Adjuvant Chemoradiotherapy versus Radiotherapy Alone for Women with High-Risk Endometrial Cancer (PORTEC-3)
Final Results of an International, Open-Label, Multicentre, Randomised, Phase 3 Trial
de Boer SM, Powell ME, Mileshkin L, et al.
Lancet Oncology 2018; 19: 295-309
Background & Rationale
- Women with high-risk endometrial cancer (15% of cases) have increased risk of recurrence and cancer-related death
- Standard adjuvant treatment has been pelvic external beam radiotherapy (EBRT)
- Radiotherapy shown to delay pelvic recurrence but not improve overall survival
- Chemotherapy shown to delay distant metastases but also without survival benefit
- RTOG 9708 phase 2 trial explored combination of EBRT with concurrent and adjuvant chemotherapy with promising results
- PORTEC-3 trial initiated to determine whether addition of chemotherapy to radiotherapy improves outcomes in high-risk endometrial cancer
Study Design
686 patients with high-risk endometrial cancer randomized 1:1
↓
Radiotherapy Alone
↓
EBRT (48.6 Gy in 1.8 Gy fractions)
5 days/week
Chemoradiotherapy
↓
EBRT (48.6 Gy) + concurrent cisplatin
50 mg/m² in week 1 & 4
↓
Adjuvant chemotherapy
4 cycles carboplatin AUC5 + paclitaxel 175 mg/m²
686 patients with high-risk endometrial cancer randomized 1:1
↓
Radiotherapy Alone
↓
EBRT (48.6 Gy in 1.8 Gy fractions)
5 days/week
Chemoradiotherapy
↓
EBRT (48.6 Gy) + concurrent cisplatin
50 mg/m² in week 1 & 4
↓
Adjuvant chemotherapy
4 cycles carboplatin AUC5 + paclitaxel 175 mg/m²
- Open-label, international, randomized phase 3 trial
- 103 centers in 6 clinical trial groups collaborating in the Gynaecological Cancer Intergroup
- Randomization stratified by participating center, lymphadenectomy, stage, and histological type
