Lee et al.
Journal of Clinical Oncology 2024
30 ROC Trial (NCT03323463)
Background
- HPV-related oropharyngeal cancers have favorable outcomes but cause significant long-term toxicity
- Standard treatment: 70 Gy chemoradiotherapy results in dysphagia, xerostomia, dental complications
- Previous de-escalation trials failed:
- Cetuximab substitution → worse outcomes (RTOG 1016, De-ESCALaTE)
- Dose reduction to 60 Gy → inferior oncologic outcomes (NRG HN-002)
- Tumor hypoxia causes radioresistance through reduced free radical production
- FMISO PET can measure tumor hypoxia
De-escalation Trials in HPV+ OPC: Learning from Our Failures
Why De-escalate?
- HPV+ OPC has 85-95% cure rates with standard therapy
- Younger patients living decades with treatment toxicity
- Dysphagia, xerostomia, dental complications affect QOL
- Goal: Maintain cure rates while reducing morbidity
Three Main De-escalation Strategies:
1. Replace Cisplatin
- RTOG 1016
- De-ESCALaTE
- Result: FAILED
2. Reduce RT Dose
- NRG HN-002 (Phase II)
- NRG HN-005
- Result: MIXED
3. Surgery + Reduced Adjuvant
- ECOG 3311
- MC1675
- PATHOS
- Result: PROMISING
Key Learning: Not all de-escalation strategies are equal. Biology-based selection (like 30 ROC) may succeed where unselected de-escalation failed.
RTOG 1016 & De-ESCALaTE: Cetuximab Cannot Replace Cisplatin
RTOG 1016 (n=849)
Design: 70 Gy (6 weeks) + Cisplatin vs Cetuximab
5-year OS: 85% vs 78% (p=0.01)
5-year PFS: 78% vs 67%
5-year LRF: 10% vs 17%
Toxicity: Similar (no benefit)
De-ESCALaTE (n=304)
Design: 70 Gy + Cisplatin vs Cetuximab
2-year OS: 98% vs 89%
2-year recurrence: 6% vs 16%
Toxicity: Similar (no benefit)
Note: Excellent 94% control with cisplatin
Clear Conclusions:
- Cetuximab is inferior to cisplatin - period
- No toxicity benefit despite hopes
- Standard chemoRT achieves 90% local control
- Message: Keep cisplatin for HPV+ disease
The 60 Gy Story: Context for 30 ROC's Bold Design
Timeline: The Optimistic Era of Dose Reduction
- 2017: HN-002 shows promising 60 Gy results → optimism builds
- 2017: 30 ROC opens (NCT03323463) - betting on biology over empiric reduction
- 2018: HN-005 launches with 60 Gy arm based on HN-002
- 2024: HN-005 60 Gy arm closed for futility
The 60 Gy Hope (2017 Perspective)
HN-002 Phase II Results:
- Selected patients (T1-2, ≤10 PY)
- 2-yr PFS 90.5% with 60 Gy
- Met acceptability criteria
- 14% dose reduction seemed safe
"If 60 Gy works, why not go further?"
The 30 ROC Vision (2017)
Different Philosophy:
- Don't reduce dose empirically
- Use biology (hypoxia) to select
- 57% reduction needs safety net
- Test mechanism, not just dose
"Some tumors need 70 Gy, some don't"
Two Parallel Philosophies Emerge:
Empiric Dose Reduction (HN-005)
- Treat everyone the same
- Hope selection criteria work
- Modest 14% reduction
- Result: Failed (selection? dose?)
Biology-Driven Selection (30 ROC)
- Test each tumor's biology
- Personalize based on hypoxia
- Radical 57% reduction if safe
- Result: 95% local control
The Key Insight:
30 ROC investigators understood something fundamental: successful de-escalation requires knowing WHY you can de-escalate, not just hoping a lower dose works.
HN-005's struggles (patient selection? dose? both?) vindicate the 30 ROC approach of using tumor biology rather than clinical criteria alone.
