Toxicity Profiles and Survival Outcomes: IMPT vs IMRT for Nonmetastatic Nasopharyngeal Carcinoma
Li et al.
JAMA Network Open 2021
Journal Club Presentation
Introduction
- Nasopharyngeal carcinoma (NPC) is endemic to East and Southeast Asia
- Primary treatment: radiotherapy ± chemotherapy with curative intent
- IMRT has improved outcomes but 50-75% still experience grade 3-4 acute toxicities
- 10-20% of survivors experience serious late complications
- Proton therapy theoretical advantage: minimal exit dose beyond target
- Limited data on IMPT for NPC due to sporadic incidence in West and lack of proton centers in endemic regions
Study Design
- Retrospective cohort study at Memorial Sloan Kettering Cancer Center
- January 2016 - December 2019
- 77 patients with newly diagnosed nonmetastatic NPC
- Compared IMPT (n=28) vs IMRT (n=49)
- Propensity score matching for 48 EBV-positive patients (1:1)
- Primary outcomes: acute/chronic adverse events and oncologic outcomes (LRFS, PFS, OS)
- Median follow-up: 30.3 months overall
Eligibility Criteria
- Adults ≥18 years old
- Newly diagnosed nonmetastatic NPC
- Treated with curative intent RT ± chemotherapy
- Excluded: palliative RT or no follow-up after RT completion
- IMPT offered as alternative to IMRT off trial or when IMRT could not be safely delivered
- Barriers to IMPT: patient preference, insurance denial, logistics (proton center ~50 miles away)
Patient Characteristics
- Median age: 48.7 years (similar between groups)
- Male predominance: 67.5%
- 89.6% had EBV-positive disease
- 89.6% had WHO type 2b (nonkeratinizing undifferentiated carcinoma)
- Most had excellent performance status (KPS 90-100)
- IMPT group had more T4 disease (28.6% vs 12.2%, p=0.14)
- IMPT group received more high-dose cisplatin (57.1% vs 24.5%, p=0.004)
Radiation Protocol
- Dose to gross tumor volume: 69.96-70 GyE in 33-35 fractions
- High-risk anatomic sites: 56-63 GyE
- Low-risk anatomic sites: 54.12-56 GyE
- Concurrent chemotherapy:
- Weekly cisplatin 40 mg/m² (up to 7 cycles) OR
- Q3 week cisplatin 100 mg/m² (up to 3 cycles)
- Stage I: RT alone; Stage II-IVA: concurrent chemoRT ± adjuvant chemotherapy
Dosimetric Comparison
- IMPT achieved significantly lower doses to organs at risk:
