GOG 99: Surgery with or without Adjunctive External Pelvic Radiation Therapy in Intermediate Risk Endometrial Adenocarcinoma
Keys HM, Roberts JA, Brunetto VL, et al.
Gynecologic Oncology 2004;92:744-751
Study Design & Methods
- Phase III randomized trial of Gynecologic Oncology Group (GOG)
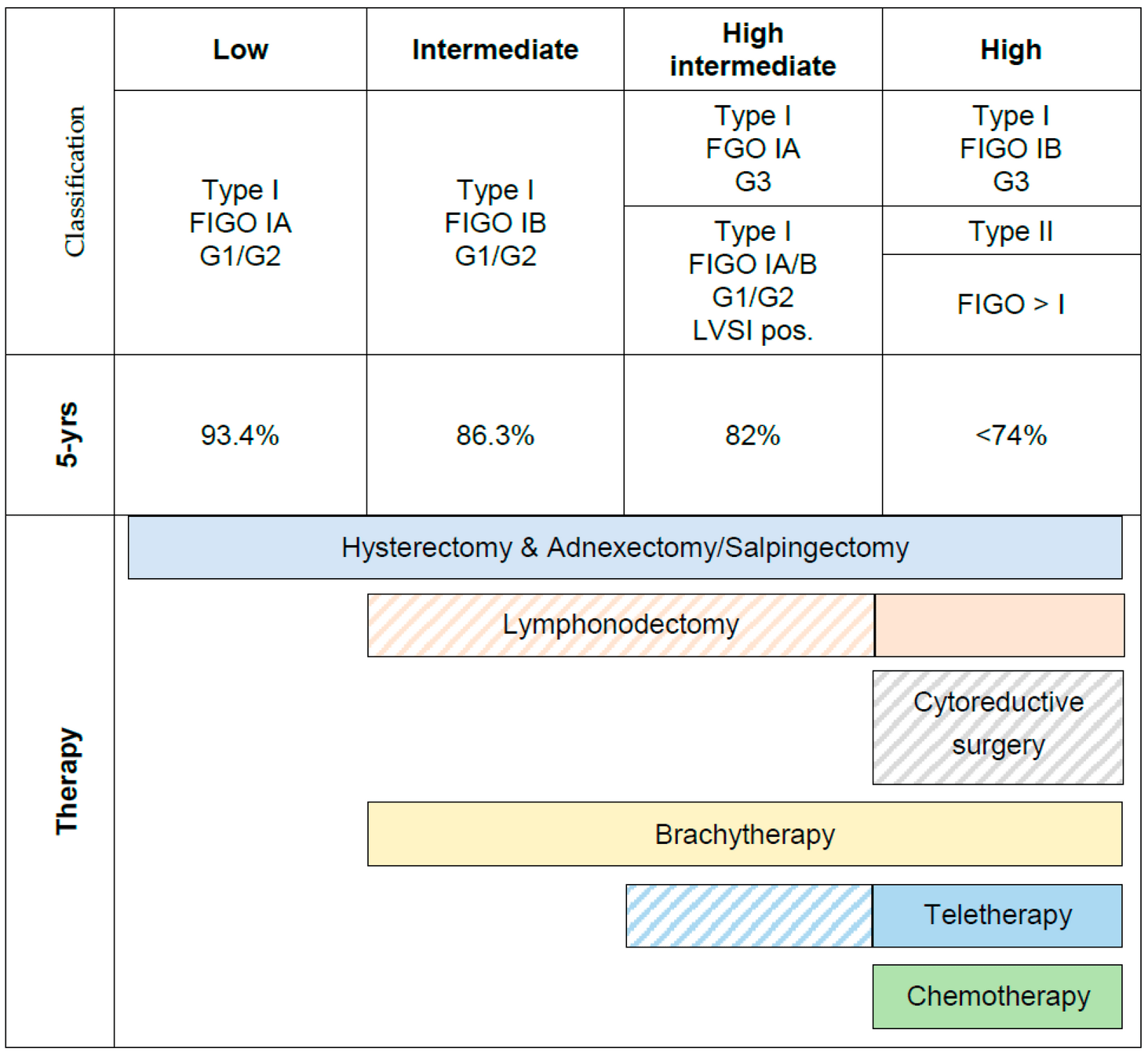
- Population: Surgically staged endometrial adenocarcinoma with "intermediate risk"
- Initial definition: Any degree of myometrial invasion, no lymph node metastasis
- FIGO stages IB, IC, IIA (occult), and IIB (occult)
- Expected 5-year recurrence rate of 20-25%
- Excluded: Clear cell and papillary serous histologies
Inclusion Criteria & Risk Stratification
Initial Inclusion Criteria
- FIGO stages IB, IC, and II (occult)
- Endometrioid adenocarcinoma (any grade)
- Complete surgical staging
- Negative nodes
- Negative peritoneal cytology
- No papillary serous or clear cell histology
Post-hoc Risk Stratification Analysis
