then every 24 weeks thereafter.
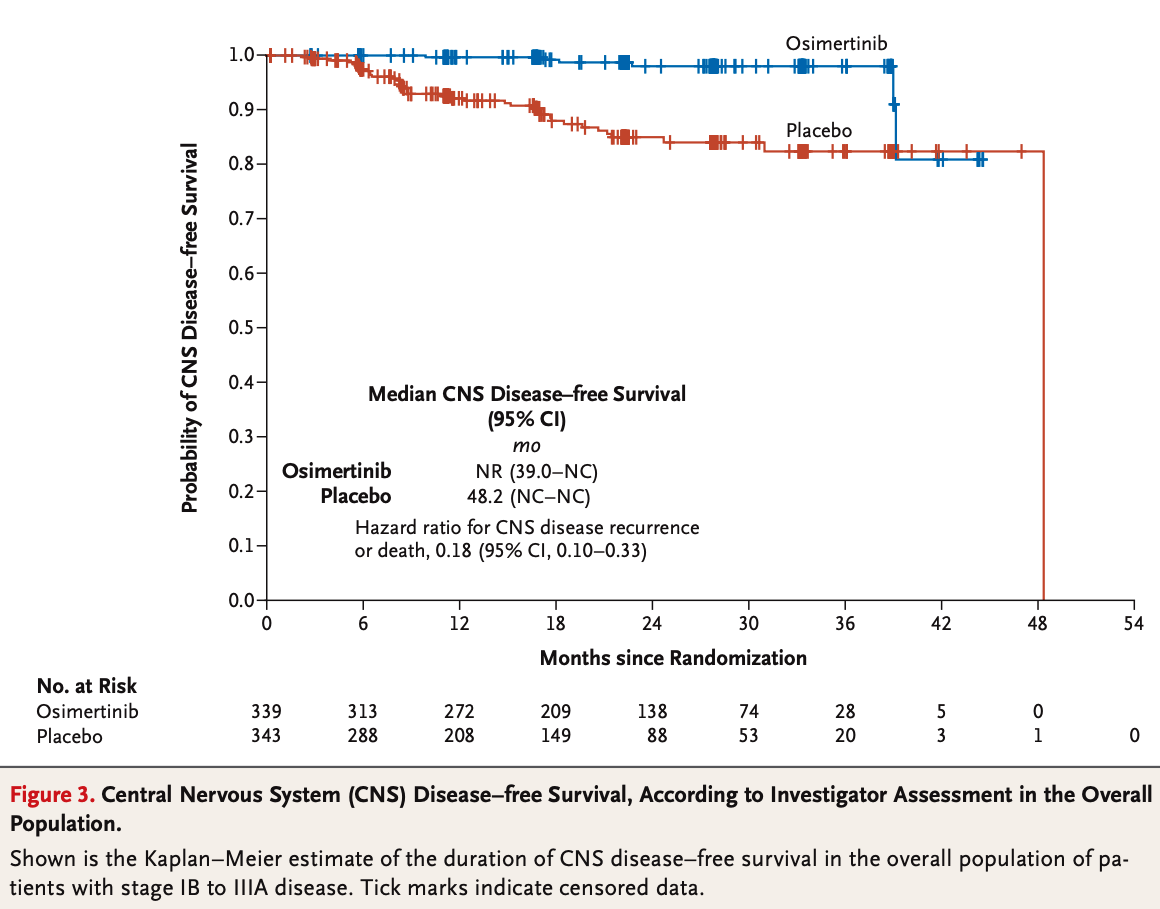
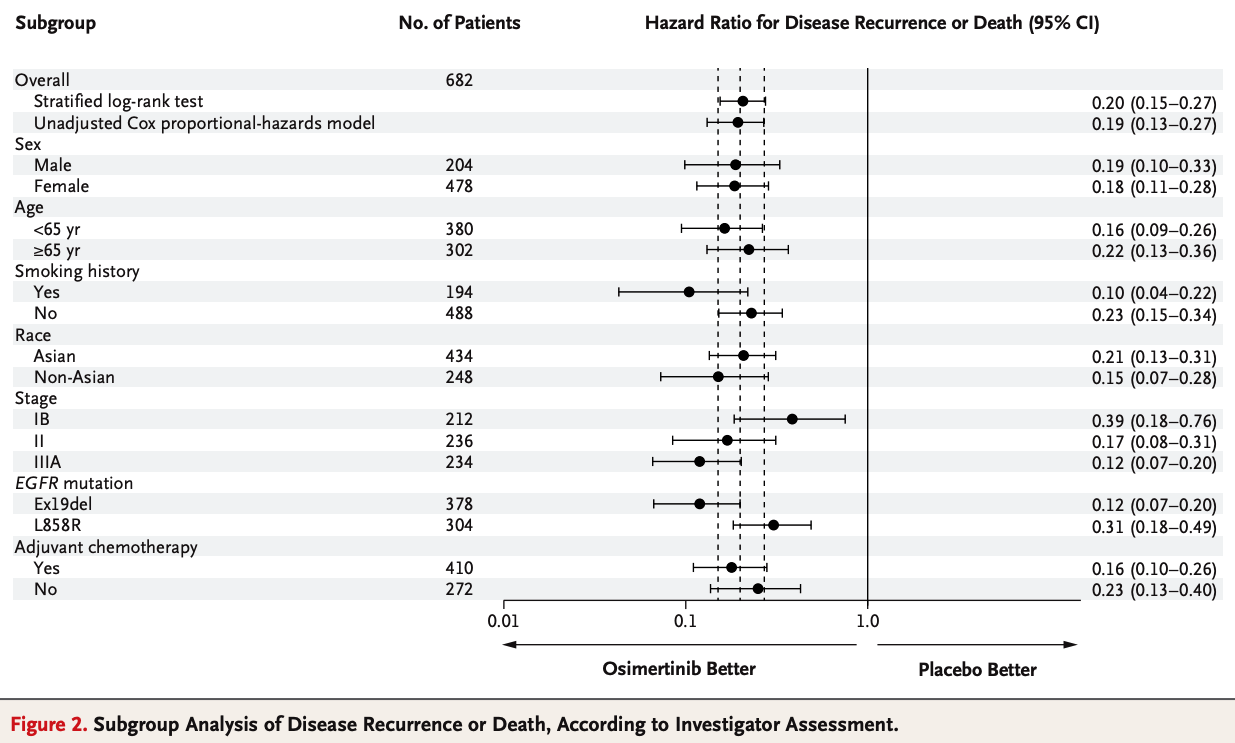
Neoadjuvant TKIs: Reducing Tumor Burden
- Trial Design: Phase II trial evaluated neoadjuvant osimertinib in Stage II-IIIA EGFR-mutated NSCLC.
Inclusion Criteria:
- Stage IB-IIIA EGFR-mutated NSCLC.
- No prior systemic therapy or TKI use.
- Age ≥18 years with ECOG performance status ≤1.
Exclusion Criteria:
- Presence of unresectable or metastatic disease.