- Co-primary endpoints: overall survival and failure-free survival
- Vaginal brachytherapy boost allowed if cervical involvement (46% in chemoRT vs 48% in RT)
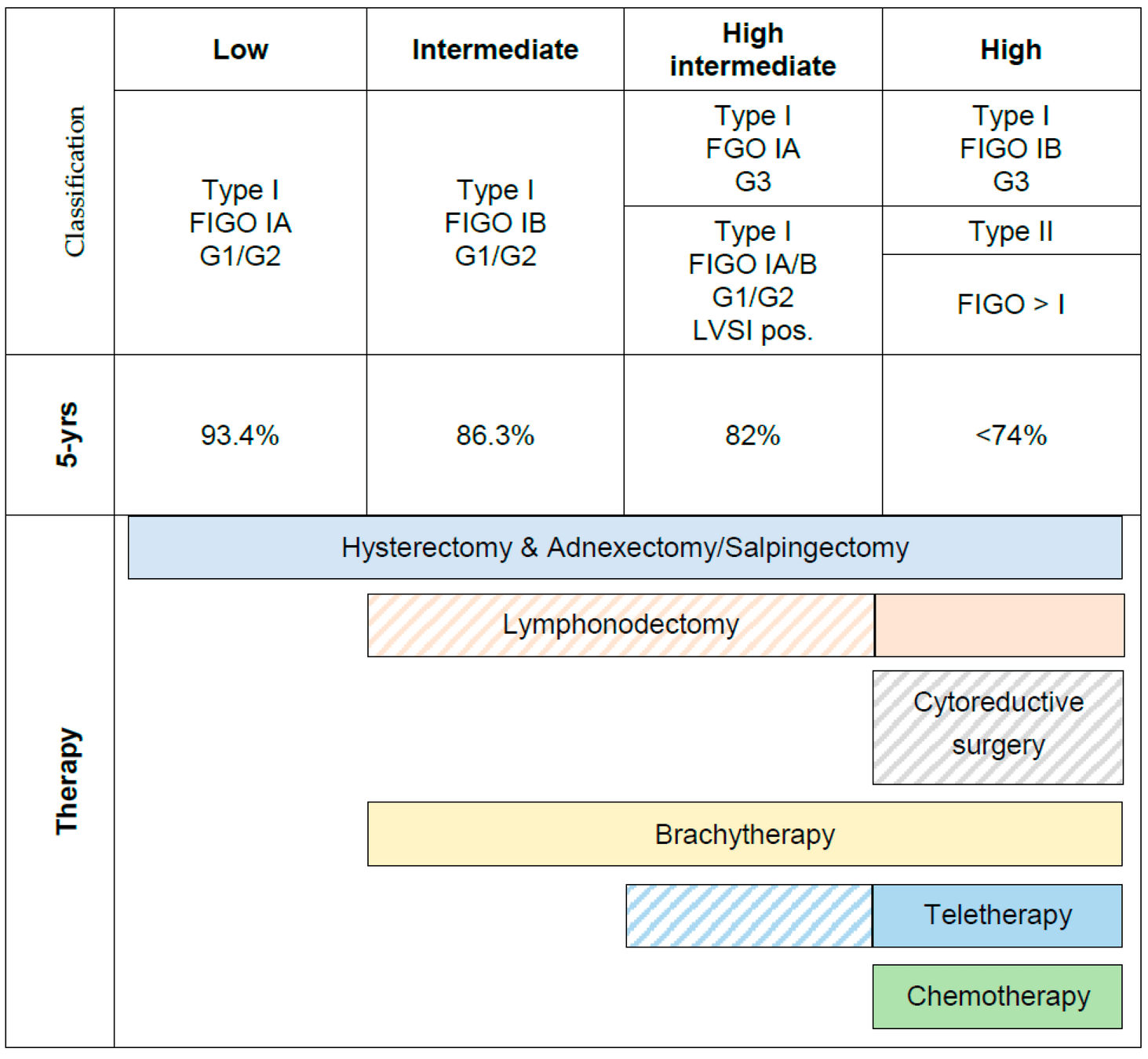
Eligibility Criteria
- Endometrioid endometrial cancer:
FIGO 2009 stage IA grade 3 with documented LVSI
FIGO stage IB grade 3
FIGO stage II
FIGO stage IIIA, IIIB (parametrial), or IIIC
FIGO stage IB grade 3
FIGO stage II
FIGO stage IIIA, IIIB (parametrial), or IIIC
- Serous or clear cell histology:
Stage IA with invasion
Stage IB, II, or III
Stage IB, II, or III
- Additional criteria:
WHO performance status 0-2
Adequate bone marrow, liver, and kidney function
Age ≥18 years (no upper limit)
Adequate bone marrow, liver, and kidney function
Age ≥18 years (no upper limit)
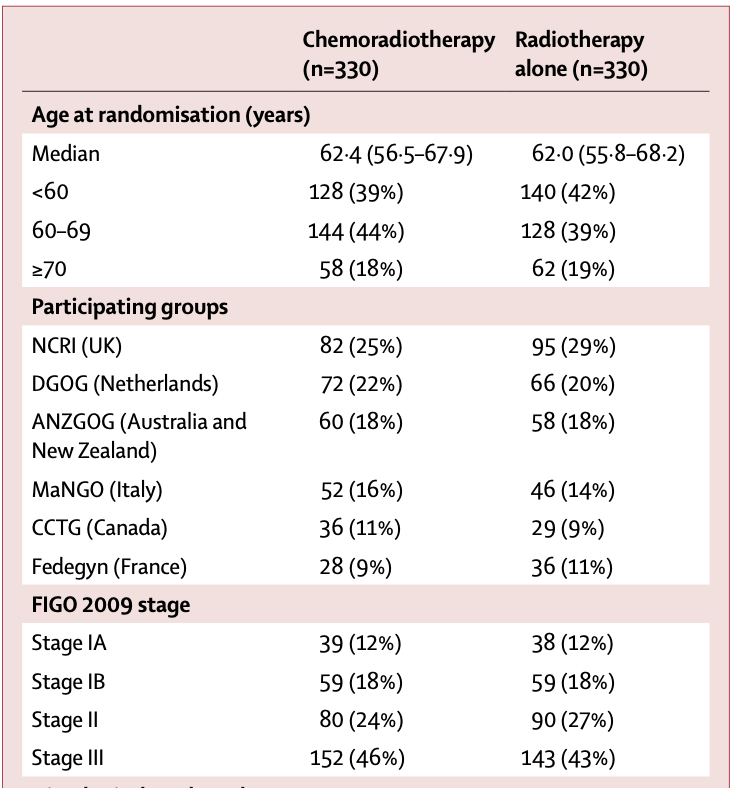
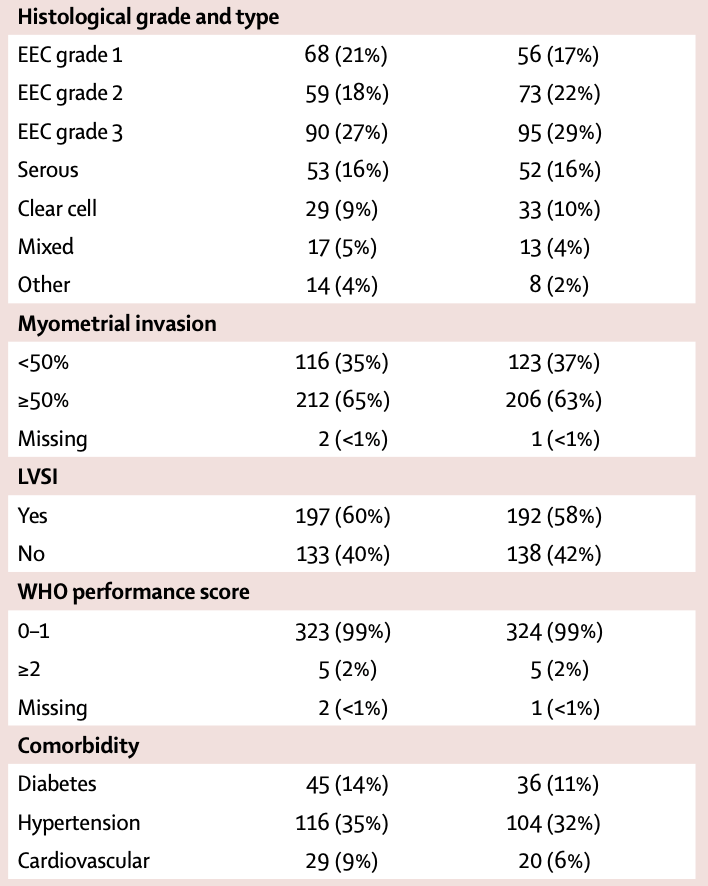
Patient Characteristics
- Median age: 62 years (56-68)
- FIGO 2009 stage:
- Stage I: 30%
- Stage II: 25%
- Stage III: 45%
- Histological type:
- Endometrioid grade 1: 19%
- Endometrioid grade 2: 20%
- Endometrioid grade 3: 28%
- Serous: 16%
- Clear cell: 9%
- Mixed/other: 8%
- Myometrial invasion ≥50%: 64%
- LVSI present: 59%
- WHO performance score 0-1: 99%
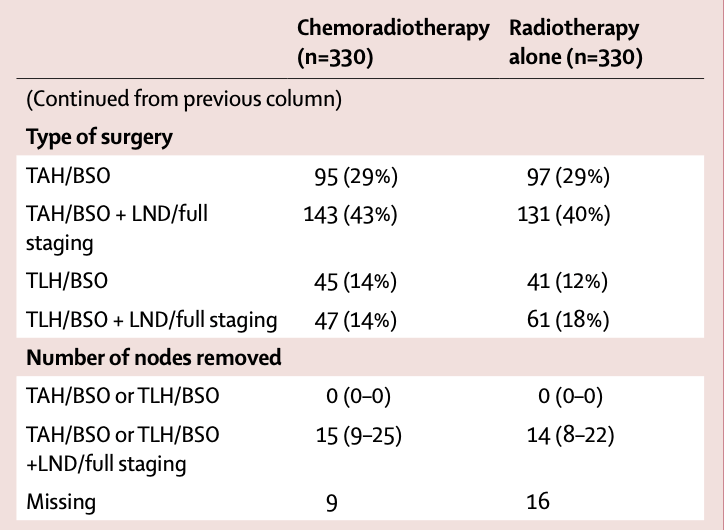
- Surgery:
- Total abdominal/laparoscopic hysterectomy with BSO: 29%
- Total abdominal/laparoscopic hysterectomy with BSO + lymphadenectomy/staging: 71%
- Median nodes removed when lymphadenectomy performed: 14-15
660 eligible patients analyzed (330 in each arm)
Baseline characteristics well balanced between groups
Patient Characteristics
Treatment Completion
| Treatment | Chemoradiotherapy n=330 |
Radiotherapy n=330 |
|---|---|---|