Study Hypothesis
- Tumors without hypoxia can be treated with significantly lower radiation doses
- FMISO PET can identify patients eligible for de-escalation from 70 Gy to 30 Gy
- 30 Gy dose based on curative dose for HPV-related anal cancer
- Pilot study (n=19): 87% pathologic response with 30 Gy in non-hypoxic tumors
Methods: Study Design
- Phase II, single-arm trial
- Primary endpoint: 2-year locoregional control
- Non-inferiority design with 7% margin (historical control 95%)
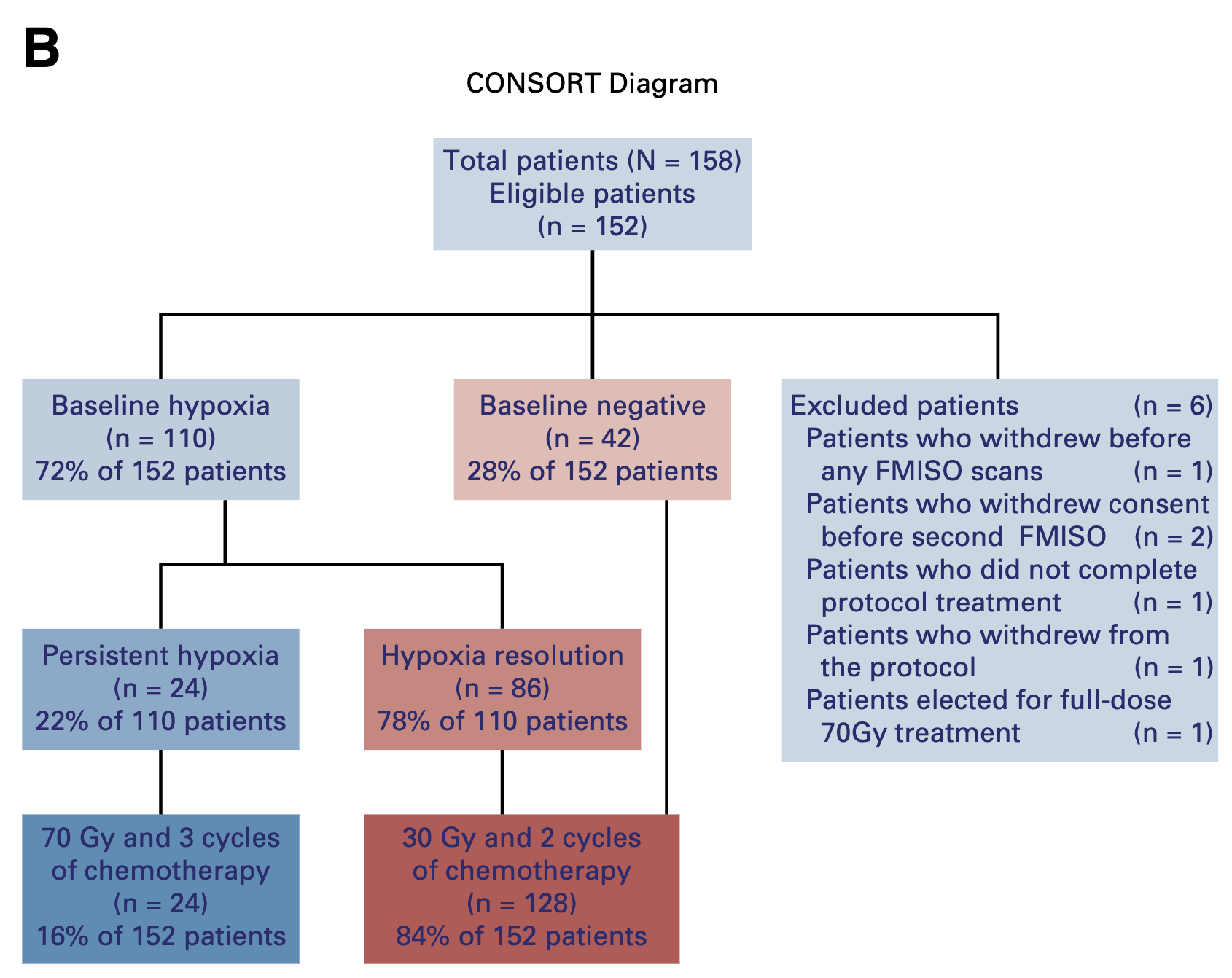
- 158 patients enrolled (152 eligible)
- Multi-site within Memorial Sloan Kettering network
Eligibility Criteria
- HPV-positive oropharyngeal carcinoma (p16+ or HPV RNA ISH+)
- T0-2, N1-2c, M0 (AJCC 7th edition)
- Age ≥18 years
- ECOG 0-2
- Able to receive high-dose cisplatin or carboplatin/5-FU
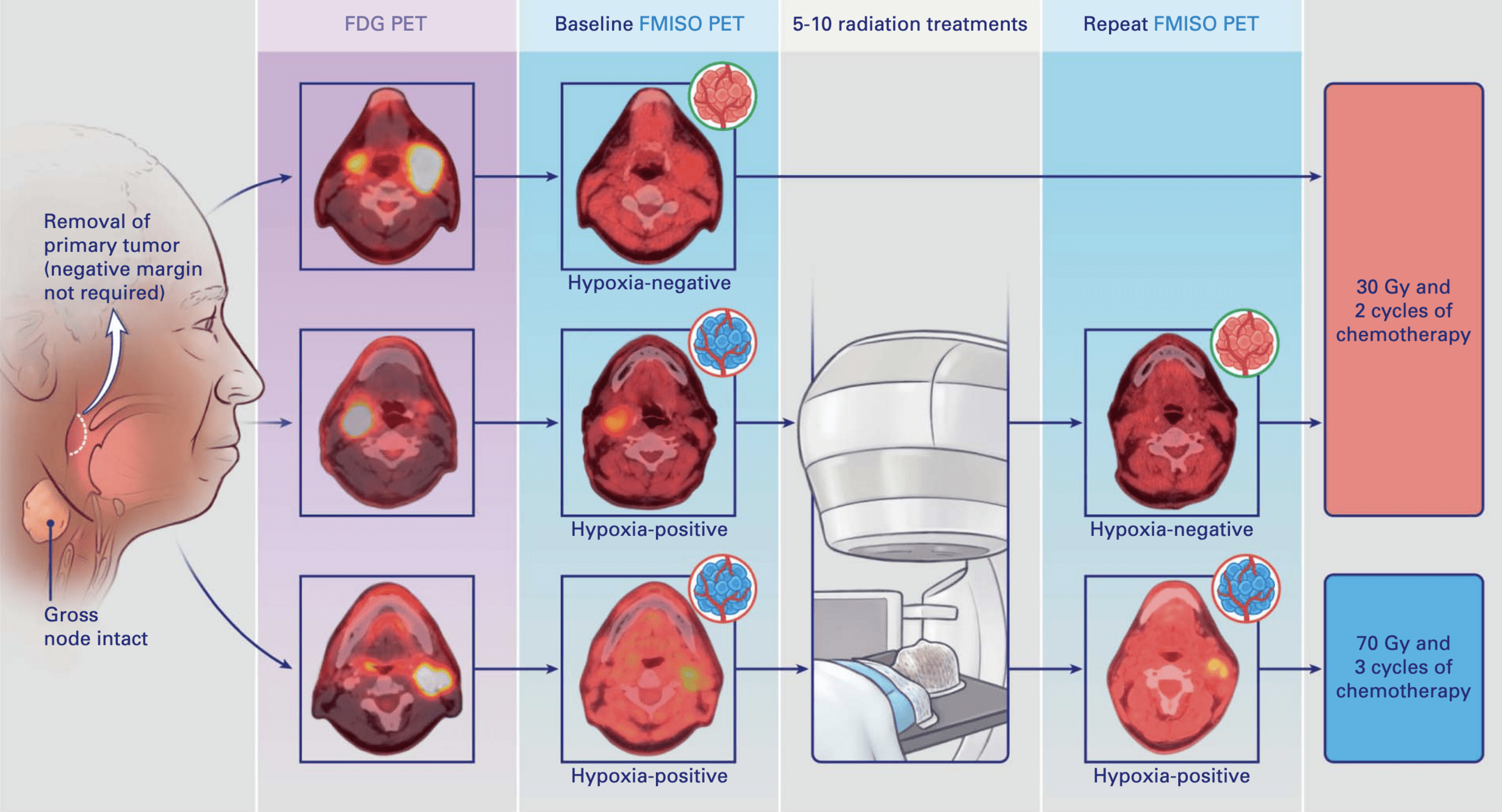
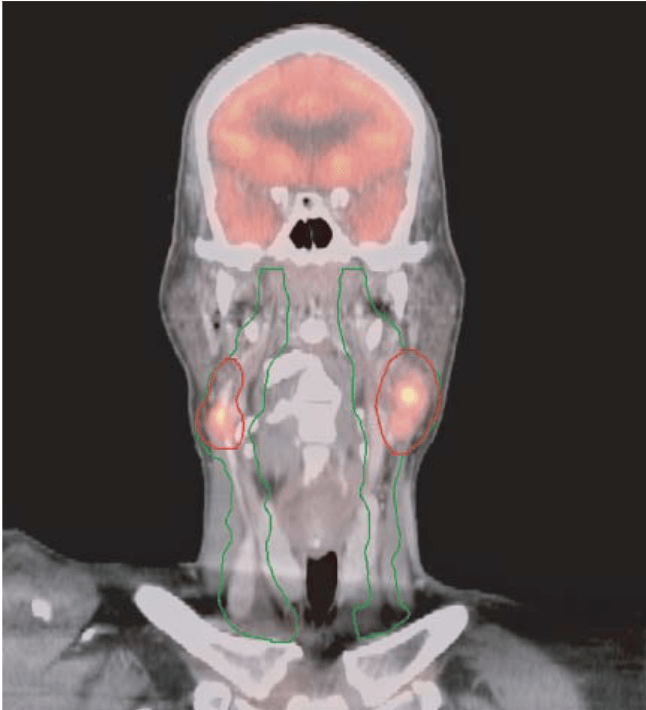
What is FMISO?