- Inability to tolerate TKIs or planned surgery.
- Active, uncontrolled systemic illnesses.
Key Results:
- Pathologic Complete Response (pCR): 0%.
- Major Pathologic Response: Observed in 15% of patients.
- Potential DFS Benefits: Extended progression-free survival with osimertinib.
Take away: Lack of pathologic complete responses (pCR) highlights need for combination strategies.
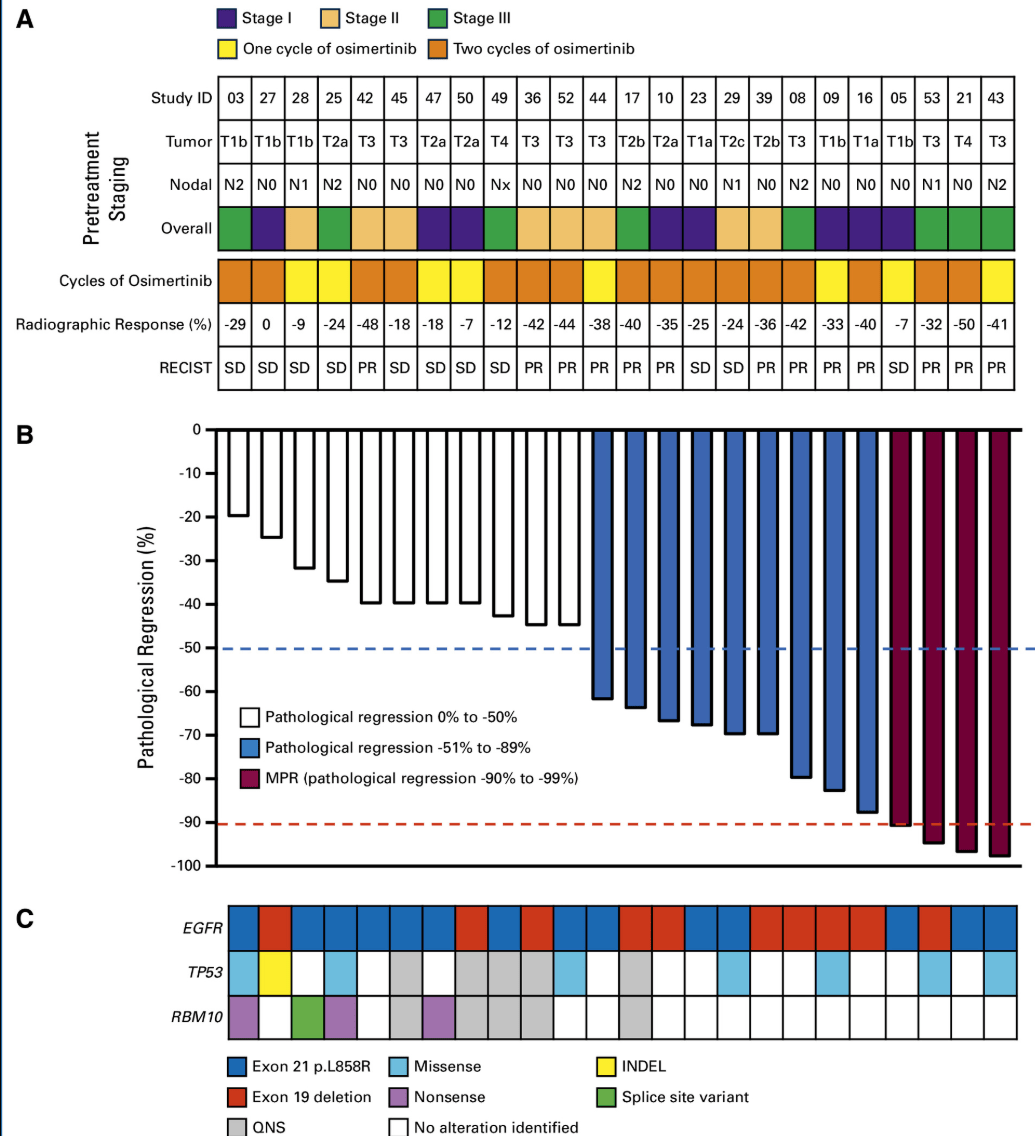
Blakely Trial: Methods and Patient Flow
Methods:
- Study Design: Phase II, single-arm trial evaluating neoadjuvant osimertinib for resectable EGFR-mutated NSCLC.
- Population: Stage II-IIIA EGFR-mutated NSCLC (Exon 19 deletions or L858R mutations).
-
Local Therapy Requirements:
- Patients must have resectable disease.
- Definitive surgical resection planned following neoadjuvant therapy.
- Adjuvant therapy was determined based on post-surgical outcomes.
- Intervention: Neoadjuvant osimertinib (80 mg daily) for 6-12 weeks prior to surgery.
-
Endpoints:
- Primary: Pathologic response rate, including complete and partial pathologic responses.
- Secondary: Radiographic response, disease-free survival (DFS), and overall survival (OS).
- Monitoring: Imaging at baseline, during treatment, and before surgery. Pathologic assessment post-resection.

The Role of TKIs in Locally Advanced NSCLC
-
Current NCCN Standard:
- Concurrent chemoradiotherapy (CCRT) is the preferred treatment for all patients with locally advanced NSCLC.
- Consolidation immunotherapy with durvalumab is the standard for patients without contraindications.
-
TKIs in EGFR/ALK-Positive NSCLC:
- Molecular profiling is strongly recommended, but targeted therapy with TKIs is not yet integrated into the concurrent chemoradiation paradigm.
- Emerging studies like LAURA are investigating the role of adjuvant osimertinib post-CCRT for EGFR-mutated NSCLC.
-
Future Directions:
- Trials are evaluating whether TKIs can replace or augment current standards such as durvalumab.
- Key challenge: Balancing systemic and local control with minimal toxicity.
Peled Trial: Design and Patient Flow
Inclusion Criteria:
- Stage III EGFR-mutant NSCLC (Exon 19 deletion or L858R mutation).
- Performance status 0–1.
- Amenable to definitive chemoradiation or surgery.
Exclusion Criteria:
- EGFR TKI-resistant mutations (e.g., exon 20 insertion).
- Significant comorbidities (e.g., interstitial lung disease).
- Major surgery within 4 weeks before enrollment.
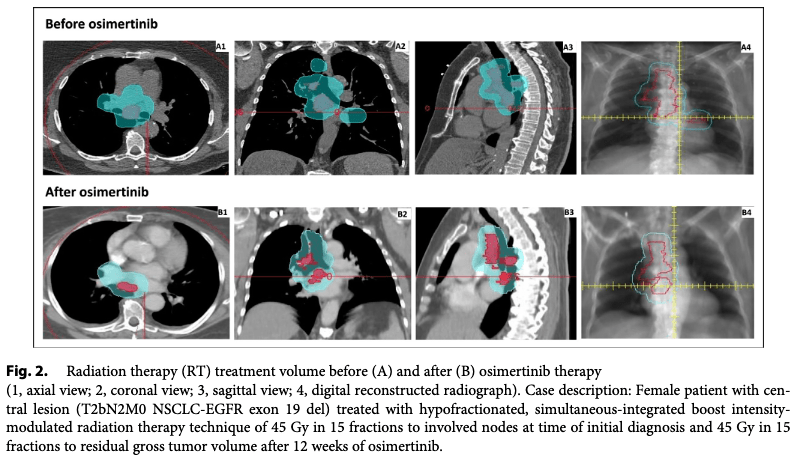
Timing of Radiation Therapy:
- Radiation therapy initiated after 12 weeks of neoadjuvant osimertinib for responders or stable disease.