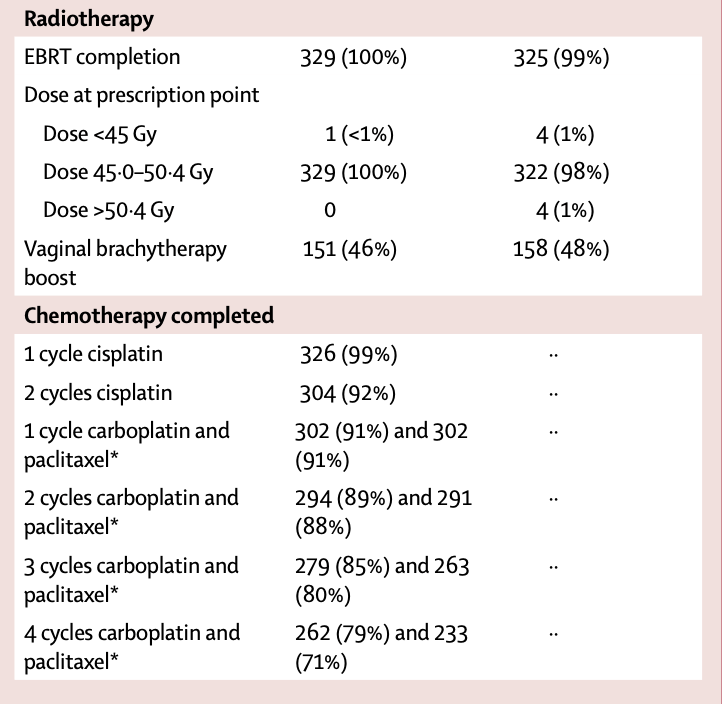
| EBRT completed | 329 (100%) | 325 (99%) |
| Vaginal brachytherapy boost | 151 (46%) | 158 (48%) |
| 2 cycles cisplatin completed | 304 (92%) | NA |
| 4 cycles carboplatin completed | 262 (79%) | NA |
| 4 cycles paclitaxel completed | 233 (71%) | NA |
- Chemotherapy was discontinued in 61 patients (18%):
- Due to toxicity: 31 (9%)
- Patient decision: 20 (6%)
- Disease progression: 7 (2%)
- Other reasons: 3 (1%)
- Dose reductions:
- Cisplatin (40 mg/m²): 5 patients (2%)
- Carboplatin (AUC5 to AUC4): 36 patients (11%)
- Paclitaxel (175 mg/m² to 135 mg/m²): 50 patients (15%)
Survival Outcomes
| Outcome at 5 years | Chemoradiotherapy n=330 |
Radiotherapy n=330 |
Hazard Ratio (95% CI) | p value* |
|---|---|---|---|---|
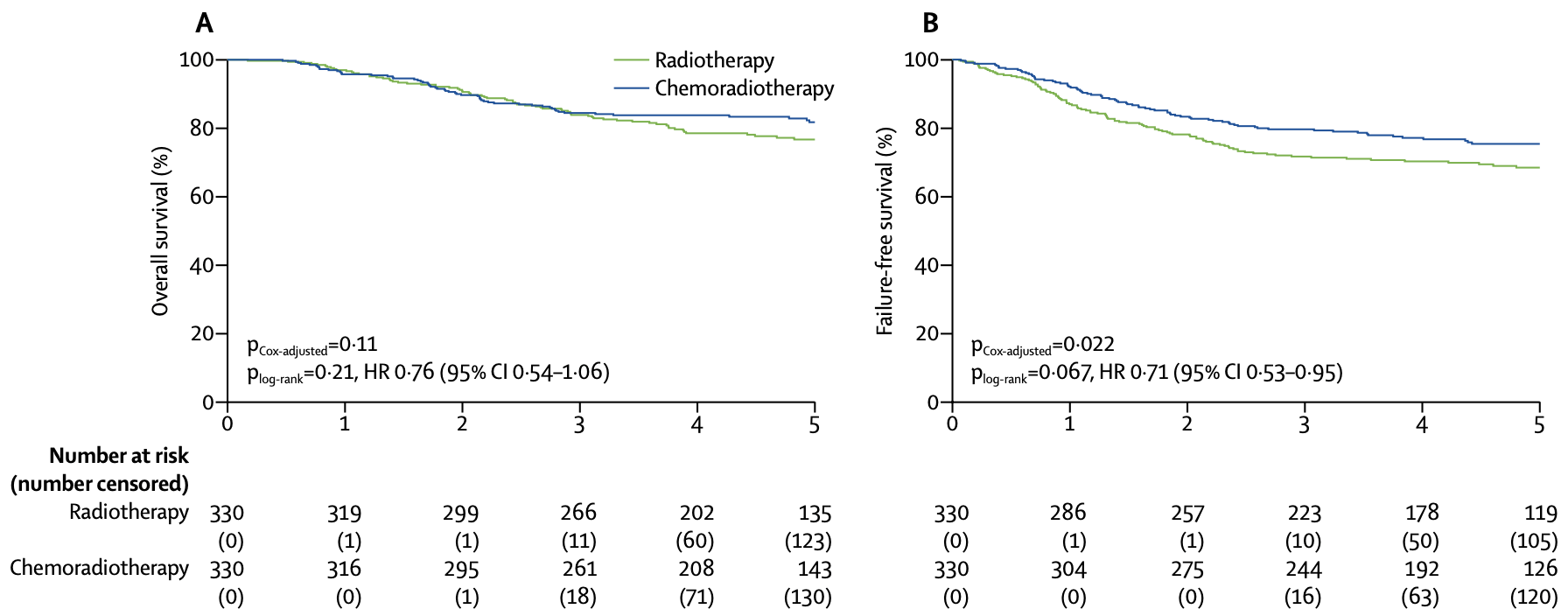
| Overall survival | 81.8% (77.5-86.2) | 76.7% (72.1-81.6) | 0.76 (0.54-1.06) | 0.109 |
| Failure-free survival | 75.5% (70.3-79.9) | 68.6% (63.1-73.4) | 0.71 (0.53-0.95) | 0.022 |
*Adjusted for stratification factors
Survival Outcomes
| Outcome at 5 years | Chemoradiotherapy n=330 |
Radiotherapy n=330 |
Hazard Ratio (95% CI) | p value* |
|---|---|---|---|---|
| Overall survival | 81.8% (77.5-86.2) | 76.7% (72.1-81.6) | 0.76 (0.54-1.06) | 0.109 |
| Failure-free survival | 75.5% (70.3-79.9) | 68.6% (63.1-73.4) | 0.71 (0.53-0.95) | 0.022 |
*Adjusted for stratification factors

Recurrence Patterns
| Recurrence Type | Chemoradiotherapy n=330 |
Radiotherapy n=330 |
p value |
|---|---|---|---|
| First recurrence | |||
| Vaginal recurrence | 0.3% | 0.3% | 0.999 |
| Pelvic recurrence | 1.0% | 1.5% | 0.473 |
| Distant metastases | 22.4% | 28.3% | 0.108 |
| Total recurrence (includes multiple sites) | |||