- Mean oral cavity dose: 15.4 vs 32.8 GyE (p<0.001)
- Mean larynx dose: 16.0 vs 29.6 GyE (p<0.001)
- Mean parotid gland dose: 22.5 vs 25.2 GyE (p=0.01)
- These dosimetric advantages translated to clinical benefits in acute toxicity
Acute Toxicity Results
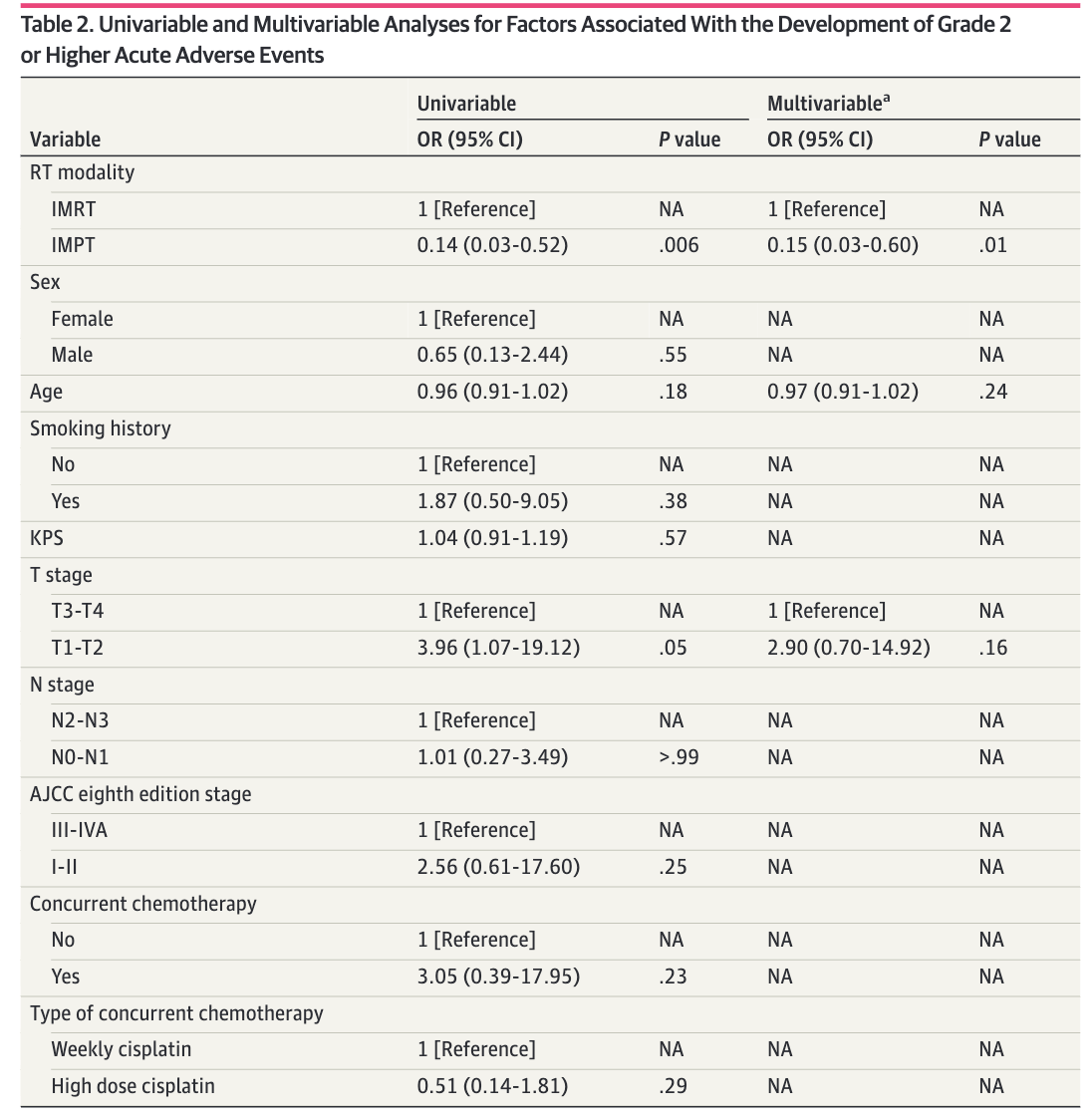
Late Toxicity Results
Figure: Kaplan-Meier Survival Curves
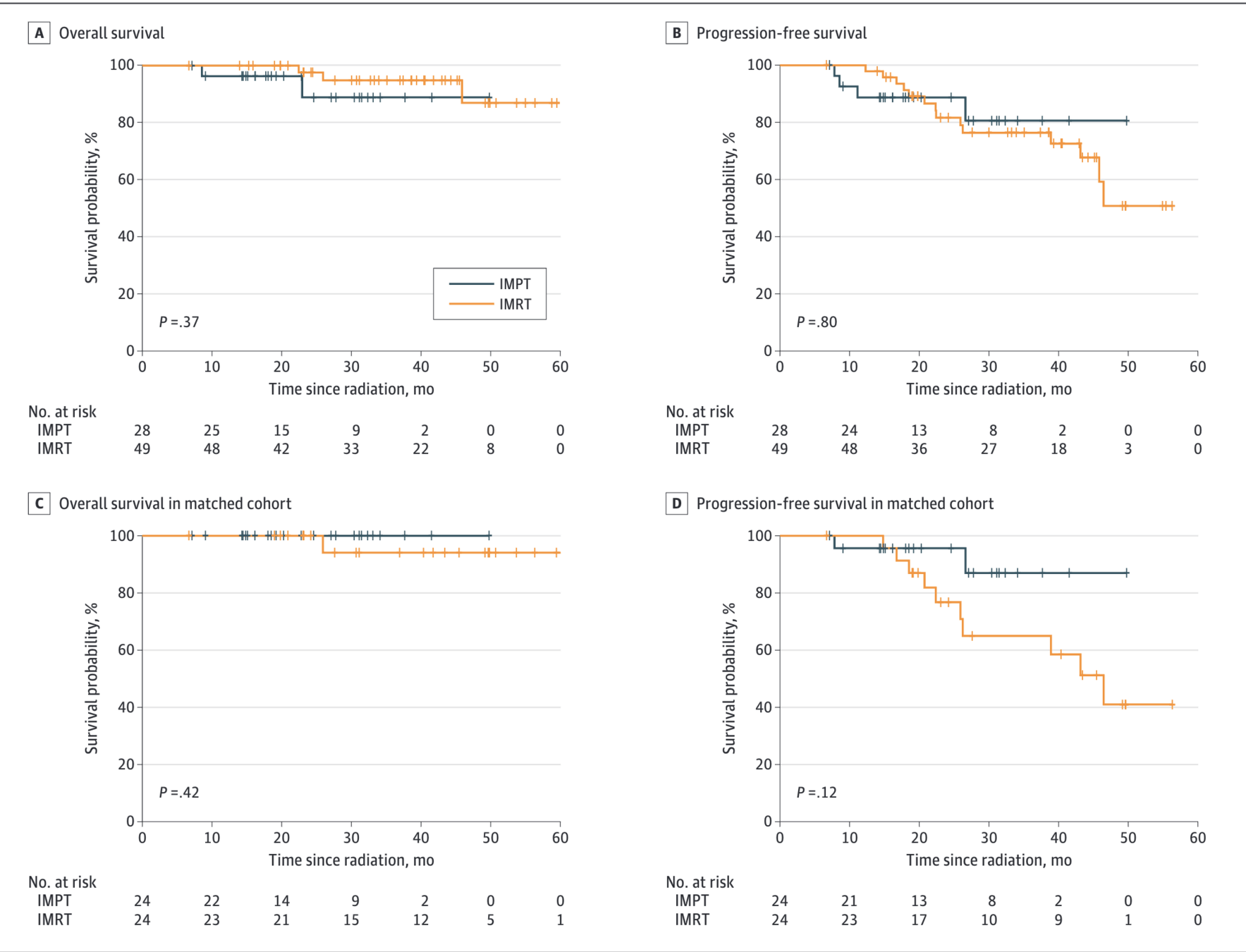
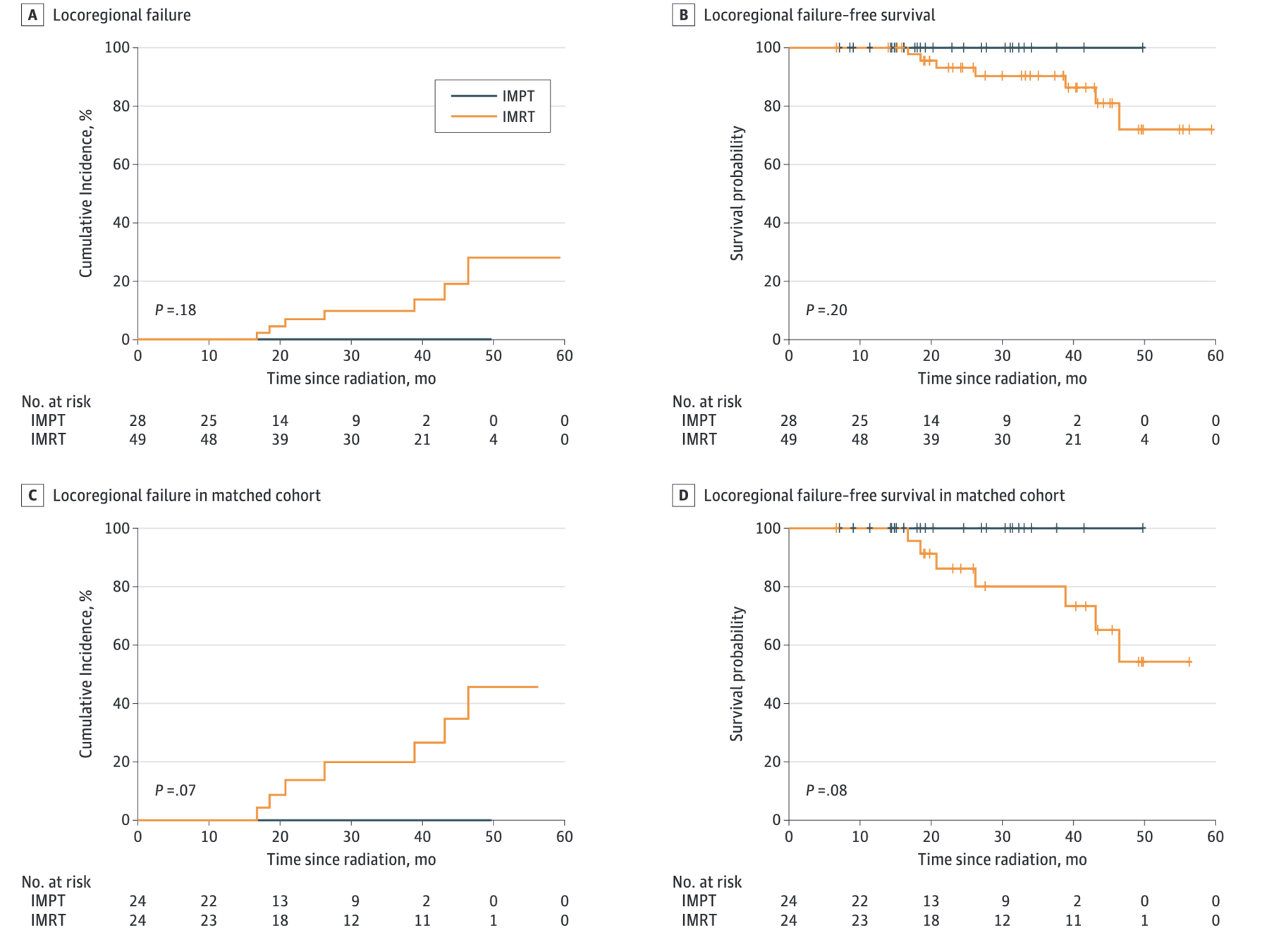
Figure: Locoregional Control
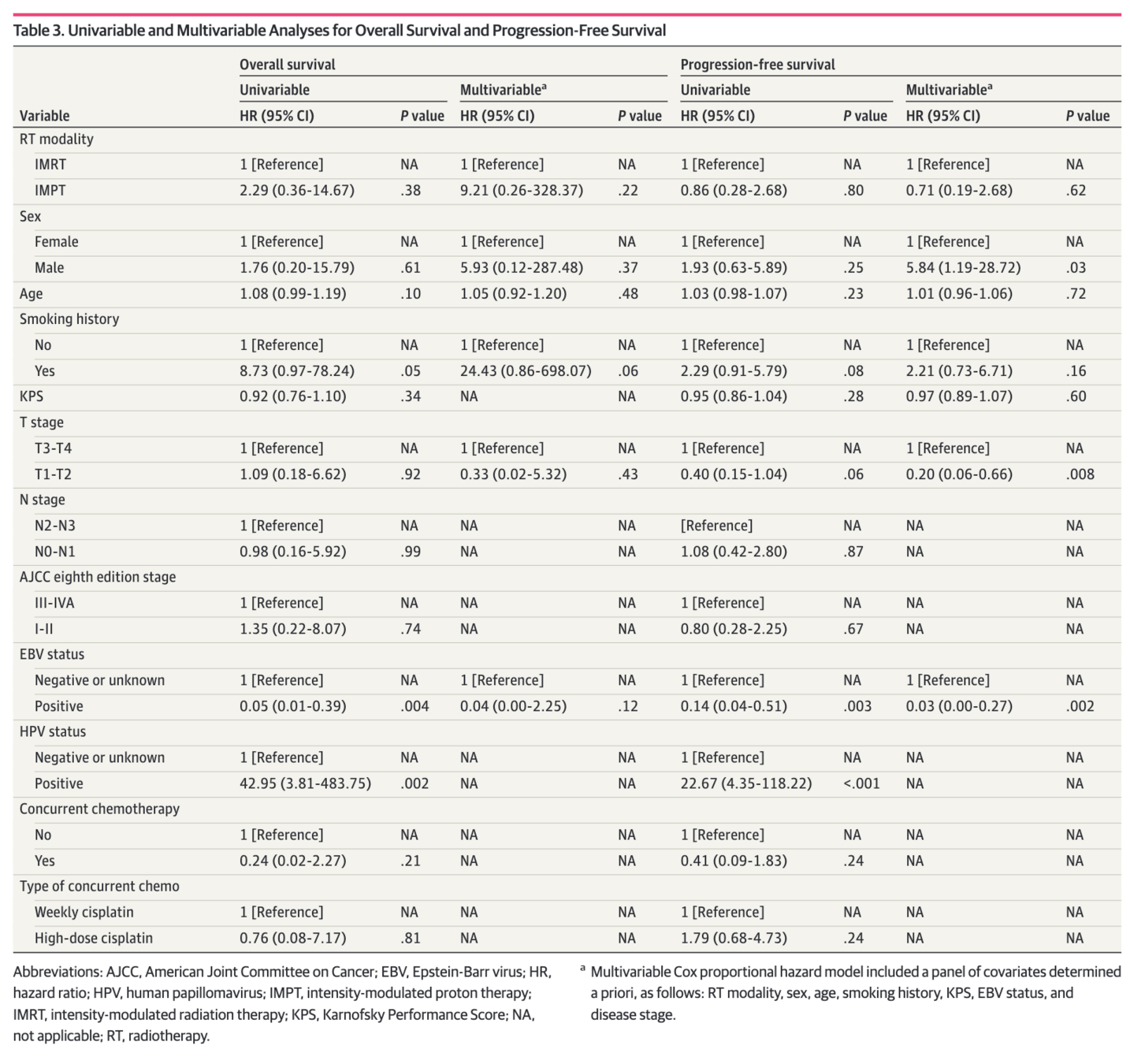
Oncologic Outcomes - Full Cohort
- Locoregional failure: 0 (IMPT) vs 7 (IMRT)
- Cumulative incidence of LRF at 30 months: 0% vs 9.6% (p=0.18)
- No significant differences in survival outcomes:
- LRFS: HR 0.00 (p<0.001) - no events in IMPT
- PFS: HR 0.86 (95% CI 0.28-2.68, p=0.80)
- OS: HR 2.29 (95% CI 0.36-14.67, p=0.37)
- Smoking history associated with poor LRFS and PFS
- EBV-positive status associated with better OS

Propensity Score-Matched Analysis
- 48 patients (24 IMPT vs 24 IMRT) with EBV-positive disease
- Matched on: T4 disease, nonsmoking status, high-dose cisplatin