- RT planning adapted to changes in tumor and nodal volume (based on GTV and PTV reduction).
- Patients with limited response received chemoradiation instead.
Peled Trial: Methods and Protocols
- Intervention: Osimertinib 80 mg daily for 12 weeks.
- Endpoints Primary: Objective response rate (ORR) via RECIST v1.1.
- Secondary: Gross tumor volume (GTV) reduction, circulating tumor DNA (ctDNA) dynamics, and safety.
-
Definitive Therapy:
- RT and/or surgery based on post-osimertinib response. (60Gy/30fx or 45Gy/15fx)
- Patients with limited response received chemoradiation.
- Monitoring: PET-CT scans and ctDNA at baseline, 6 weeks, and 12 weeks.
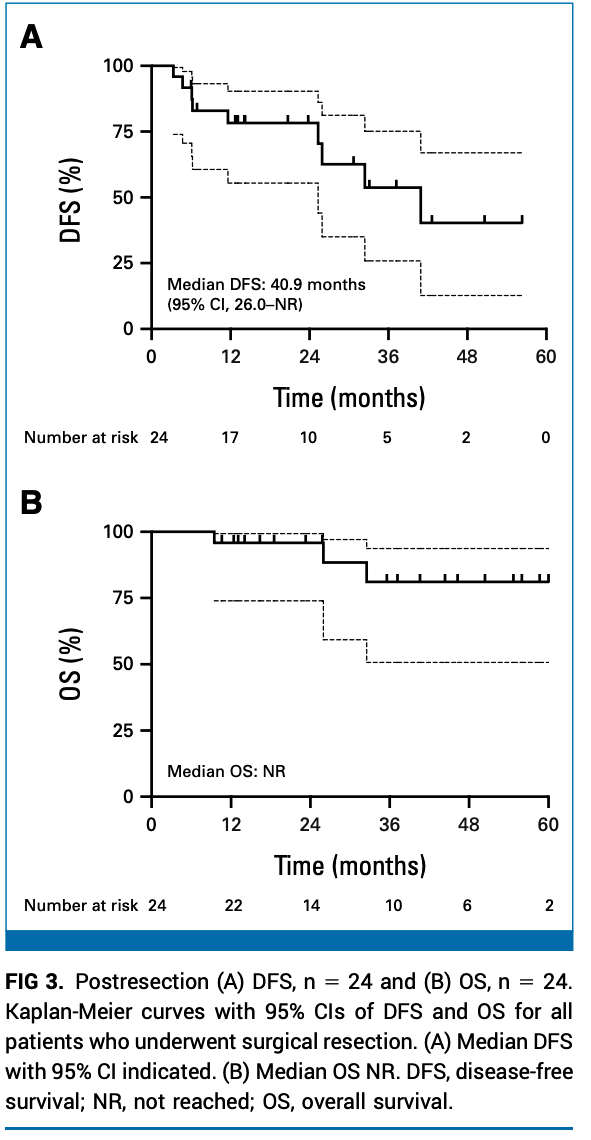
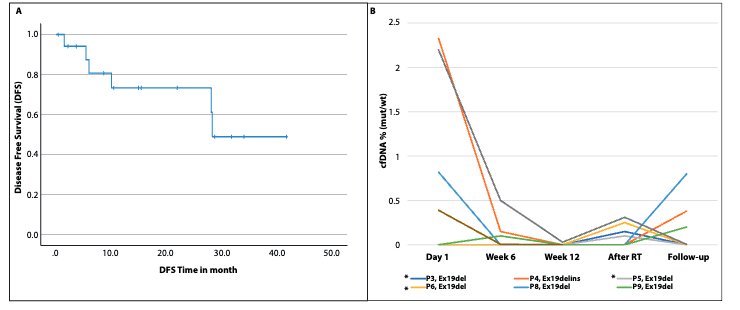
Peled Trial: Key Results
- ORR: 95.2% (17 partial responses, 2 complete responses).
-
Tumor Volume Reduction:
- Median GTV reduction: 48% (P = 0.02).
- Median PTV reduction: 31% (P = 0.01).
-
Surgical Outcomes:
- 3 patients underwent surgery; 1 achieved pathologic complete response.
-
ctDNA:
- Detected in 5 patients; 4 became negative during osimertinib therapy.
Critiques of the Peled Trial
-
Strengths:
- High ORR (95.2%) demonstrates osimertinib's efficacy as neoadjuvant therapy.
- Significant reduction in tumor volumes improved RT planning.
-
Limitations:
- Small sample size (N=24).
- No control arm to compare against standard chemoradiation or surgery outcomes.
-
Unanswered Questions:
- Long-term survival data are still limited.
- How to integrate osimertinib with existing treatments (e.g., durvalumab).
LAURA Trial: Design and Enrollment
Inclusion Criteria:
- Unresectable stage III NSCLC with EGFR exon 19 deletions or exon 21 L858R mutation.
- Completed platinum-based chemoradiotherapy (concurrent or sequential).
- No progression during or after definitive chemoradiotherapy.
- WHO performance status 0-1.
Exclusion Criteria:
- History of interstitial lung disease or unresolved grade ≥2 adverse effects post-chemoradiotherapy.
- Symptomatic pneumonitis after chemoradiotherapy.
- Patients with mutations other than EGFR exon 19 or exon 21.