| Vaginal recurrence | 2.1% | 2.1% | 0.995 |
| Pelvic recurrence | 4.9% | 9.2% | 0.026 |
| Distant metastases | 23.1% | 29.7% | 0.077 |
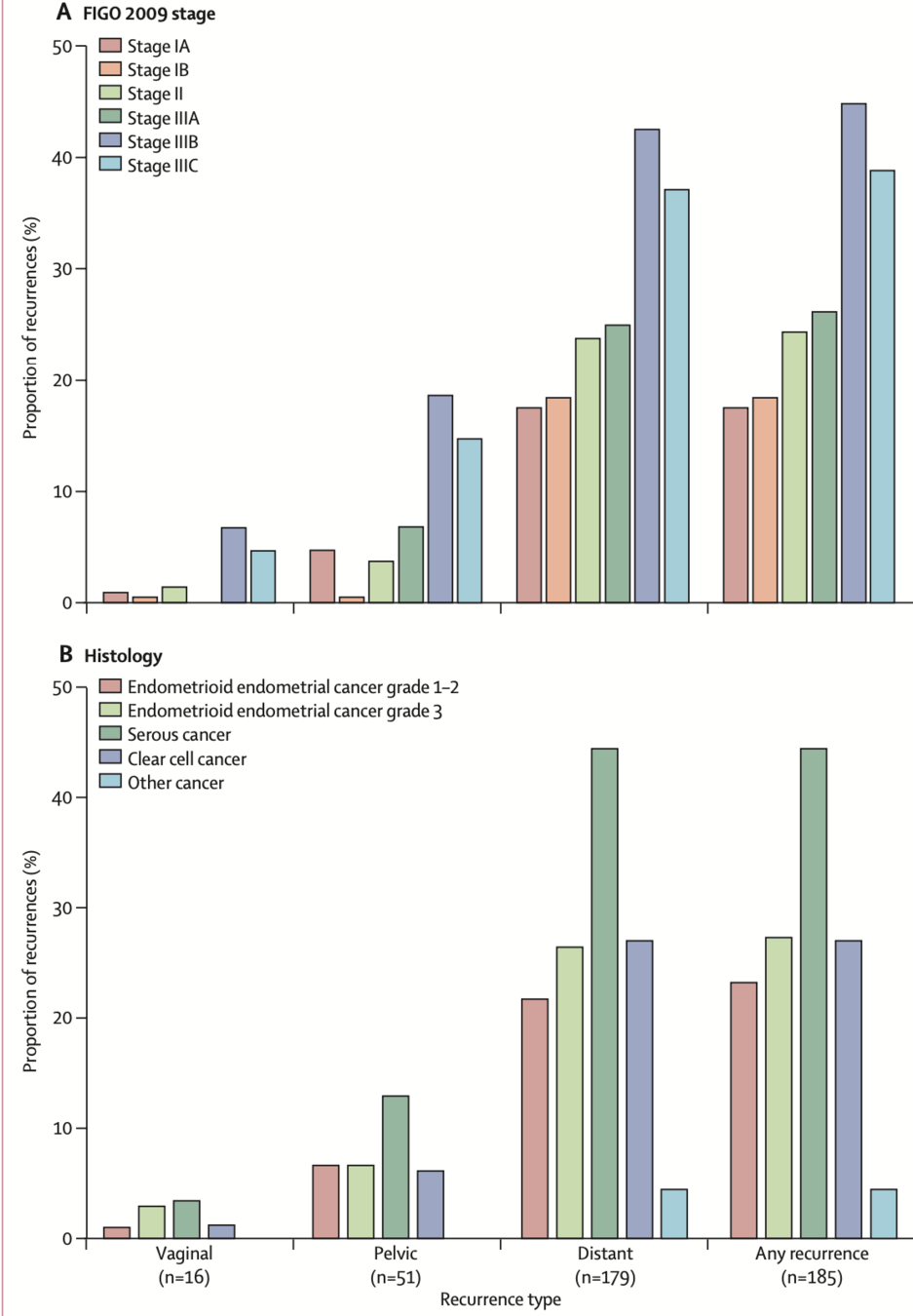
- Most recurrences were distant metastases in both arms
- Isolated vaginal and pelvic recurrences were rare
- Significant reduction in total pelvic recurrences with chemoradiotherapy
- Trend toward reduction in distant metastases with chemoradiotherapy
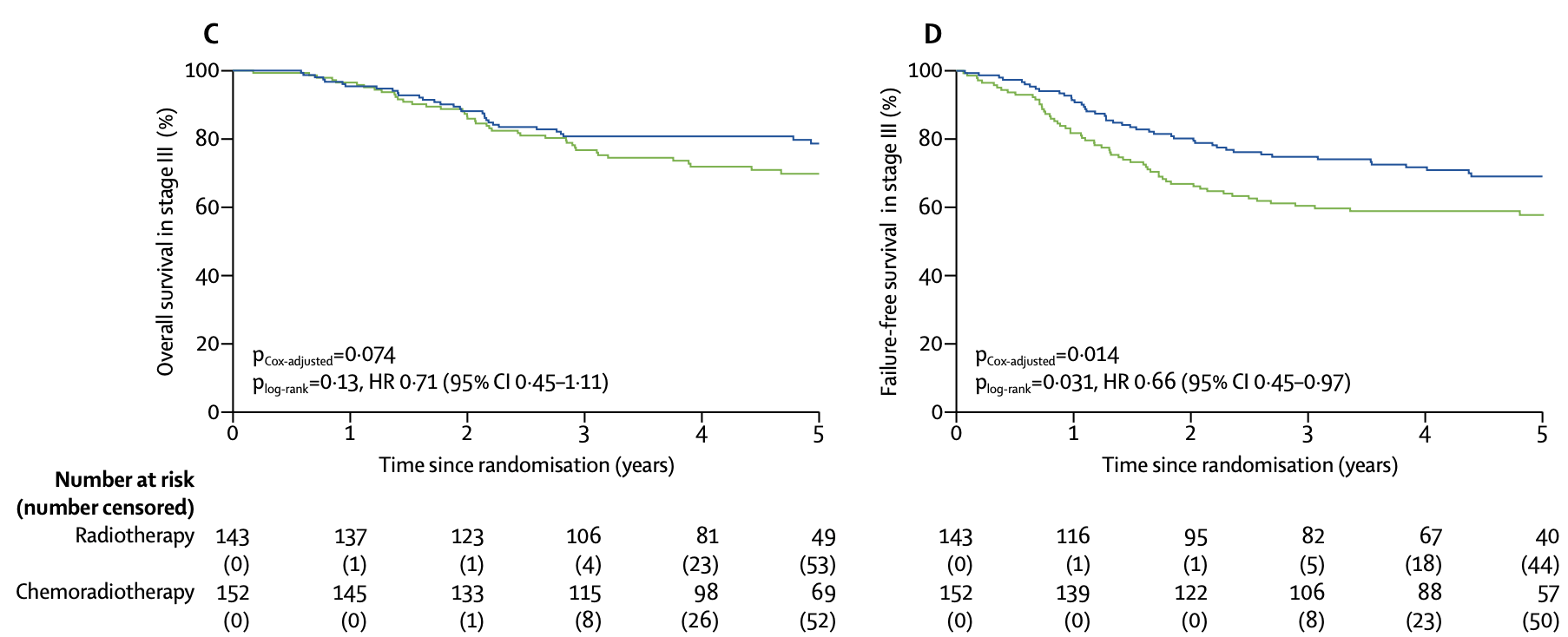
Subgroup Analysis - Stage III
| Outcome by Stage | Chemoradiotherapy | Radiotherapy | Hazard Ratio (95% CI) | p value |
|---|---|---|---|---|
| Stage III (n=295) | ||||
| 5-year overall survival | 78.7% (72.2-85.7) | 69.8% (62.4-78.1) | 0.71 (0.45-1.11) | 0.074* |
| 5-year failure-free survival | 69.3% (61.1-76.2) | 58.0% (49.3-65.7) | 0.66 (0.45-0.97) | 0.014* |
| Stage I-II (n=365) | ||||
| 5-year failure-free survival | 80.8% (74.1-86.0) | 76.6% (69.5-82.2) | 0.85 (0.54-1.33) | 0.47 |
*Adjusted for stratification factors
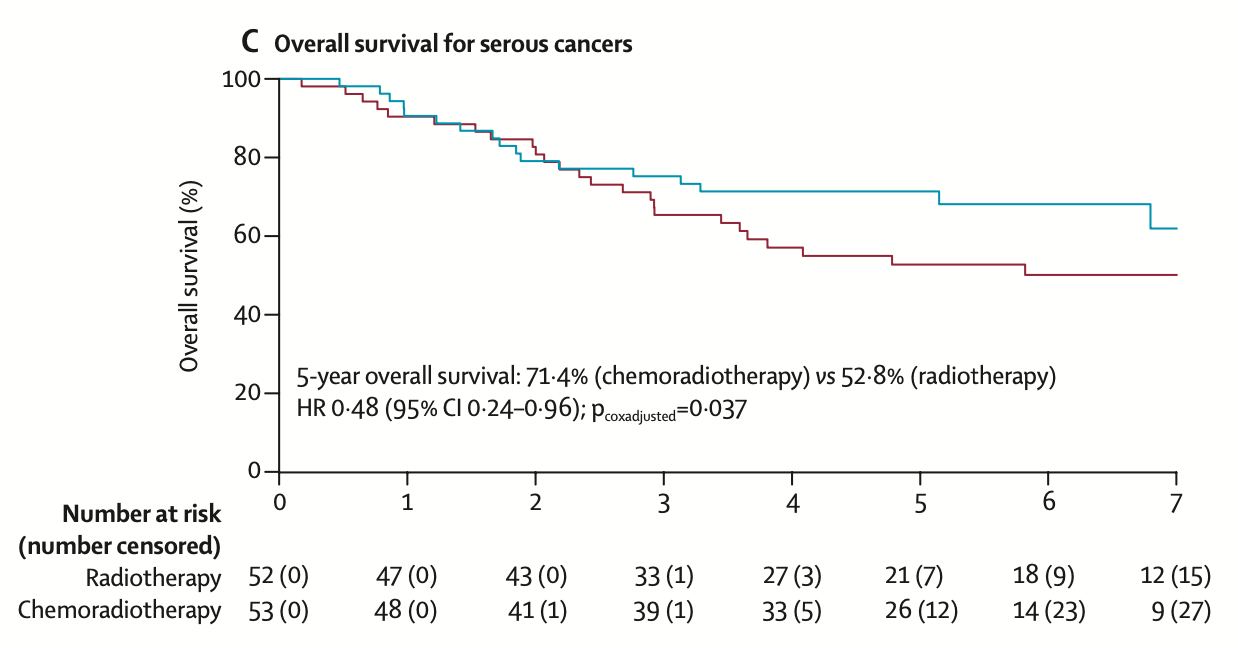
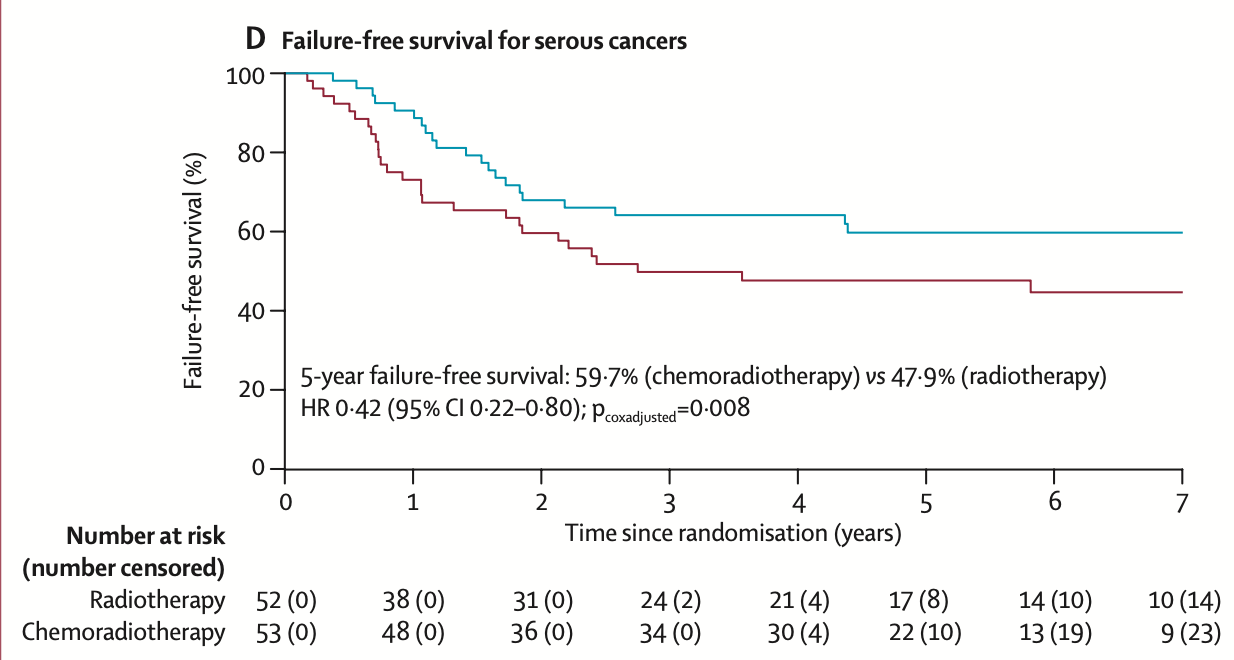
Subgroup Analysis - Serious
| Outcome by Histology | Chemoradiotherapy | Radiotherapy | Hazard Ratio (95% CI) | p value |
|---|---|---|---|---|
| Serous Cancer (n=105) | ||||
| 5-year overall survival | 71.4% (60.1–84.7) | 52.8% (40.6–68.6) | 0.48 (0.24–0.96) | 0.037 |
| 5-year failure-free survival | 59.7% (45.1–71.6) | 47.9% (33.9–60.6) | 0.42 (0.22–0.80) | 0.008 |
| Recurrence risk: 44.8% in serous cancers vs 27.7% in grade 3 endometrioid and 27.4% in clear cell cancers | ||||
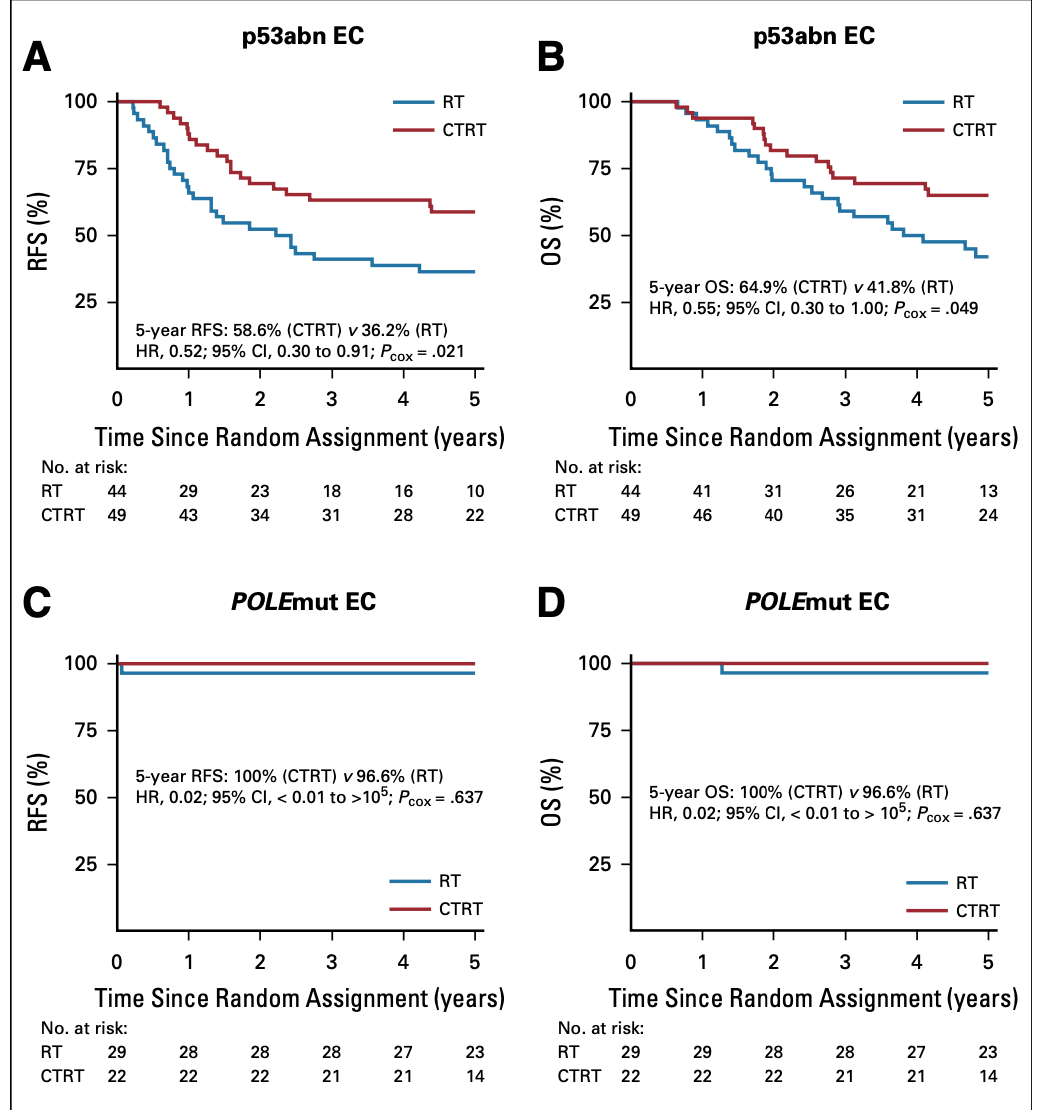
Subgroup Analysis - Mutation
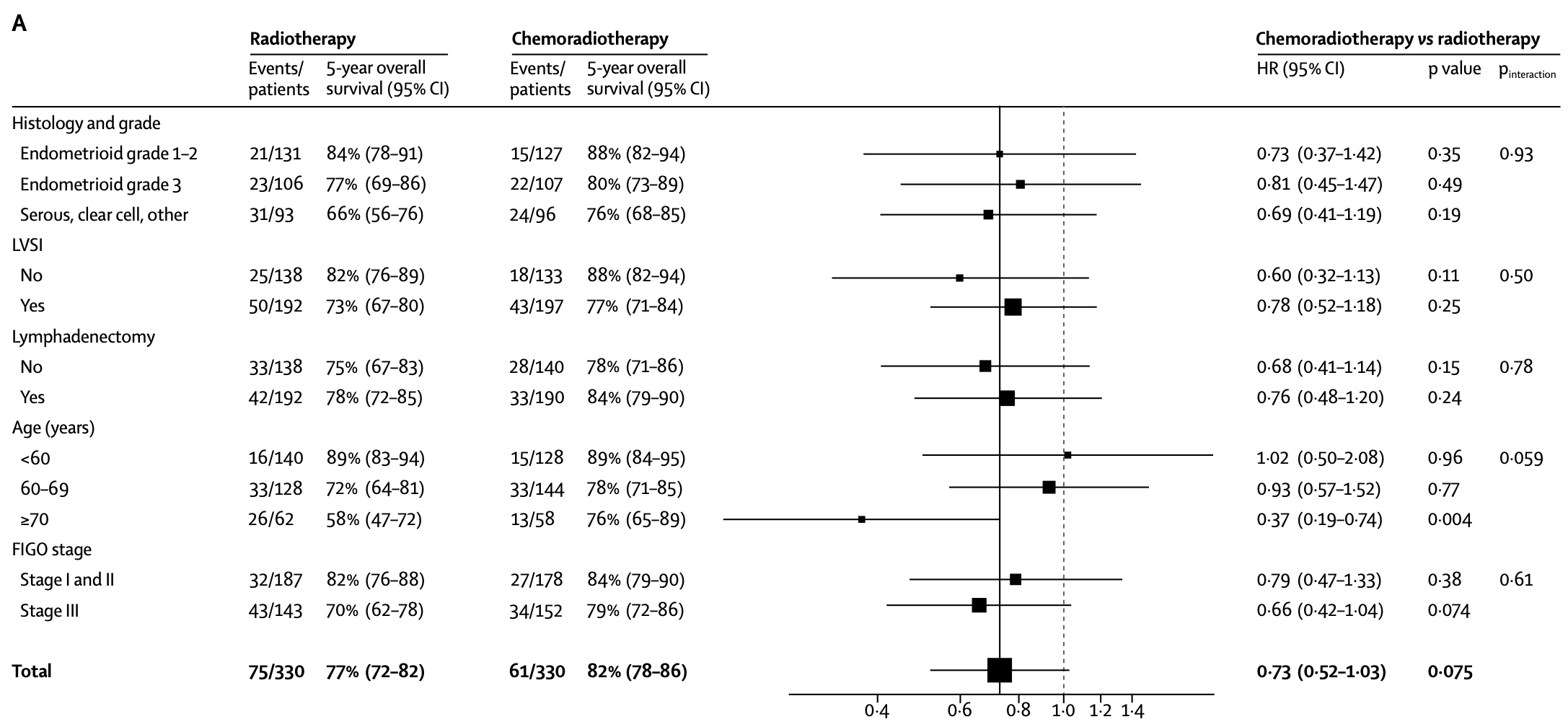
Prognostic Factors - Multivariable Analysis