During the study, researchers found the actual recurrence rate was lower than expected (12% at 2 years in observation arm vs expected 20-25%)
This prompted development of a more precise definition of risk groups
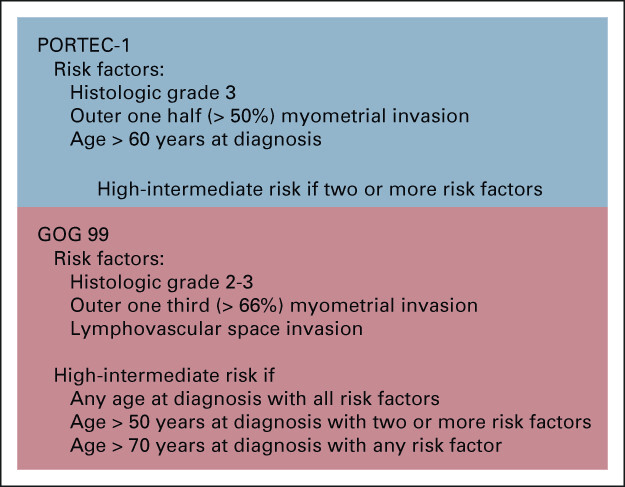
High Intermediate Risk (HIR) Definition
Requires ANY ONE of these criteria:
- Age ≥70 years with ONE additional risk factor
- Age ≥50 years with ANY TWO additional risk factors
- Any age with ALL THREE additional risk factors
Additional risk factors:
- Grade 2-3 tumor
- Presence of lymphovascular invasion
- Outer third myometrial invasion
Impact of Stratification
- HIR group: ~1/3 of patients (132)
- 2-year recurrence: 26% in NAT vs 6% in RT
- Accounted for ~2/3 of all recurrences
- Low Intermediate Risk (LIR): remaining patients
- 2-year recurrence: 5% in NAT vs 2% in RT
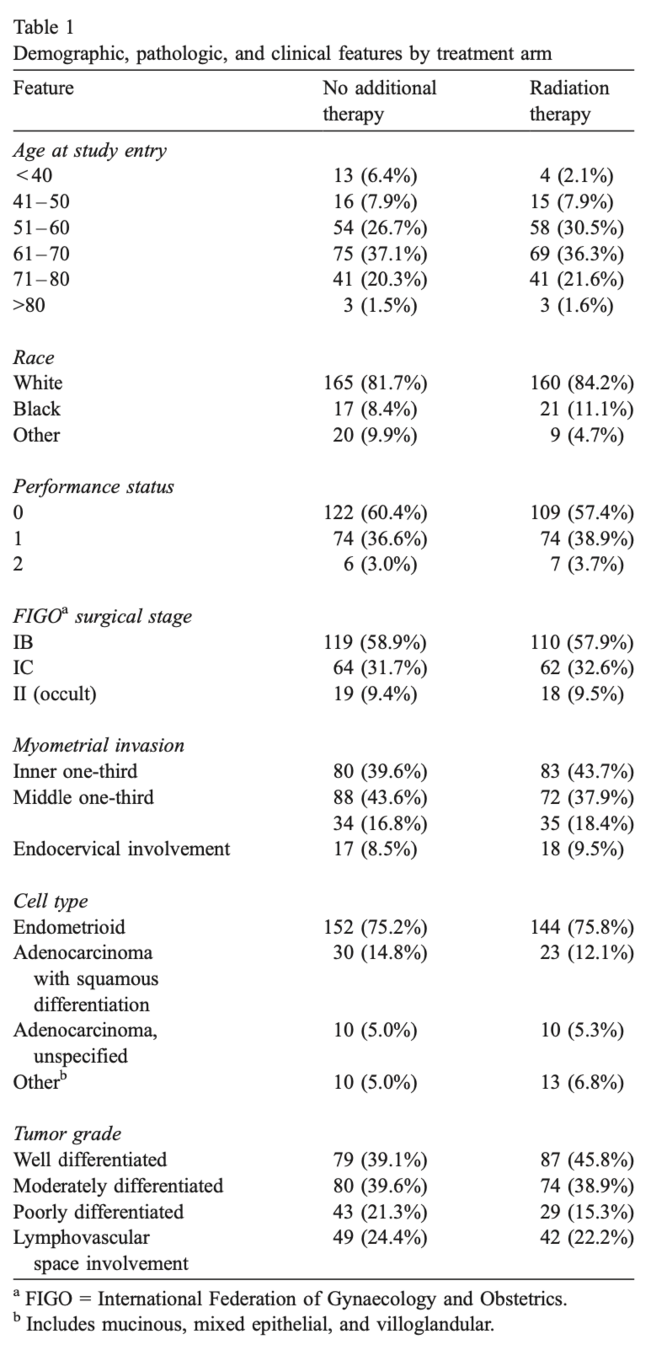
Patient Characteristics
- 448 women entered; 392 (88%) determined eligible
- 202 in No Additional Treatment (NAT) arm
- 190 in Radiation Therapy (RT) arm