Patient Characteristics:
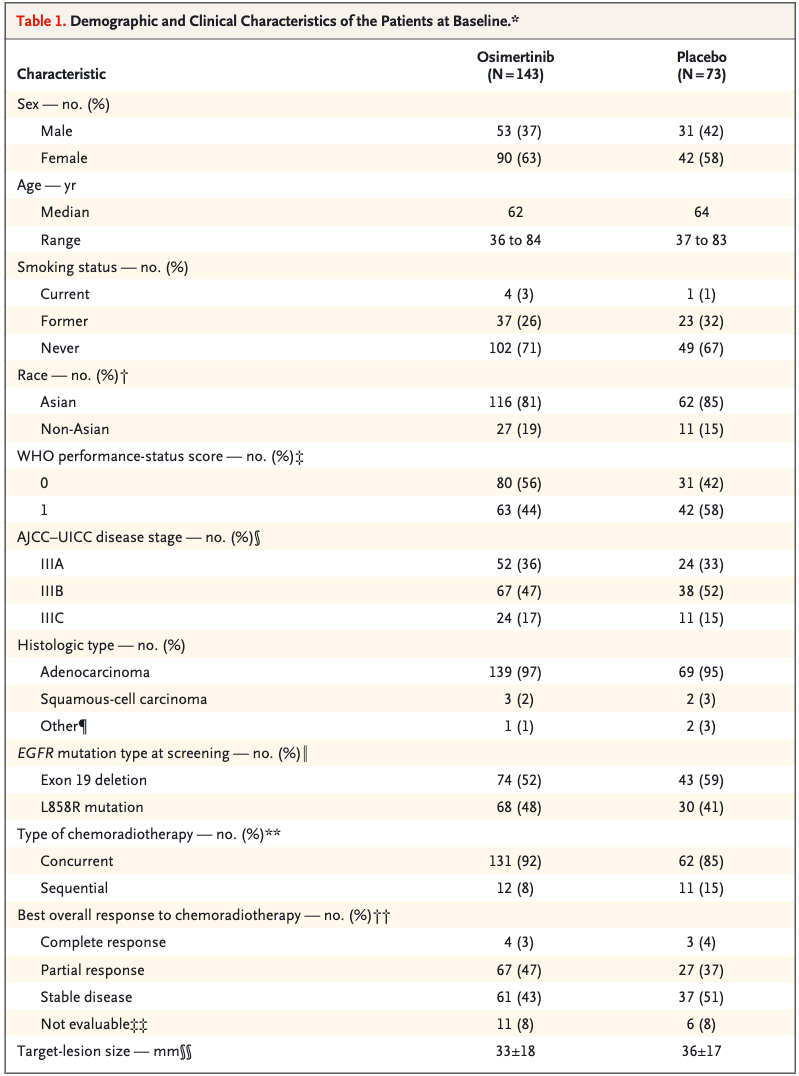
- Total patients: 216 randomized (143 osimertinib, 73 placebo).
- Most received **concurrent chemoradiotherapy**: 92% (osimertinib), 85% (placebo).
- Majority were Asian (81% osimertinib, 85% placebo).
- Performance status of 0: 56% (osimertinib) vs. 42% (placebo).
LAURA Trial: Design and Patient Flow
Timing of TKI Initiation in LAURA Trial
- Timing: TKI started within 6 weeks of completing chemoradiotherapy.
- Concurrent Chemoradiotherapy: 92% of osimertinib and 85% of placebo patients received concurrent therapy.
- Sequential Chemoradiotherapy: Smaller proportion: 8% (osimertinib) vs. 15% (placebo).
- Post-Treatment Monitoring: Regular imaging every 8 weeks (up to 48 weeks) and every 12 weeks thereafter.
Impact of Timing:
- Patients initiating osimertinib immediately after chemoradiotherapy achieved significant CNS progression-free survival benefits.
- No subgroup differences identified between concurrent and sequential chemoradiotherapy timing.
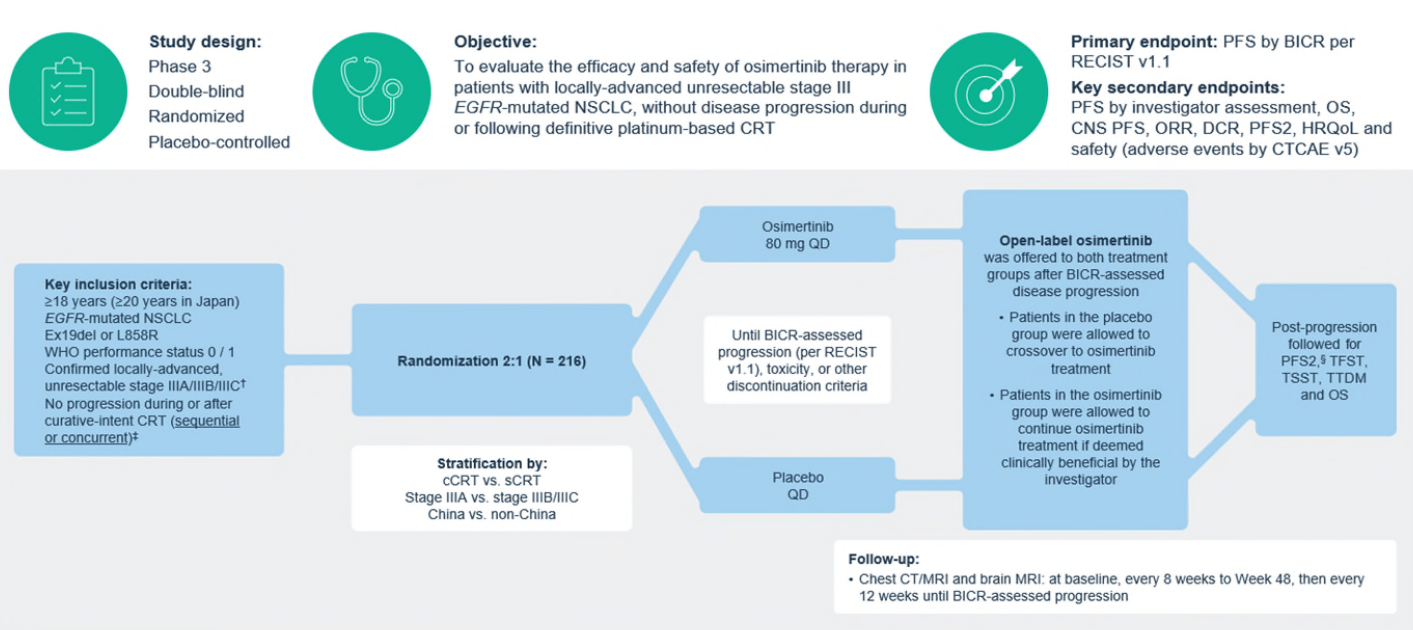
LAURA Trial: Key Results
- PFS: Median 39.1 months (osimertinib) vs. 5.6 months (placebo); HR 0.16 (P<0.001).
- CNS Progression: New brain metastases: 8% (osimertinib) vs. 29% (placebo).
- Safety: Grade ≥3 adverse events: 35% (osimertinib) vs. 12% (placebo).
- OS (Interim): 84% (osimertinib) vs. 74% (placebo) at 36 months (HR 0.81, P=0.53).