Only age group was found to be predictive of treatment effect (pinteraction=0.012)
Women aged ≥70 years had the greatest benefit from chemoradiotherapy
Women aged ≥70 years had the greatest benefit from chemoradiotherapy
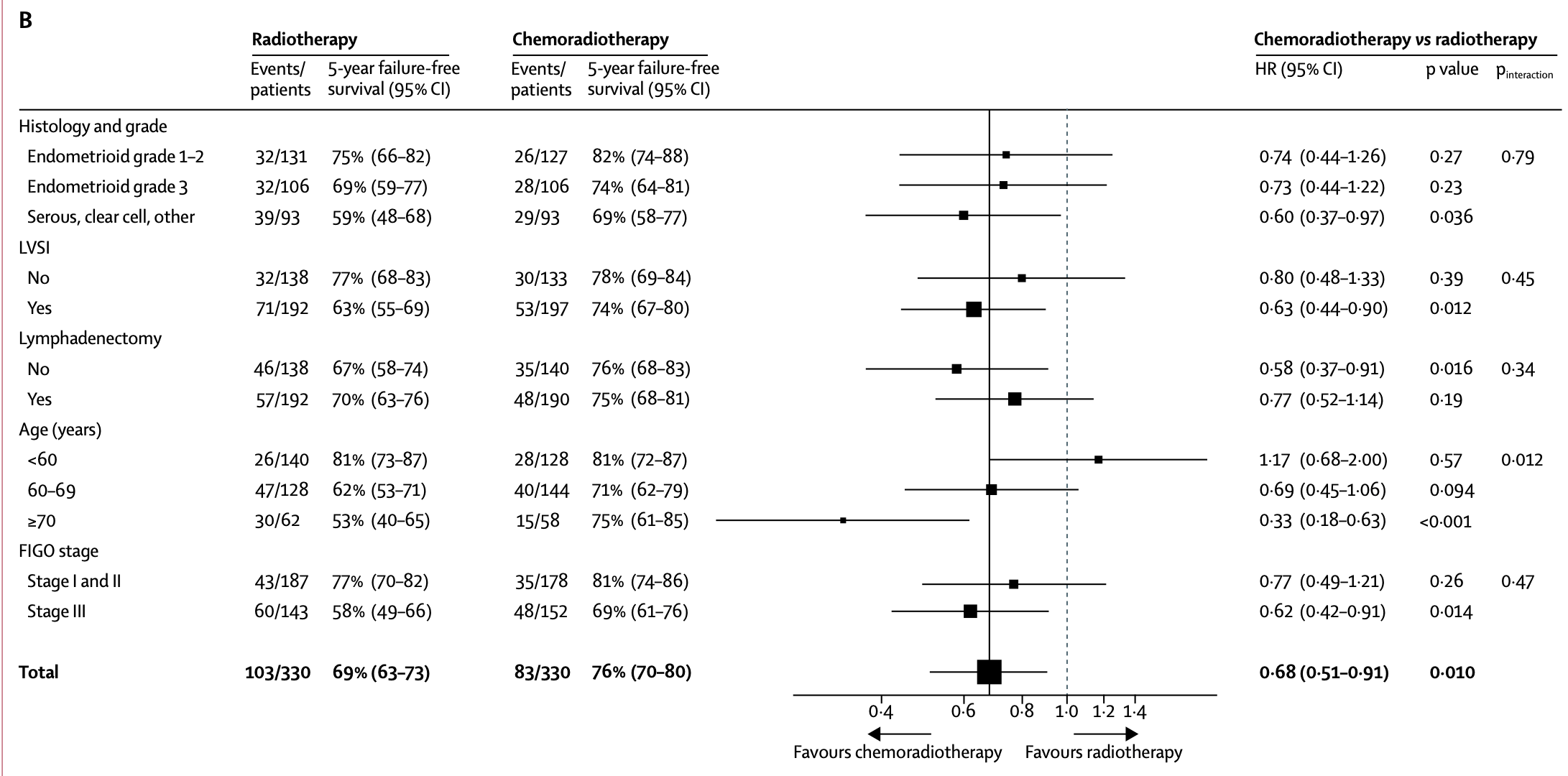
Prognostic Factors - Multivariable Analysis
| Prognostic Factor | 5-year OS HR (95% CI) |
p value | 5-year FFS HR (95% CI) |
p value |
|---|---|---|---|---|
| Age ≥70 vs <60 years | 3.29 (1.99-5.44) | <0.0001 | 2.14 (1.41-3.25) | <0.0001 |
| Age 60-69 vs <60 years | 2.31 (1.48-3.59) | <0.0001 | 1.74 (1.23-2.46) | <0.0001 |
| Stage III vs I-II | 2.41 (1.66-3.51) | <0.0001 | 2.62 (1.90-3.61) | <0.0001 |
| Grade 3 endometrioid vs grade 1-2 | 1.76 (1.10-2.81) | <0.0001 | 1.56 (1.06-2.30) | <0.0001 |
| Serous/clear cell vs grade 1-2 endometrioid | 2.35 (1.48-3.72) | <0.0001 | 2.15 (1.46-3.16) | <0.0001 |
| LVSI present vs absent | 1.36 (0.93-1.98) | 0.11 | 1.36 (0.99-1.87) | 0.054 |
Only age group was found to be predictive of treatment effect (pinteraction=0.012)
Women aged ≥70 years had the greatest benefit from chemoradiotherapy
Women aged ≥70 years had the greatest benefit from chemoradiotherapy
Prognostic Factors
Only age group was found to be predictive of treatment effect (pinteraction=0.012)
Women aged ≥70 years had the greatest benefit from chemoradiotherapy
Women aged ≥70 years had the greatest benefit from chemoradiotherapy
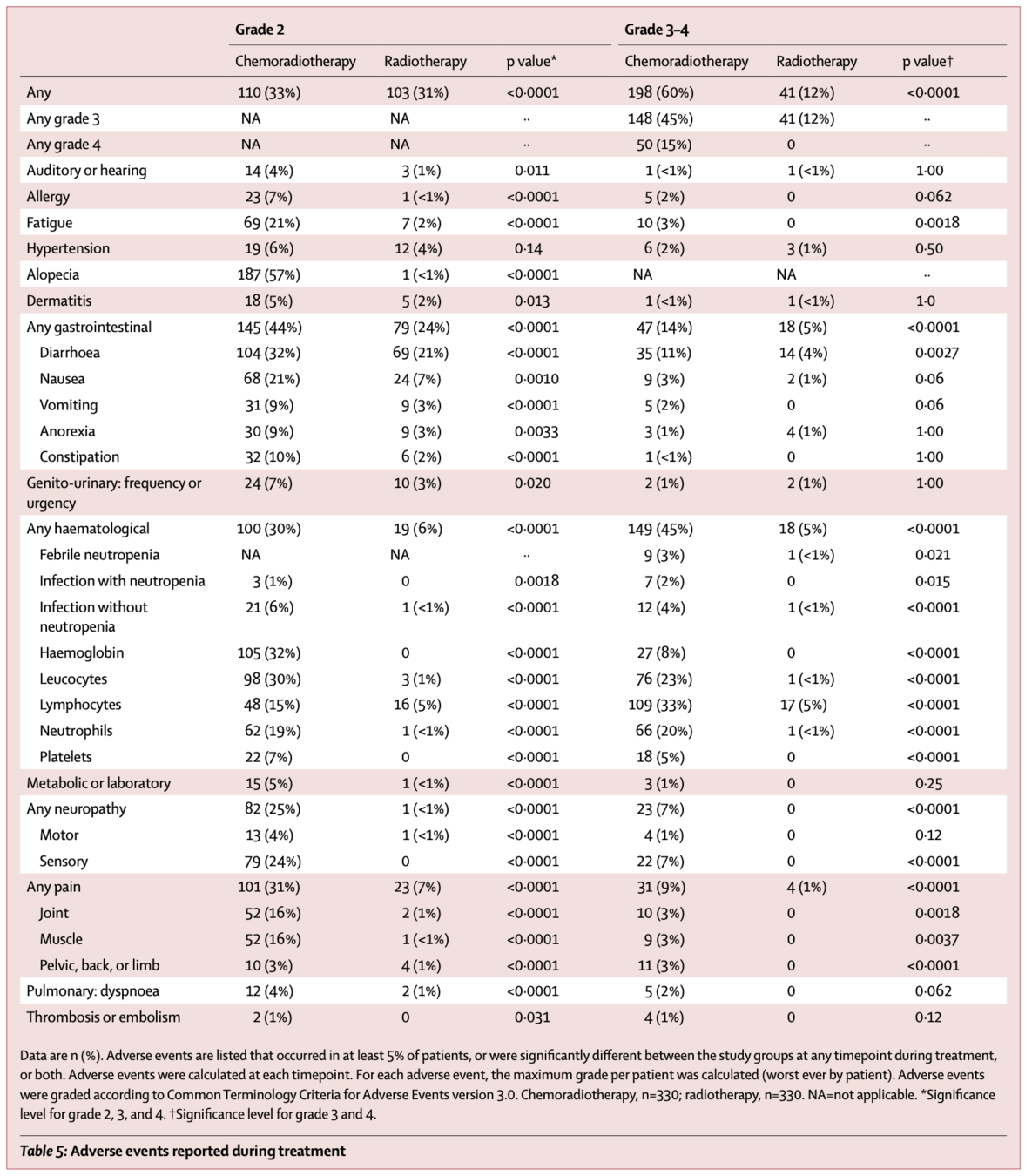
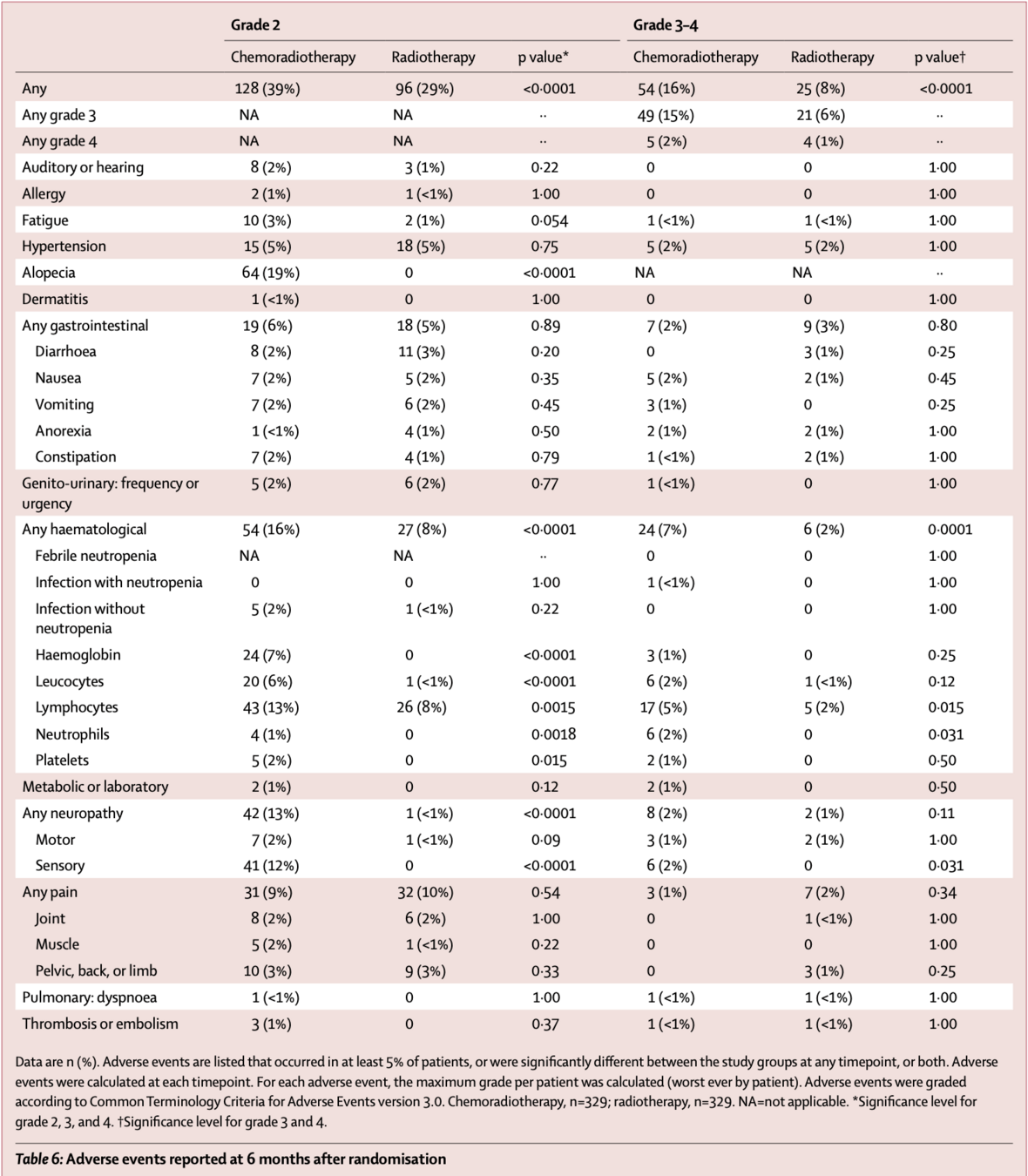
Toxicity During Treatment
| Adverse Events | Chemoradiotherapy n=330 |
Radiotherapy n=330 |
p value |
|---|---|---|---|
| Grade 2 or worse | 308 (93%) | 144 (43%) | <0.0001 |
| Grade 3 or worse | 198 (60%) | 41 (12%) | <0.0001 |