- 2-year LRFS: 100% (IMPT) vs 86.2% (IMRT), p=0.08
- 2-year PFS: 95.7% (IMPT) vs 76.7% (IMRT), HR 0.31, p=0.14
- 3-year OS: 100% (IMPT) vs 94.1% (IMRT), p=0.42
- No locoregional recurrence or death in IMPT group
- Smoking remained significant predictor of poor outcomes
Conclusions
- IMPT was associated with significantly reduced acute toxicity burden vs IMRT
- Rare late complications with IMPT (median follow-up ~2 years)
- Excellent oncologic outcomes with 100% locoregional control at 2 years in IMPT group
- Results suggest IMPT should be discussed as potential primary RT modality when available
- Prospective trials warranted to optimize patient selection
- Particularly relevant as proton centers expand in endemic regions
Strengths
- Largest comparative analysis of IMPT vs IMRT for primary NPC treatment
- Contemporary cohort with modern techniques
- Comprehensive toxicity assessment with CTCAE grading
- Propensity score matching to address selection bias
- Detailed dosimetric comparisons
- Consistent treatment protocols at single institution
Limitations
- Retrospective design with inherent selection bias
- Small sample size, especially in IMPT group (n=28)
- Imbalanced follow-up time: 23.0 months (IMPT) vs 37.0 months (IMRT)
- Socioeconomic factors not captured (insurance status)
- Limited patient-reported outcome data
- Single institution experience
- May miss late recurrences and toxicities occurring after 2 years
Discussion Points
- How should we select patients for IMPT vs IMRT for NPC? What factors should guide this decision?
- Is the follow-up adequate to capture meaningful late toxicity differences? What late effects are we most concerned about?
- How do we balance the dosimetric advantages with practical barriers (access, cost, insurance)?
- Should IMPT be standard of care for NPC when available, or do we need randomized data first?
- What is the potential impact as proton centers expand in Asia where NPC is endemic?
IMPT vs IMRT for Nonmetastatic Nasopharyngeal Carcinoma
By RadMedSkiier
IMPT vs IMRT for Nonmetastatic Nasopharyngeal Carcinoma
Journal club presentation on toxicity profiles and survival outcomes comparing intensity-modulated proton therapy vs intensity-modulated radiation therapy
- 54