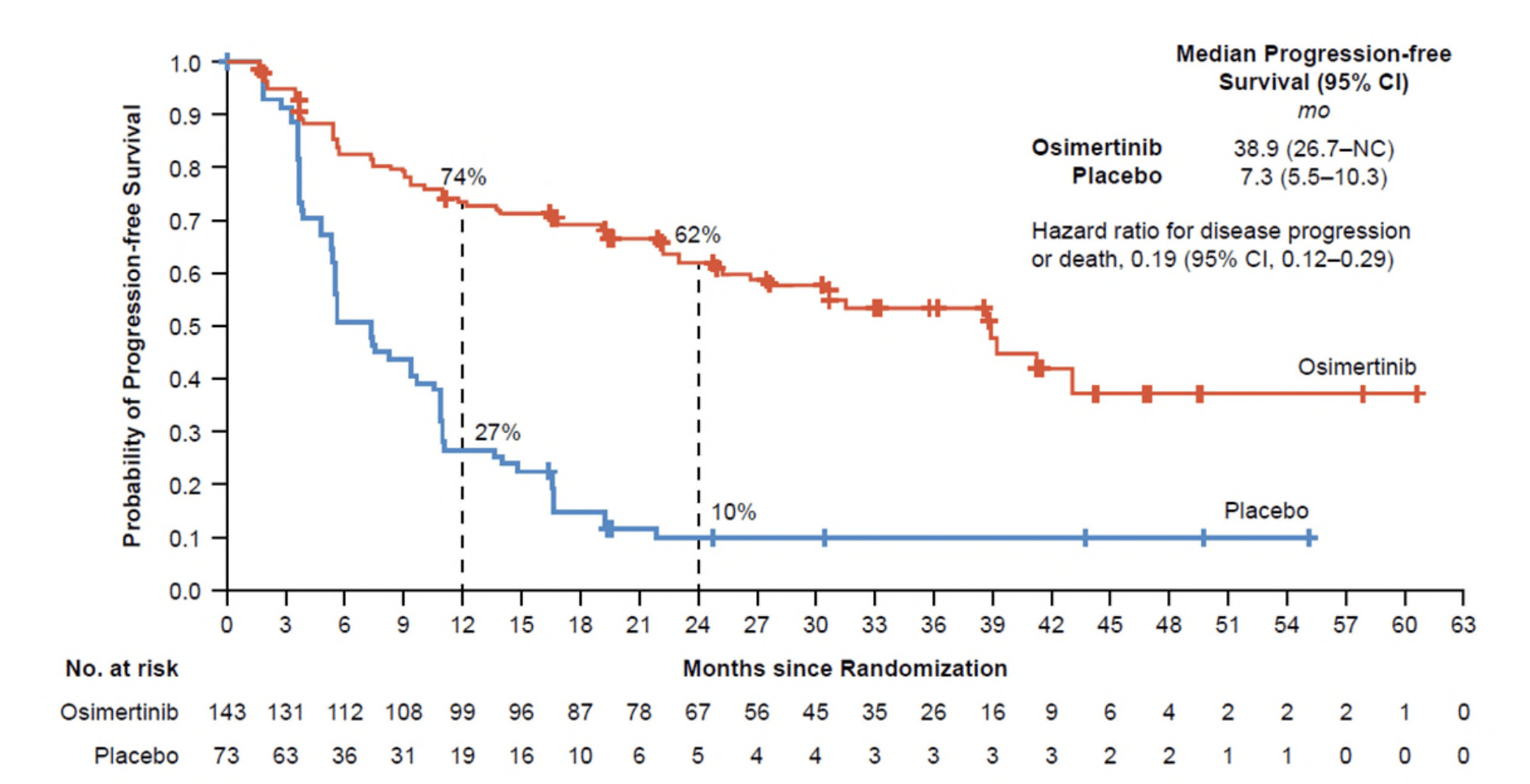
LAURA Trial: Key Results
- PFS: Median 39.1 months (osimertinib) vs. 5.6 months (placebo); HR 0.16 (P<0.001).
- CNS Progression: New brain metastases: 8% (osimertinib) vs. 29% (placebo).
- Safety: Grade ≥3 adverse events: 35% (osimertinib) vs. 12% (placebo).
- OS (Interim): 84% (osimertinib) vs. 74% (placebo) at 36 months (HR 0.81, P=0.53).
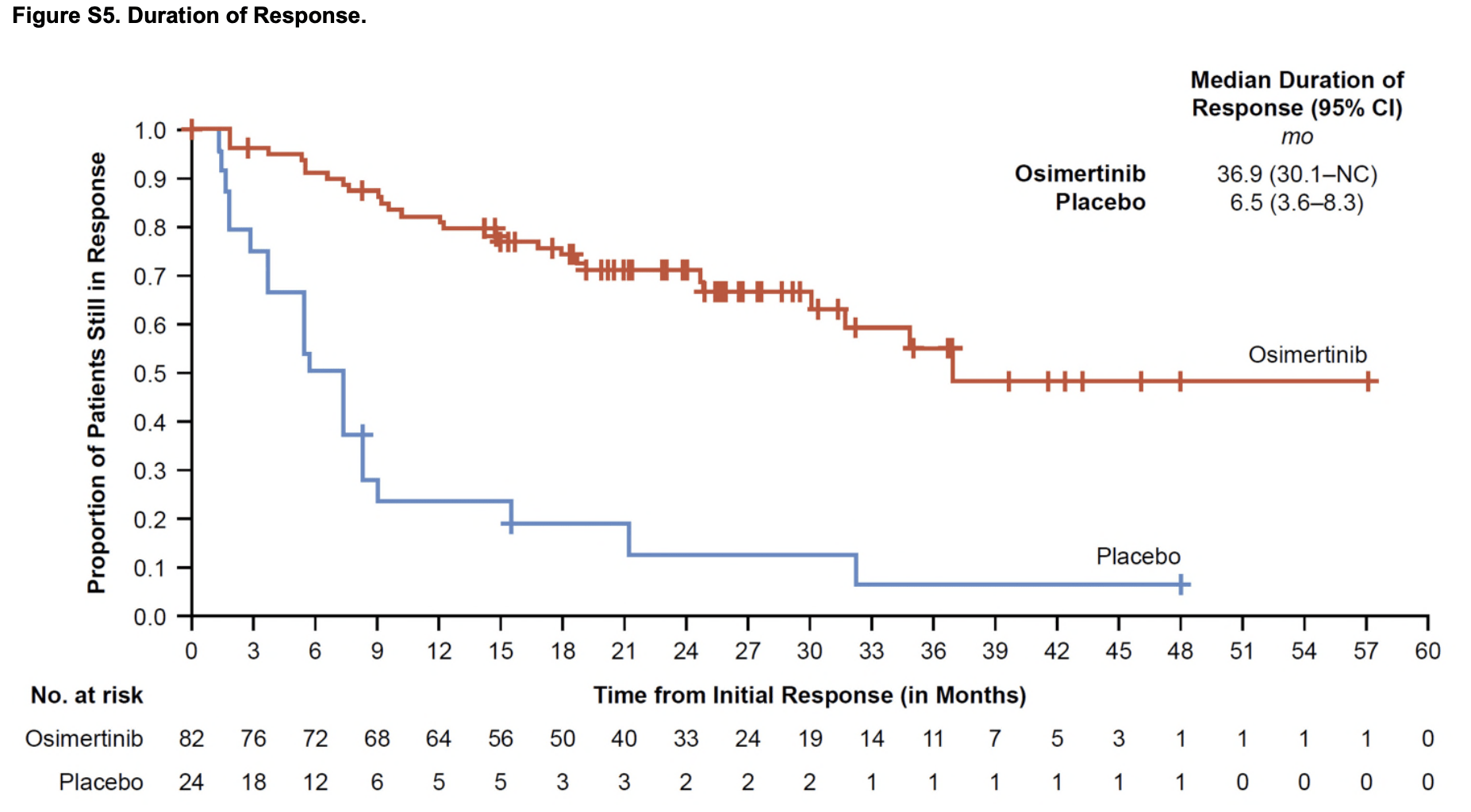
LAURA Trial: Key Results
- PFS: Median 39.1 months (osimertinib) vs. 5.6 months (placebo); HR 0.16 (P<0.001).