- Median follow-up: 69 months
- Well-balanced groups for high-risk factors:
- Outer 1/3 myometrial invasion: ~17%
- Grade 3 histology: ~18%
- Lymphovascular space involvement: ~23%
- Age >70 years: ~22%
- Occult cervical involvement: ~9%
Trial Schema
-
Start within 8 weeks post-surgery
-
Cobalt60 or linear accelerator ≥ 4 MeV
-
4-field or AP/PA technique
-
Superior border: L4-L5 interspace
-
No pelvic organs blocked
-
No vaginal brachytherapy
- Total abdominal hysterectomy
- Bilateral salpingo-oophorectomy
- Selective bilateral pelvic and para-aortic lymphadenectomy
- Removal of any enlarged/suspicious nodes
- Negative lymph nodes
- Negative peritoneal cytology
- Normal lab values (WBC, platelets, hepatic, renal)
- GOG performance status ≤ 2
- No prior malignancy except skin (excluding melanoma)
- No prior radiation or chemotherapy
High Intermediate Risk (HIR) Subgroup
- During study, initial definition of intermediate risk needed refinement
- Factors associated with increased recurrence risk were identified:
- Increasing age
- Moderate to poorly differentiated tumor grade
- Presence of lymphovascular invasion
- Outer-third myometrial invasion
- High Intermediate Risk (HIR) subgroup defined as:
- Age ≥70 with any one risk factor OR
- Age ≥50 with any two risk factors OR
- Any age with all three risk factors
- All other patients considered Low Intermediate Risk (LIR)
- About 1/3 of patients (132) were in the HIR subgroup
- This group accounted for ~2/3 of recurrences (28/44) and cancer-related deaths (22/32)
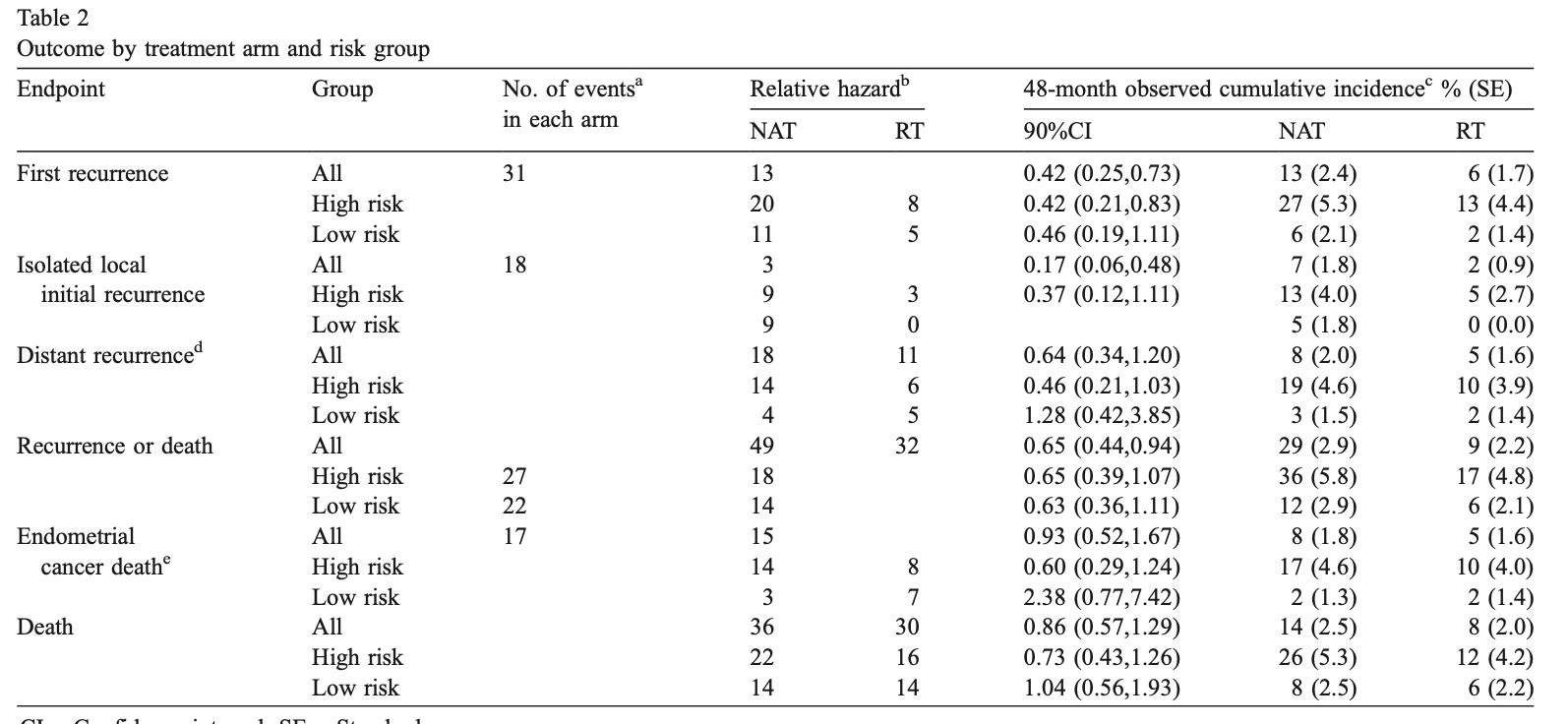
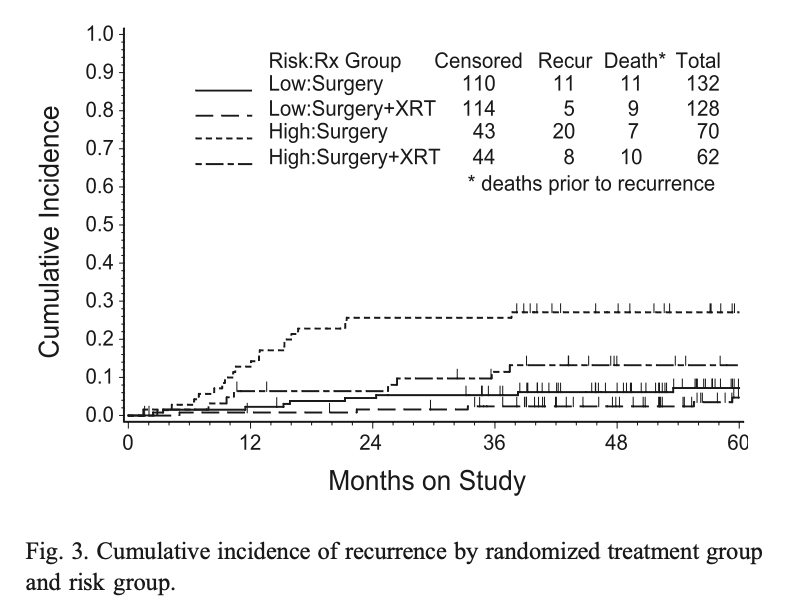
Primary Outcome: Recurrence
- Total recurrences: 44 (31 in NAT arm, 13 in RT arm)
- Radiation reduced recurrence hazard by 58% (RH=0.42, p=0.007)
- 24-month estimated cumulative incidence of recurrence:
- NAT arm: 12% (90% CI=0.09-0.17)
- RT arm: 3% (90% CI=0.02-0.06)
- Most recurrences occurred within 18 months of diagnosis
- Major difference seen in vaginal recurrences:
- NAT arm: 13 vaginal recurrences
- RT arm: 2 vaginal recurrences (both patients refused RT)
- Overall impact on pelvic/vaginal recurrences: 18 in NAT vs 3 in RT
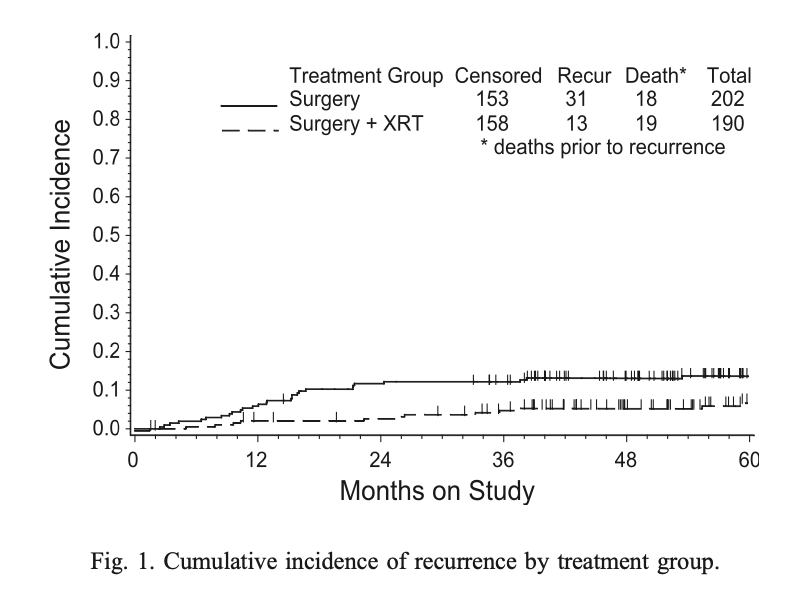
Sites of Recurrence
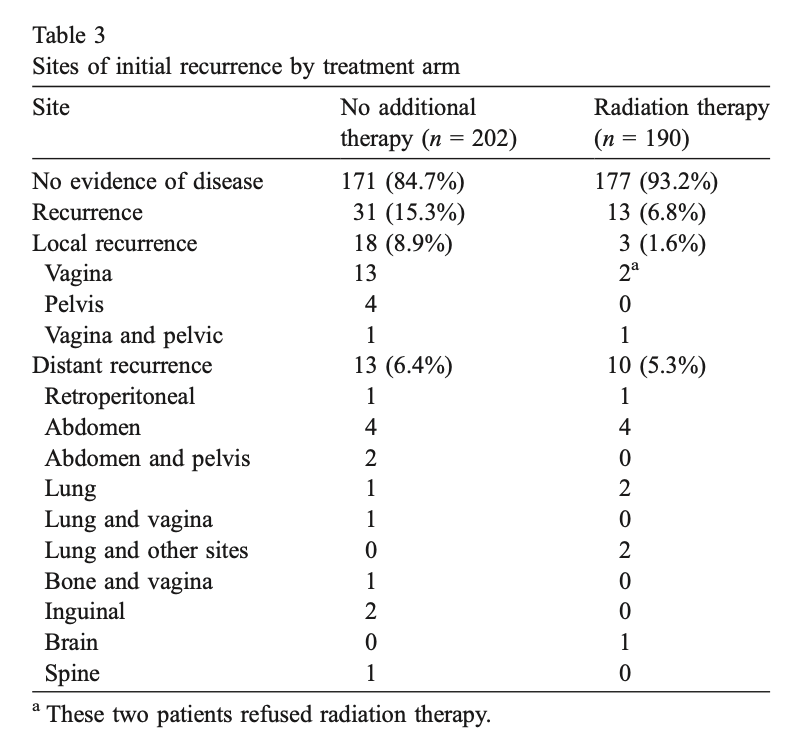
Outcomes by Risk Group
- High Intermediate Risk (HIR) Subgroup:
- 24-month recurrence rate: 26% in NAT vs 6% in RT
- Treatment showed substantial impact (Relative Hazard = 0.42)
- Absolute reduction of 19% at 24 months
- Low Intermediate Risk (LIR) Subgroup:
- 24-month recurrence rate: ~5% in NAT vs ~2% in RT
- Similar relative effect but small absolute benefit (~4%)
- The benefit was consistent across both local control and distant metastasis
- HIR subgroup showed some survival benefit with RT (not statistically significant)
- No apparent survival benefit in LIR subgroup
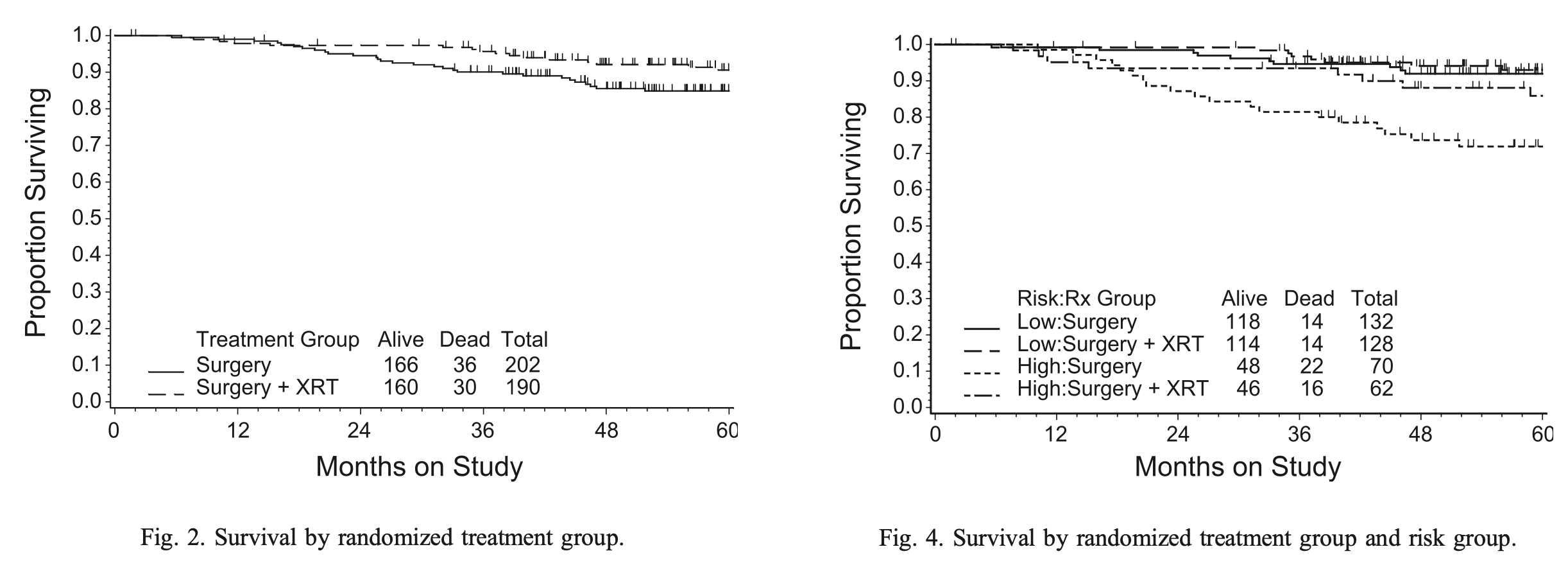
Secondary Outcome: Survival
- Total deaths: 66 (36 in NAT arm, 30 in RT arm)