- 18F-Fluoromisonidazole - a 2-nitroimidazole radiotracer
- Most validated PET tracer for hypoxia imaging
- Half-life: 109.8 minutes (ideal for delayed imaging)
- Moderate lipophilicity allows cellular uptake
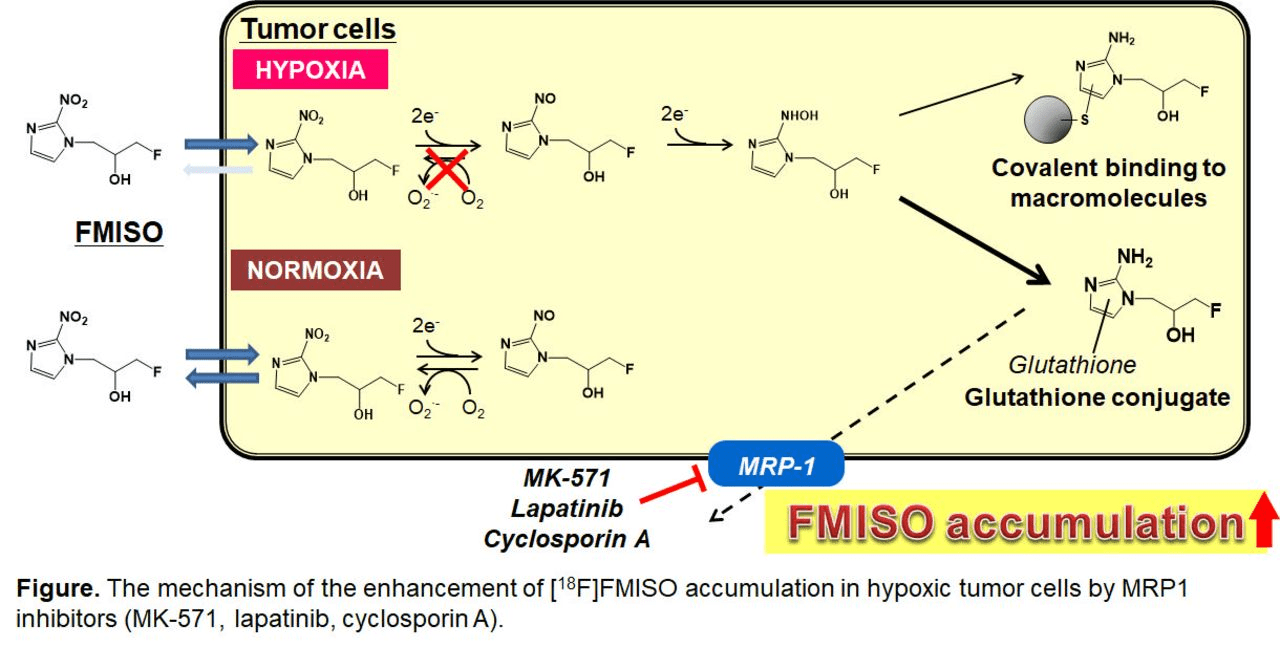
Mechanism of Action
- Enters cells via passive diffusion
- In normoxic cells (pO₂ >10 mmHg):
→ Re-oxidized and washed out - In hypoxic cells (pO₂ <10 mmHg):
→ Undergoes nitroreductase-mediated reduction
→ Forms reactive intermediates
→ Covalently binds to macromolecules
→ Trapped in viable hypoxic cells
Imaging Protocol
- Patient prep: 4-6 hour fast, good hydration
- Dose: 2-4 MBq/kg (150-400 MBq typical)
-
Critical: 2-4 hour delay before imaging
→ Allows clearance from normoxic tissue
→ Maximizes hypoxic contrast - 10-20 minute acquisition per bed position
Interpretation (30 ROC Trial)
- Hybrid method (visual + quantitative)
- Tumor-to-background ratio (TBR) >1.3
- Visual assessment of 4 image characteristics
- Visual prevails if disagreement
- Excellent inter-observer agreement (κ = 0.859)
Key Point: FMISO specifically accumulates in viable hypoxic cells (not necrotic tissue), providing a functional map of radioresistant tumor regions that can guide personalized dose escalation or de-escalation strategies.