- CNS Progression: New brain metastases: 8% (osimertinib) vs. 29% (placebo).
- Safety: Grade ≥3 adverse events: 35% (osimertinib) vs. 12% (placebo).
- OS (Interim): 84% (osimertinib) vs. 74% (placebo) at 36 months (HR 0.81, P=0.53).
LAURA Trial: Toxicities
Radiation Pneumonitis
-
Incidence:
- Osimertinib: 48%
- Placebo: 38%
-
Severity:
- Most cases were Grade 1–2.
- Grade 3: 2% (osimertinib) vs. 0% (placebo).
- No Grade 4 or 5 events were reported.
- Statistical Significance: Difference in incidence was not statistically significant.
Interstitial Lung Disease (ILD)
-
Incidence:
- Osimertinib: 8%
- Placebo: 1%
-
Severity:
- Most cases were Grade 1–2.
- Attributed primarily to pneumonitis.
- Statistical Significance: Not specified, but clinically relevant difference noted.
Management:
- Most radiation pneumonitis and ILD cases resolved with supportive care.
- Guidelines allowed continuation of osimertinib for mild to moderate symptoms.
Critiques of the LAURA Trial
-
Unanswered Questions:
- Is osimertinib superior to durvalumab for this population?
- What is the optimal duration of osimertinib therapy?
-
Applicability Concerns:
- Heavy Asian patient representation; results may not generalize globally.
- Exclusion of patients with unresolved post-chemoradiotherapy toxicities.
-
Strengths:
- Robust CNS control and extended PFS benefit.
- Well-designed phase III trial with placebo control.
TKIs and Radiotherapy in Oligometastatic NSCLC
- Established Role: TKIs remain standard for oncogene-driven NSCLC (EGFR, ALK, ROS1 mutations).
-
Focus: Synergistic integration of TKIs and radiotherapy:
- Upfront Radiotherapy: Prolonged survival demonstrated in trials combining SBRT with systemic TKIs.
- Oligoprogression: Radiotherapy targets isolated progression sites while TKIs continue to control systemic disease.
-
Key Trials:
- SINDAS Trial: EGFR-mutant NSCLC, OS benefit with upfront SBRT + TKIs (25.5 vs. 17.4 months).
- Peled et al., 2023: Investigated SBRT with osimertinib in EGFR+ oligometastatic NSCLC
- NORTHSTAR Trial: Evaluating consolidative SBRT with osimertinib in EGFR-mutant NSCLC
SINDAS Trial: Study Design and Patient Flow
Inclusion Criteria:
- EGFR-mutated NSCLC (Exon 19 deletions or L858R mutations).
- 1-5 synchronous oligometastases (confirmed by imaging).
- No prior therapy for metastatic disease.
- ECOG performance status ≤1.
Exclusion Criteria:
- Brain metastases.
- Concurrent severe illnesses or contraindications to radiotherapy.
Enrolled Population:
- 73% of metastases were bone lesions.
- Median age: 60 years; more patients in the RT+TKI arm had >2 metastases.
Timing of Radiation Therapy:
- Radiotherapy delivered upfront to primary tumor, involved nodes, and oligometastases (25-40 Gy in 1-5 fractions).
- Radiotherapy occurred **prior to TKI initiation** in patients with synchronous metastases.
- No radiotherapy was allowed in the TKI-alone arm until disease progression.
SINDAS Trial: Trial Design and Patient Flow
Methods:
-
Radiotherapy: Delivered to all sites, including:
- Primary tumor and involved mediastinal lymph nodes.
- All oligometastatic sites (up to 5).
- Uniform Fractionation: 25-40 Gy in 5 fractions, including mediastinal nodes (unconventional for central structures).
- Techniques: SBRT, VMAT, or IMRT with constraints for adjacent organs (e.g., mean dose <16.5 Gy for trachea/bronchus).
SINDAS Trial: 5 Fx Dose Constraints
| Structure | Dose Constraint |
|---|---|
| Trachea/Bronchus | Mean dose ≤ 16.5 Gy |
| Heart | V30 ≤ 5 cc (Volume receiving ≥ 30 Gy) |
| Esophagus | V30 ≤ 5 cc |
| Spinal Cord | Maximum dose ≤ 30 Gy |
| Lungs | V20 ≤ 30% (Volume receiving ≥ 20 Gy) |
| Ribs | V30 ≤ 5 cc |
| Liver | Mean dose ≤ 28 Gy |
| Kidneys | Mean dose ≤ 20 Gy |
SINDAS Trial: Results
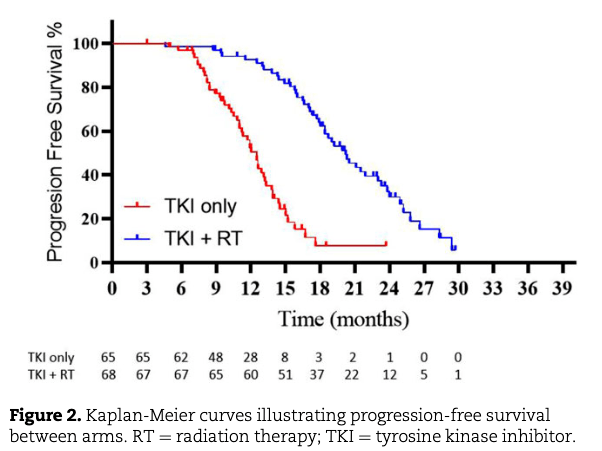
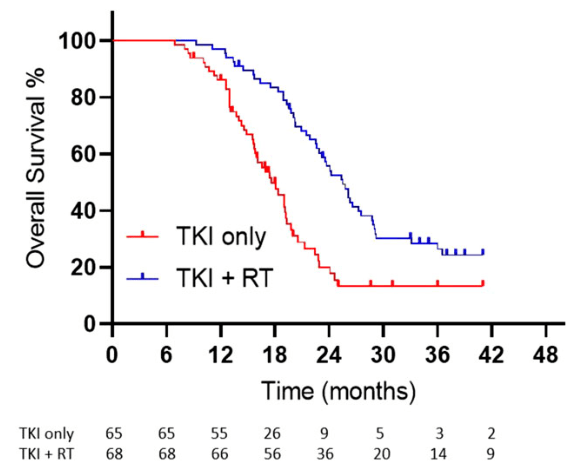
Key Results:
- TKIs Used: Gefitinib, Erlotinib, or Icotinib (Osimertinib not available).
- Median OS: 25.5 vs. 17.4 months (p < 0.001).