| Selected Grade 3-4 Adverse Events | |||
| Hematological | 149 (45%) | 18 (5%) | <0.0001 |
| Gastrointestinal | 47 (14%) | 18 (5%) | <0.0001 |
| Neuropathy | 23 (7%) | 0 (0%) | <0.0001 |
| Pain | 31 (9%) | 4 (1%) | <0.0001 |
- No treatment-related deaths
- Most adverse events were hematological
- Neuropathy grade 2+ was reported in 25% of patients in chemoradiotherapy group
- From 12 months onwards, no significant differences in grade 3-4 adverse events
- However, grade 2+ sensory neuropathy persisted in chemoradiotherapy group:
- 8% vs 1% at 3 years (p<0.0001)
- 9% vs 0% at 5 years (p<0.0001)
Toxicity After Treatment
| Adverse Events | 6 Months Post-Treatment | 3 Years | 5 Years | |||
|---|---|---|---|---|---|---|
| ChemoRT | RT | ChemoRT | RT | ChemoRT | RT | |
| Grade 2 or worse events | ||||||
| Any | 39% | 29% | 32% | 24% | 40% | 28% |
| Sensory neuropathy | 12% | 0% | 8% | 1% | 9% | 0% |
| Gastrointestinal | 6% | 5% | Detailed data for individual toxicities at 3 and 5 years not reported in this paper | |||
| Hematological | 16% | 8% | ||||
| Fatigue | 3% | 1% | ||||
| Pain | 9% | 10% | ||||
| Alopecia | 19% | 0% | ||||
| Hypertension | 5% | 5% | ||||
| Grade 3 or worse events | ||||||
| Any | 16% | 8% | No significant differences between groups from 12 months onwards | |||
Data from de Boer et al. Lancet Oncology 2018
Conclusions
- Addition of chemotherapy to radiotherapy improved 5-year failure-free survival (75.5% vs 68.6%, p=0.022) but not overall survival (81.8% vs 76.7%, p=0.109)
- Greatest benefit seen in patients with:
- Stage III disease
- Age ≥70 years
- No significant benefit observed for stage I-II disease
- Significantly higher rates of toxicity during treatment with chemoradiotherapy:
- Grade 3-4 adverse events: 60% vs 12%
- Grade 2+ sensory neuropathy persisted long-term
- Pelvic control was high with radiotherapy alone (vaginal recurrence ~2%, pelvic recurrence ~9%)
- Majority of recurrences were distant metastases in both arms
Strengths and Limitations
Strengths
- Large, international, phase 3 trial
- Strong collaborative effort across 6 clinical trial groups
- Balanced baseline characteristics
- Clear eligibility criteria
- High treatment compliance
- Central pathology review
- Comprehensive toxicity assessment
- Inclusion of elderly patients
Limitations
- Open-label design (not blinded)
- Lower event rates than expected
- Final analysis time-based rather than event-based
- Variable use of lymphadenectomy (58% of patients)
- Heterogeneous population (different histologies and stages)
- Insufficient power to detect small differences in overall survival
- No quality of life data presented in this publication
Clinical Implications
- Combined chemoradiotherapy cannot be recommended as new standard for all high-risk endometrial cancer patients
- For stage III disease, chemoradiotherapy should be considered to maximize failure-free survival
- For stage I-II disease, radiotherapy alone provides excellent pelvic control without the added toxicity of chemotherapy
- Benefits and risks of combined treatment should be individually discussed with each patient
- Age should not be a limiting factor - elderly patients derived substantial benefit
- Long-term risk of persistent neuropathy should be balanced against potential benefits
- Further follow-up needed to evaluate long-term survival outcomes
Discussion Points
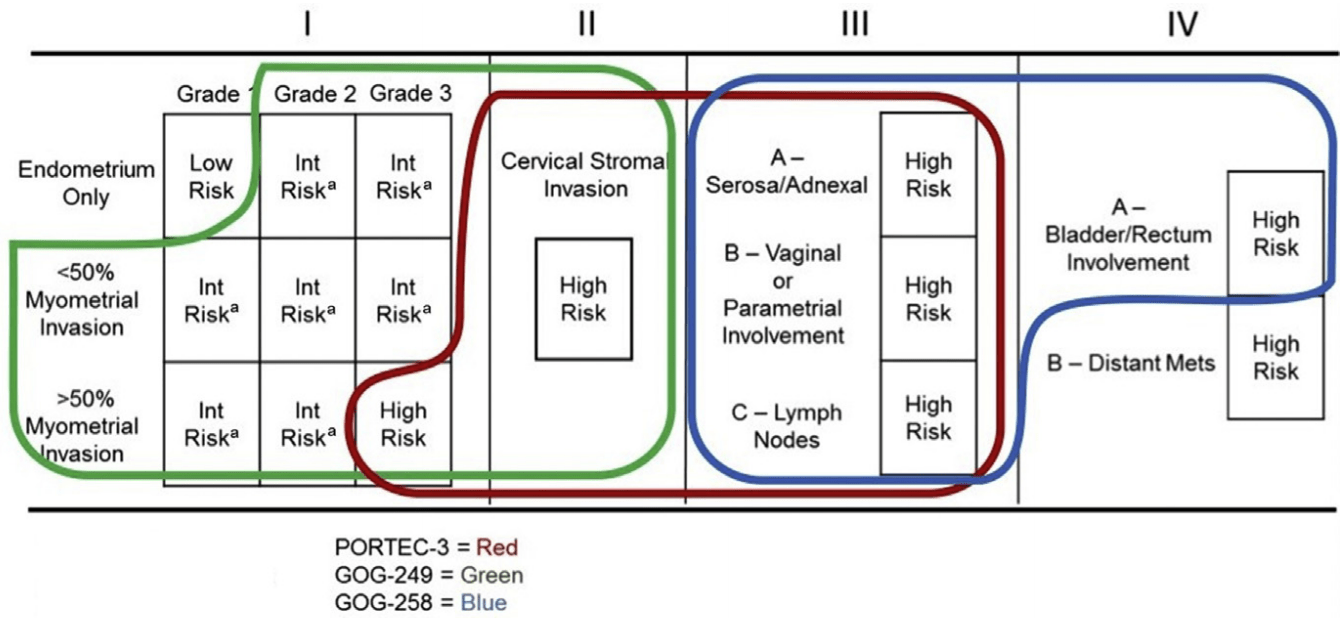