Treatment Allocation
-
30 Gy cohort (84% of patients):
- Baseline FMISO negative OR
- Hypoxia resolution on repeat FMISO
- 15 fractions with concurrent chemotherapy
-
70 Gy cohort (16% of patients):
- Persistent hypoxia on repeat FMISO
- 35 fractions with concurrent chemotherapy
- Chemotherapy: Cisplatin 100 mg/m² or Carboplatin/5-FU
- All patients had surgical removal of primary tumor
Radiation Planning: MSKCC Contouring & Dose Specifications
Target Volume Definitions
- Post-op cavity + 5-10 mm margin
- Include pre-op GTV extent
- Tonsil: Include ipsilateral pterygoid plates
- BOT: Include pre-epiglottic space
- Unknown primary: Entire oropharyngeal axis
CTV Nodal:
- Gross nodes + 5 mm (no additional margin if >3cm)
- Entire involved nodal level
- No intentional ECE coverage required
CTV Elective:
- Bilateral levels II-IV (standard)
- Level IB if oral cavity involvement
- Retropharyngeal if posterior pharyngeal wall
- Well-lateralized T1-2 tonsil + single node → ipsilateral only
Dose Prescription
ALL PATIENTS - First 30 Gy:
- PTV_3000: All volumes (primary, nodes, elective)
- 2 Gy × 15 fractions = 30 Gy
- Delivered regardless of hypoxia status
HYPOXIA NEGATIVE/RESOLVED (84%):
- Stop at 30 Gy total dose
- 3 weeks total treatment time
PERSISTENT HYPOXIA (16%):
- PTV_7000: Hypoxic gross nodes only
- Additional 2 Gy × 20 fractions = 40 Gy boost
- Total 70 Gy to hypoxic nodes
- 7 weeks total treatment time
Planning Specifications