- Median PFS: 20.2 vs. 12.5 months (p < 0.001).
- Local Control (LC): 91% (RT+TKI) vs. 55% (TKI only).
- Toxicity: Grade 3-4 pneumonitis: 6% (RT+TKI), no grade 5 toxicity.
SINDAS Trial: Toxicities
Radiation Pneumonitis
-
Incidence:
- RT+TKI Group: 6% (Grade 3–4).
- TKI-Only Group: 0% (Grade 3–4).
-
Severity:
- Most cases were mild (Grade 1–2).
- No Grade 5 events were reported.
-
Management:
- Resolved with corticosteroids and supportive care.
Other Toxicities
-
Fatigue:
- Reported in both arms, with higher rates in RT+TKI group.
-
Dermatologic and GI Toxicities:
- Consistent with known profiles of first-generation TKIs (e.g., gefitinib, erlotinib).
- Rash and diarrhea were the most common.
-
Overall Safety:
- No significant increase in Grade ≥3 toxicities between arms.
- No treatment-related deaths reported.
Overall Toxicity Profile:
- Radiotherapy in combination with TKIs was generally well-tolerated.
- Low rates of severe toxicities suggest safety of this approach with appropriate management.
Critiques of the SINDAS Trial
- Outdated Systemic Therapy: Used first-generation TKIs (gefitinib, erlotinib, icotinib) instead of third-generation osimertinib, now the standard for EGFR-mutated NSCLC.
- Early Trial Closure: Lacked prespecified stopping criteria, raising concerns about long-term data robustness.
- Unconventional RT Protocol: Uniformly used 5-fraction RT for all sites, including mediastinal lymph nodes and primary tumor, which is not standard practice.
- Exclusion of Brain Metastases: Limited applicability in an era where CNS-penetrant TKIs (e.g., osimertinib) are standard.
- Inadequate Staging: PET imaging not uniformly used, potentially missing occult metastases.
- Timing of Radiotherapy: Optimal timing of RT (upfront vs. delayed) remains unanswered.
NORTHSTAR trial
NORTHSTAR Trial: Study Design and Patient Flow
Inclusion Criteria:
- Stage IIIB-IV EGFR-mutated NSCLC (Exon 19, L858R, or T790M mutations).
- Up to 3 metastases or TKI-naive/first-line osimertinib users.
- No progression after 6-12 weeks of induction osimertinib.
- ECOG performance status ≤1.
Exclusion Criteria:
- Prior osimertinib or third-generation EGFR TKIs.
- Symptomatic CNS metastases.
- Significant comorbidities (e.g., uncontrolled hypertension).
Enrolled Population:
- Induction osimertinib given to all patients for 6-12 weeks.
- Patients randomized to continue osimertinib alone or add consolidative RT (± surgery).
Timing of Radiation Therapy:
- RT delivered **after induction osimertinib** to target residual disease or consolidate response.
- Radiotherapy timing evaluated for toxicity and synergistic effects when combined with osimertinib.
- Designed for consolidative RT, not upfront therapy like in SINDAS.
Critiques of the NORTHSTAR Trial Design
Key Questions the Design Fails to Answer:
-
Timing of Radiotherapy:
- Does the benefit of consolidative RT differ from upfront RT as in the SINDAS trial?
- Should RT be delivered only after achieving systemic control with osimertinib?
-
Selection of Patients for RT:
- How should patients with oligoprogressive disease be stratified?
- Do patients with larger metastatic burdens benefit equally from consolidative RT?
Strengths and What It Solves:
- Inclusion of CNS Disease: The trial includes patients with limited brain metastases, addressing a key gap in prior studies like SINDAS.
- Modern Systemic Therapy: Incorporates osimertinib, a third-generation EGFR TKI with superior systemic and CNS efficacy.
- Focus on Consolidative RT: Evaluates RT in a setting that mirrors real-world practice, where systemic control is often achieved before considering RT.
- Improved RT Protocols: Standardizes RT techniques and fractionation schedules, improving reproducibility.
Comparative Analysis of Key Trials
| Trial | Population | Intervention | Key Outcomes | Toxicities |
|---|---|---|---|---|
| SINDAS | EGFR+ NSCLC, 1-5 oligometastases | RT (25-40 Gy in 5 Fx) + 1st Gen TKIs |
- OS: 25.5 vs. 17.4 months (p < 0.001) - Local control: 91% vs. 55% |
Grade 3-4 pneumonitis: 6% |
| LAURA | Stage III EGFR+ NSCLC | Osimertinib vs. Placebo |
- PFS: 39.1 vs. 5.6 months (HR: 0.16) - CNS progression: 8% vs. 29% |
- Pneumonitis: 48% vs. 38% - ILD: 8% vs. 1% |
| ADAURA | Stage IB-IIIA EGFR+ NSCLC | Adjuvant Osimertinib |
- DFS: HR 0.20 (99% CI: 0.14-0.30) - Significant CNS and systemic DFS benefits |
- No Grade 4/5 toxicities - Pneumonitis and rash observed |
| Blakely | Stage II-IIIA EGFR+ NSCLC | Neoadjuvant Osimertinib |
- ORR: ~50% - GTV/Pathologic response: No complete response - Median DFS: Promising but limited data |
- Rash and diarrhea observed - Mild pneumonitis reported |
| Peled | Stage III EGFR+ NSCLC | Neoadjuvant Osimertinib (12 weeks) |
- ORR: 95.2% - GTV reduction: 48% |
Mild pneumonitis reported |
Radiation vs TKIs for CNS Disease
-
When to Prioritize Radiation:
- Symptomatic metastases causing mass effect or impending herniation.
- Patients who progress intracranially on brain-penetrant TKIs.
-
When to Defer Radiation:
- Asymptomatic patients with high CNS penetrance of TKIs like osimertinib or alectinib.
- Patients with long-term systemic disease control on TKIs.
-
Clinical Evidence:
- Phase III studies demonstrate CNS activity with osimertinib and alectinib significantly reducing intracranial progression.
- Local therapy (SRS) improves intracranial control in conjunction with TKIs for large/multiple brain lesions.
CNS Penetration of Targeted Therapies in NSCLC
EGFR TKIs
ALK TKIs
ROS1 TKIs
Other TKIs
Radiation vs TKIs for CNS Disease
-
When to Prioritize Radiation:
- Symptomatic metastases causing mass effect or impending herniation.
- Patients who progress intracranially on brain-penetrant TKIs.
-
When to Defer Radiation:
- Asymptomatic patients with high CNS penetrance of TKIs like osimertinib or alectinib.
- Patients with long-term systemic disease control on TKIs.
-
Clinical Evidence:
- Phase III studies demonstrate CNS activity with osimertinib and alectinib significantly reducing intracranial progression.
- Local therapy (SRS) improves intracranial control in conjunction with TKIs for large/multiple brain lesions.
Mutation-Specific Failure Patterns
-
EGFR Mutations:
- Exon 19 deletions: Longer PFS and OS compared to Exon 21 L858R.