- How do the PORTEC-3 results compare with other trials in high-risk endometrial cancer (GOG-258, GOG-249, NSGO-EC-9501/EORTC-55991)?
- Would a different chemotherapy regimen or sequence (e.g., sequential rather than concurrent) have resulted in different outcomes?
- How should we approach treatment decisions for specific histological subtypes, particularly serous cancers?
- What is the role of molecular classification (POLE, p53, MSI) in treatment decision-making for high-risk endometrial cancer?
- How do we balance the improved failure-free survival in stage III disease against the increased toxicity of combined treatment?
Subsequent Publications from PORTEC-3
- de Boer et al. (Lancet Oncol 2019): Toxicity and quality-of-life results
- Significantly higher symptom burden during and 3 months after treatment
- Rapid recovery of HRQOL after treatment completion
- Persistent sensory neuropathy and tingling in extremities
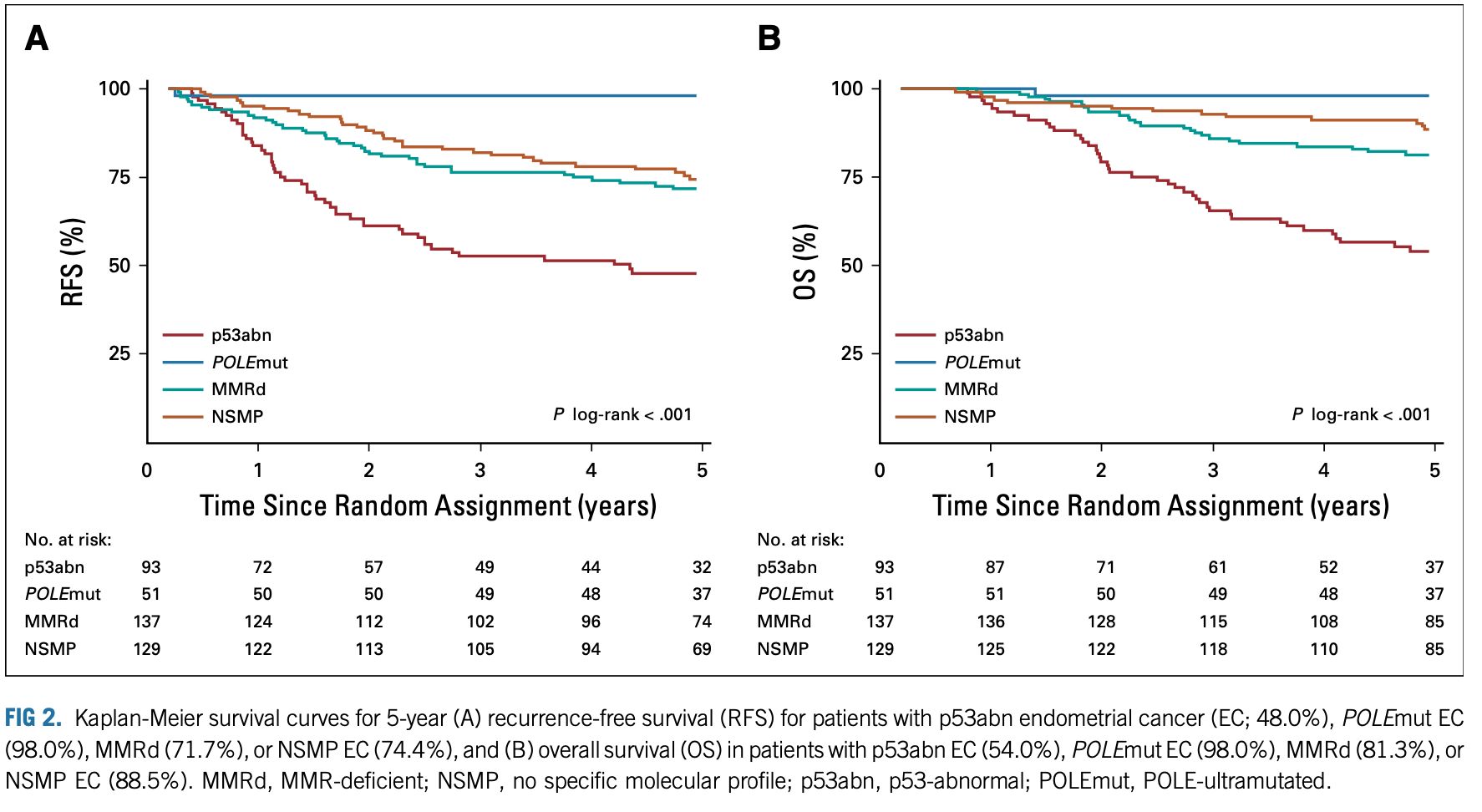
- León-Castillo et al. (JCO 2020): Molecular risk classification
- Benefit of chemoradiotherapy limited to p53-abnormal cancers
- No significant benefit in NSMP, MMRd, or POLE-mutant tumors
- POLE-mutant tumors had excellent outcomes in both arms
- Wortman et al. (IJROBP 2021): Comparison of IMRT vs. 3DCRT
- Significantly less grade 2+ GI toxicity with IMRT (15% vs. 4%)
- Improved patient-reported bowel symptoms with IMRT
PORTEC-3: Adjuvant Chemoradiotherapy versus Radiotherapy Alone for Women with High-Risk Endometrial Cancer
By RadMedSkiier
PORTEC-3: Adjuvant Chemoradiotherapy versus Radiotherapy Alone for Women with High-Risk Endometrial Cancer
Journal club presentation on the international, open-label, phase 3 randomised PORTEC-3 trial
- 92