- Cancer/treatment-related deaths: 32 (17 in NAT, 15 in RT)
- 48-month estimated overall survival:
- NAT arm: 86%
- RT arm: 92%
- Relative hazard: 0.86 (90% CI=0.57-1.29)
- Not statistically significant (p=0.55)
- About half of deaths due to causes other than endometrial cancer:
- NAT: 19 of 36 deaths (53%)
- RT: 15 of 30 deaths (50%)
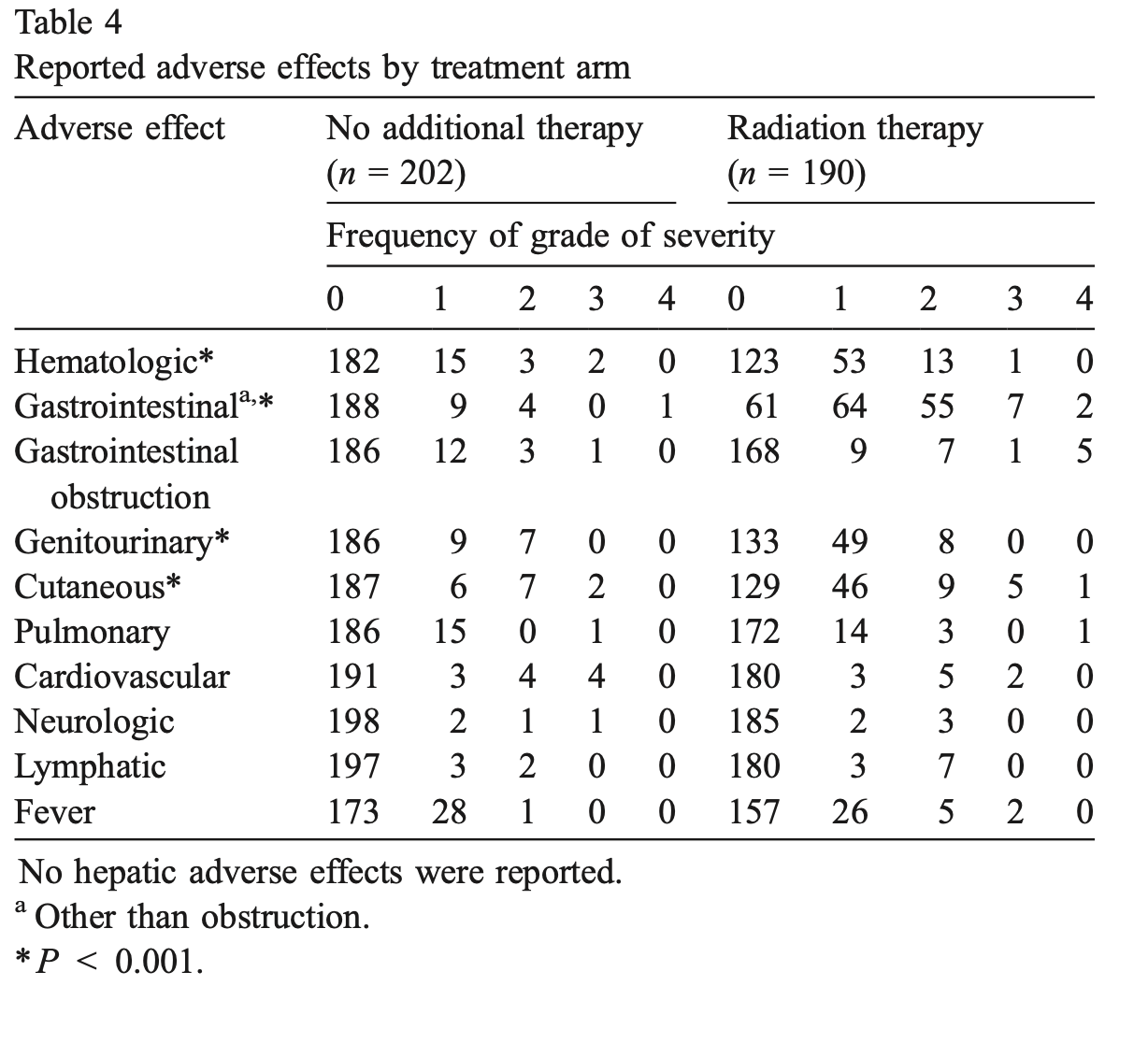
Treatment-Related Toxicity
- RT group experienced more frequent and more severe toxicities
- Two women in RT arm died from complications involving intestinal injury
- Statistically significant differences (p<0.001) in:
- Hematologic toxicity
- Gastrointestinal toxicity
- Genitourinary toxicity
- Cutaneous toxicity
- No significant difference in bowel obstructions, but higher grade obstructions in RT
- Grade 3-4 obstruction: 6 in RT arm vs 1 in NAT arm
Conclusions
- Adjuvant pelvic RT significantly reduces risk of recurrence in intermediate risk endometrial cancer
- Estimated 58% reduction in recurrence hazard with RT