- Common CNS progression due to limited blood-brain barrier penetration by early TKIs.
- ALK Rearrangements: CNS failures dominate despite newer TKIs like lorlatinib.
- ROS1 Fusions: Less CNS failure compared to ALK or EGFR mutations, especially with entrectinib.
- KRAS G12C: Primarily systemic progression. Emerging combinatorial strategies under investigation.
Duration of Control Before Failure
- EGFR: Median PFS ~19 months with osimertinib; systemic failures dominate thereafter.
- ALK: Median PFS ~35 months with next-generation TKIs (e.g., alectinib).
- Post-Failure Strategies: Oligoprogression can be managed with local radiation while continuing systemic TKIs.
Patterns of Failure in Oncogene-Addicted NSCLC
-
Distant Failures:
- EGFR-mutated NSCLC: Predominantly systemic progression (e.g., T790M resistance). Median PFS with osimertinib ~19 months.
- ALK-positive NSCLC: CNS metastases dominate. Alectinib reduces CNS progression risk but doesn’t eliminate it.
- Local Failures: Typically observed in cases of oligoprogression or resistant residual disease.
Best Practices for Oncogene-Driven NSCLC
-
Comprehensive Molecular Testing:
- Test for EGFR, ALK, ROS1, RET, MET, NTRK, KRAS mutations.
- Incorporate liquid biopsies and ctDNA for real-time monitoring.
-
Integrating Radiation and Systemic Therapy:
- For oligometastatic disease, consider RT + TKIs (e.g., SINDAS trial).
- In locally advanced settings, can use concurrent RT (e.g., LAURA trial).
-
CNS Management:
- Use CNS-penetrant therapies (e.g., osimertinib, lorlatinib).
- Consider SRS over WBRT where appropriate.
-
Toxicity Management:
- Monitor and manage pneumonitis and ILD proactively.
- Adapt treatment plans to minimize adverse events.
-
Oligometastatic Disease:
- Continued role for SBRT to enhance local control in patients responding to TKIs.
- Potential synergy between RT and systemic therapies in CNS metastases management.
-
Locally Advanced Disease:
- Consolidative RT remains critical in patients with partial responses to systemic therapies.
- Exploration of concurrent TKI and RT to minimize disease recurrence.
-
Future Research Directions:
- Defining the optimal timing and sequencing of local and systemic therapies.
- Randomized trials to assess long-term survival benefits of RT in patients on modern TKIs.
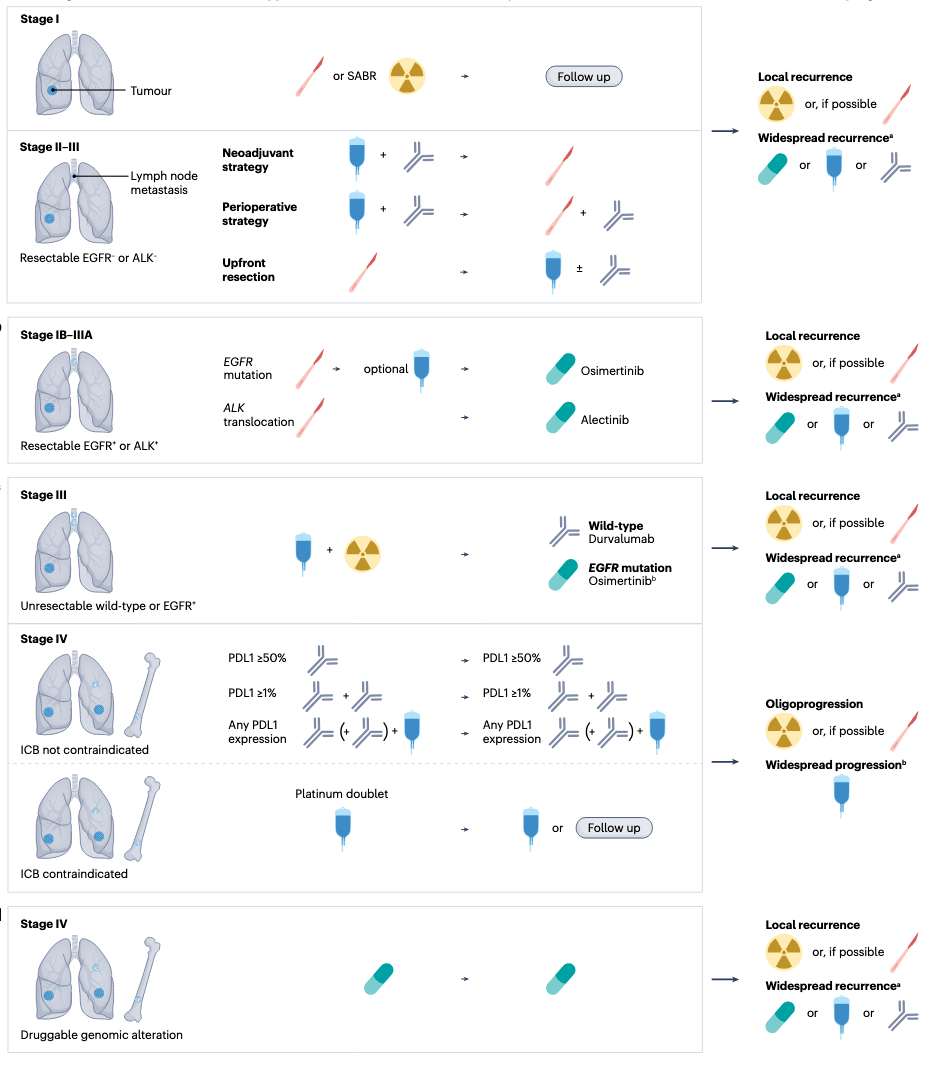
The Future of Oncogene-Driven NSCLC
-
Combining Systemic Therapies:
- Exploring the synergistic potential of TKIs with immunotherapies, particularly in oligometastatic and locally advanced disease.
- Optimal sequencing and timing of immunotherapy alongside radiation and targeted therapies.
-
Refining Patient Selection:
- Biomarker-driven approaches to identify patients who benefit most from combination therapies.
- Real-time monitoring using liquid biopsies to adapt treatment strategies dynamically.
-
Understanding Resistance Mechanisms:
- Further study of resistance patterns to TKIs and immunotherapies.
- Integration of local therapies to manage resistance-driven recurrences.
Final Thoughts
The role of local therapies, like radiation, remains critical in managing oncogene-driven NSCLC, especially in oligometastatic and locally advanced settings. While combining TKIs, immunotherapy, and radiation is the future, radiation is still a cornerstone of treatment today.
Targeting Beyond the Tumor: Radiation's Evolving Role in Oncogene-Driven Lung Cancer
By RadMedSkiier
Targeting Beyond the Tumor: Radiation's Evolving Role in Oncogene-Driven Lung Cancer
Explore the exciting advancements in targeted therapies for oncogene-driven lung cancer, highlighting novel approaches and trial results that promise to redefine treatment strategies and improve patient outcomes. Discover what the future holds!
- 29