- Primary benefit seen in reducing vaginal apex recurrences
- Absolute benefit varies dramatically by risk group:
- High Intermediate Risk: 19% absolute reduction at 24 months
- Low Intermediate Risk: 4% absolute reduction at 24 months
- Adjuvant RT should be limited to patients whose risk factors fit the High Intermediate Risk definition
High Intermediate Risk (HIR) Definition
Requires ANY ONE of these criteria:
- Age ≥70 years with ONE additional risk factor
- Age ≥50 years with ANY TWO additional risk factors
- Any age with ALL THREE additional risk factors
Additional risk factors:
- Grade 2-3 tumor
- Presence of lymphovascular invasion
- Outer third myometrial invasion
Impact of Stratification
- HIR group: ~1/3 of patients (132)
- 2-year recurrence: 26% in NAT vs 6% in RT
- Accounted for ~2/3 of all recurrences
- Low Intermediate Risk (LIR): remaining patients
- 2-year recurrence: 5% in NAT vs 2% in RT
Conclusions
High Intermediate Risk (HIR) Definition
Requires ANY ONE of these criteria:
- Age ≥70 years with ONE additional risk factor
- Age ≥50 years with ANY TWO additional risk factors
- Any age with ALL THREE additional risk factors
Additional risk factors:
- Grade 2-3 tumor
- Presence of lymphovascular invasion
- Outer third myometrial invasion
Impact of Stratification
- HIR group: ~1/3 of patients (132)
- 2-year recurrence: 26% in NAT vs 6% in RT
- Accounted for ~2/3 of all recurrences
- Low Intermediate Risk (LIR): remaining patients
- 2-year recurrence: 5% in NAT vs 2% in RT

GOG 99: Surgery with or without Adjunctive External Pelvic Radiation Therapy in Intermediate Risk Endometrial Adenocarcinoma
By RadMedSkiier
GOG 99: Surgery with or without Adjunctive External Pelvic Radiation Therapy in Intermediate Risk Endometrial Adenocarcinoma
A presentation of the Gynecologic Oncology Group phase III trial comparing surgery alone versus surgery with adjuvant pelvic radiation therapy in intermediate risk endometrial cancer
- 90