- Technique: IMRT (VMAT acceptable)
- PTV margins: 3-5 mm from CTV
- Coverage: 95% of PTV receives 95% of Rx dose
- Hot spots: <107% within PTV, <110% point max
- Plan review: 2 radiation oncologists (N.Y.L. and N.R.)
Critical Innovation: Universal elective dose of 30 Gy (vs historical 50-63 Gy) dramatically reduces toxicity while maintaining excellent regional control. This represents a paradigm shift in elective nodal treatment.
Patient Characteristics
- Median age: 59 years (90% male)
- Primary site: Tonsil 55%, BOT 27%, Unknown 18%
- Stage: 89% stage IVA (AJCC 7th)
- Never smokers: 53%
- Baseline hypoxia: 72% (110/152 patients)
- Hypoxia resolution: 78% (86/110 patients)
- Smokers 3x more likely to have persistent hypoxia (p=0.02)


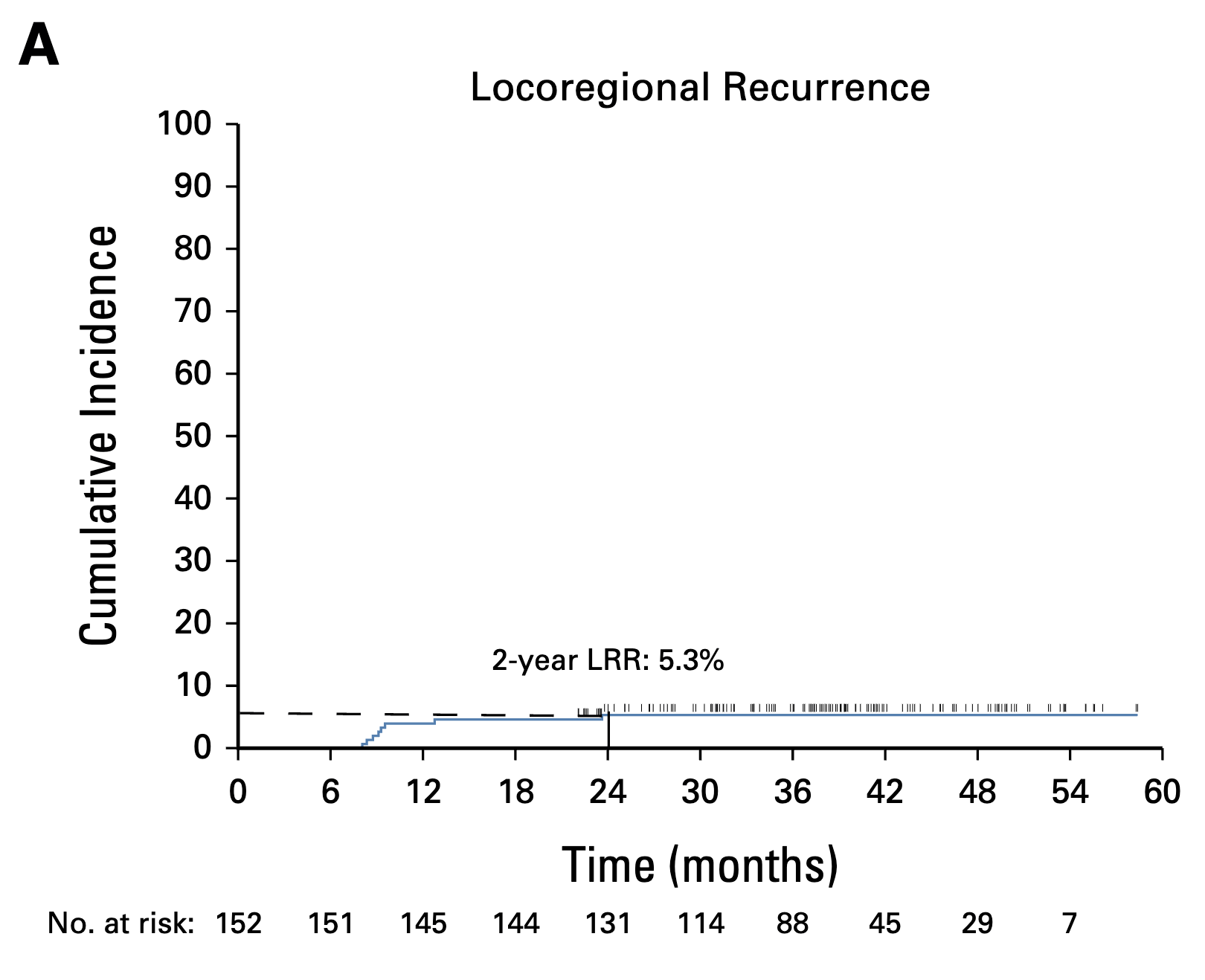
Primary Outcome: Locoregional Control
- 2-year LRC: 94.7% (95% CI: 89.8-97.7)
- Met primary objective (>88% lower bound)
- Median follow-up: 38.3 months
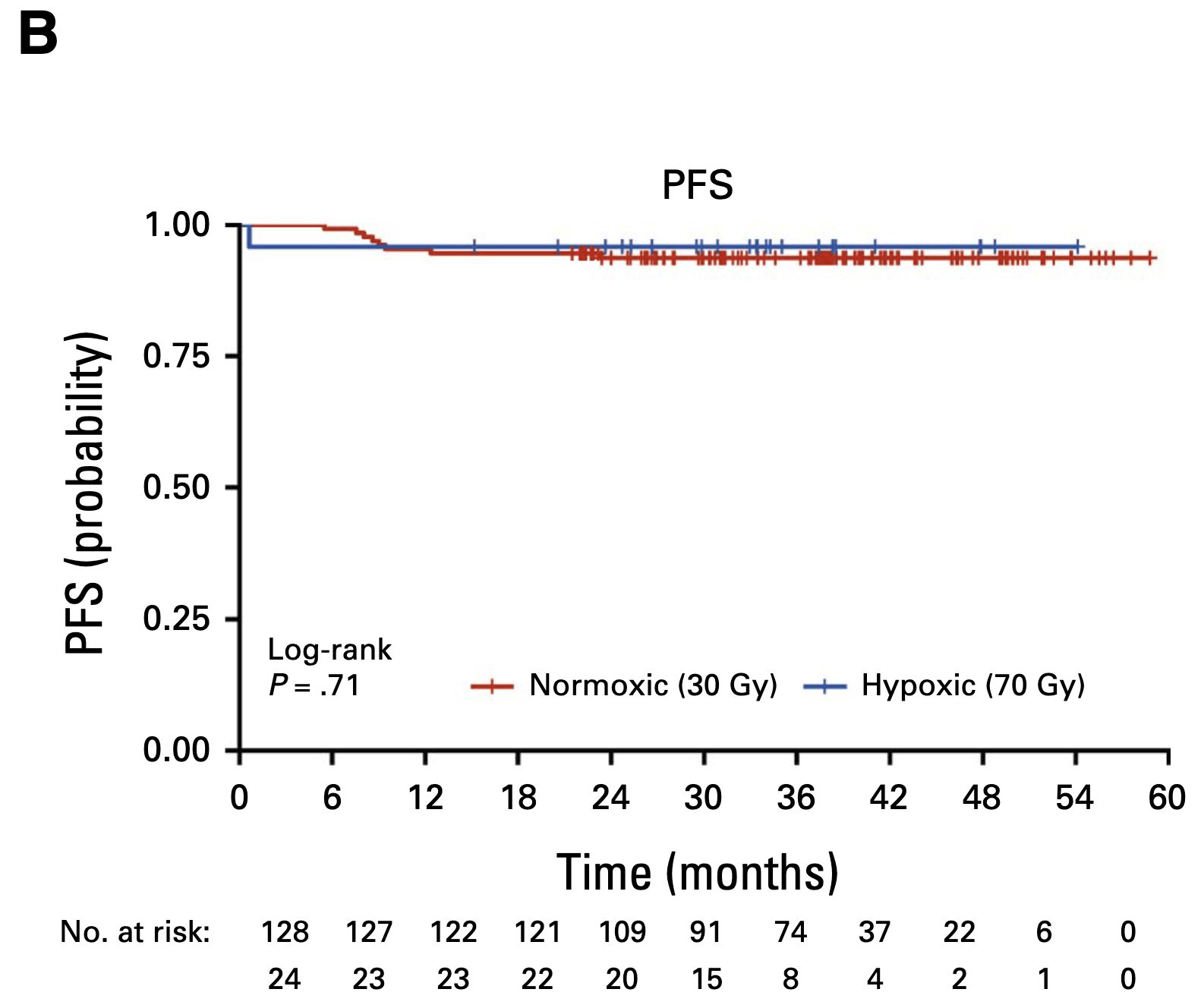
Secondary Outcomes
-
30 Gy cohort (n=128):
- 2-year PFS: 94%
- 2-year OS: 100%
- 8 nodal recurrences (all salvaged with neck dissection)
- 1 distant metastasis
-
70 Gy cohort (n=24):
- 2-year PFS: 96%
- 2-year OS: 96%
- No locoregional failures
- No primary site recurrences in either cohort
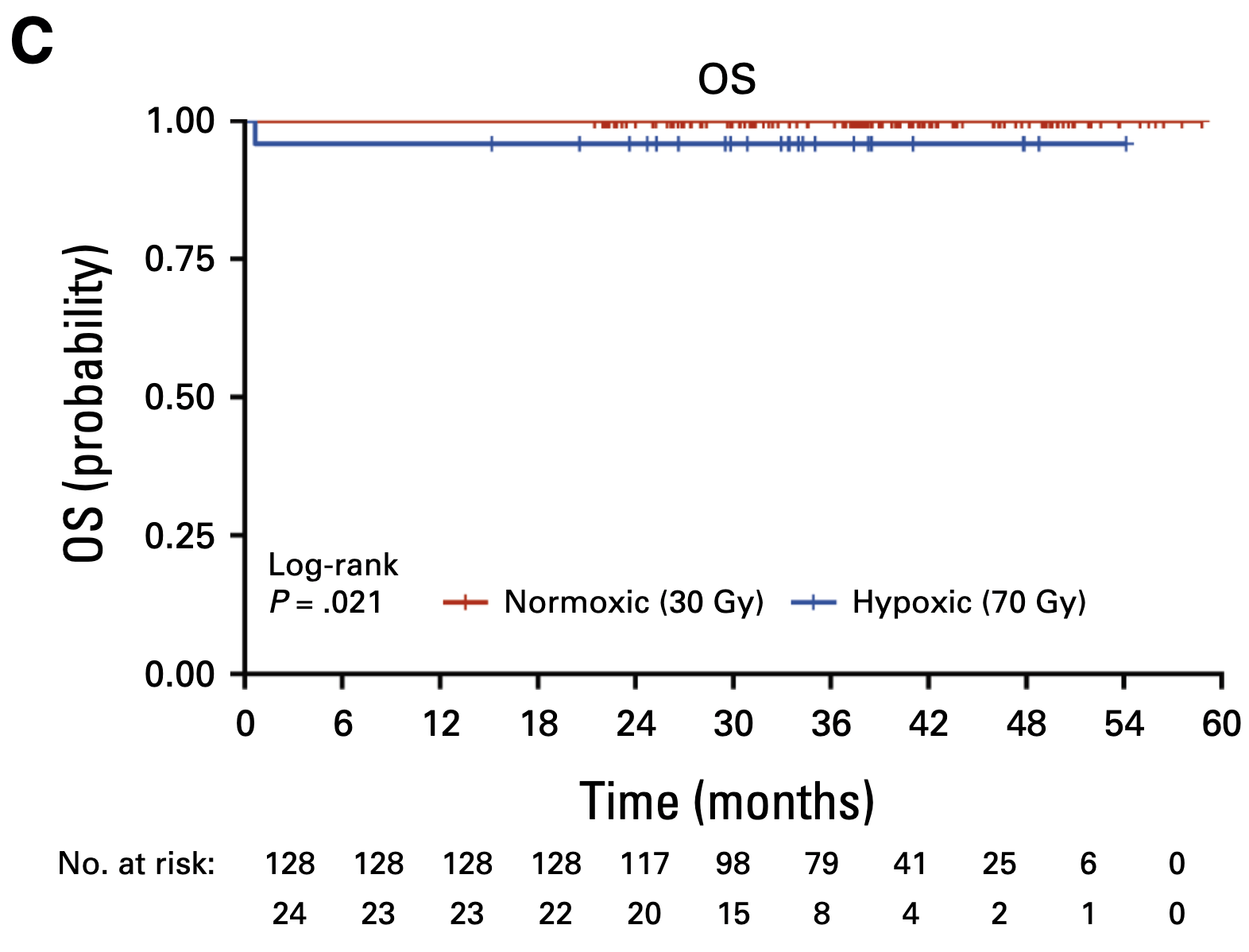
Acute Toxicity
- Grade 3-4 acute toxicity: 32% (30 Gy) vs 58.3% (70 Gy), p=0.02
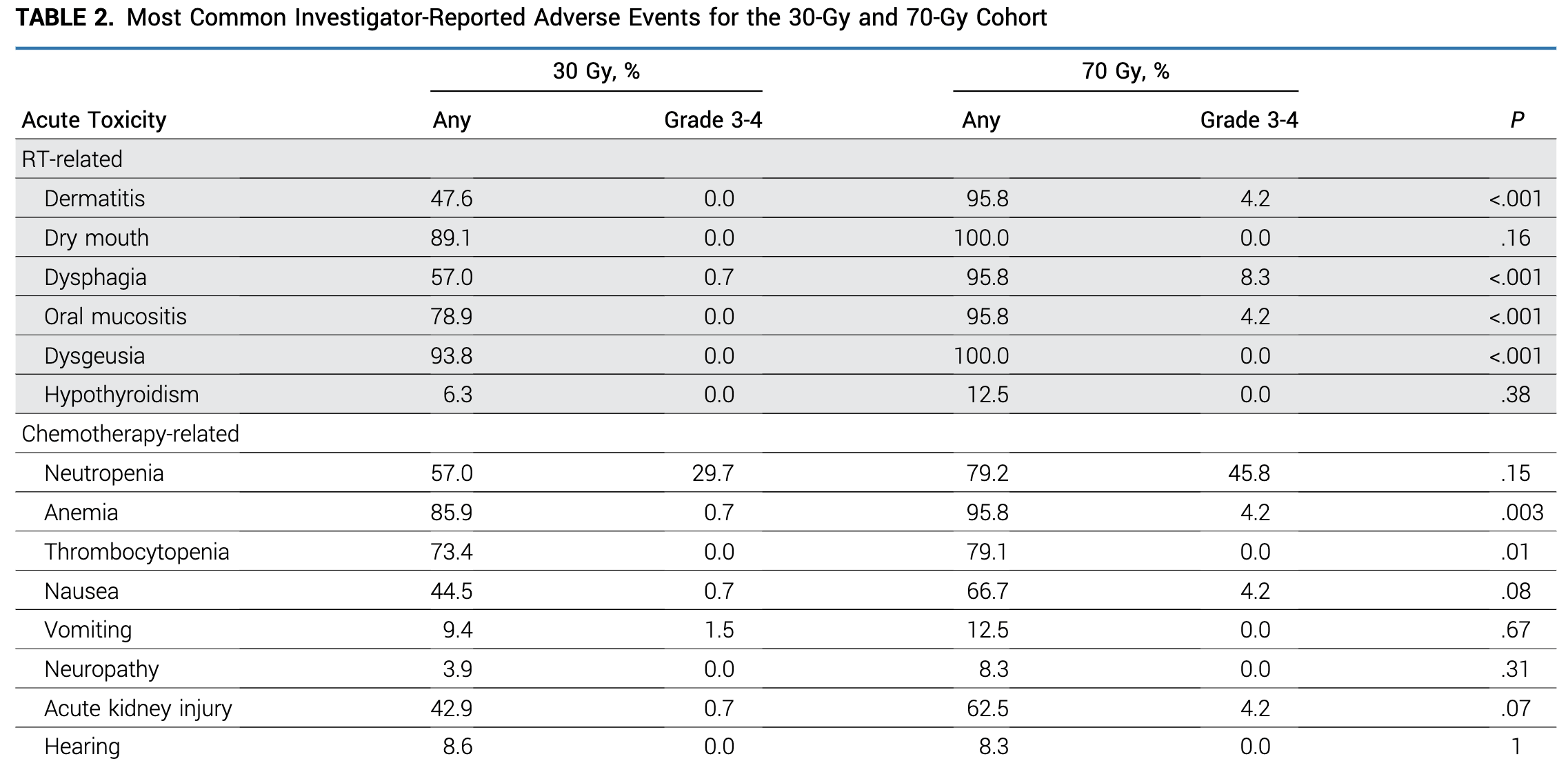
Late Toxicity and PROs
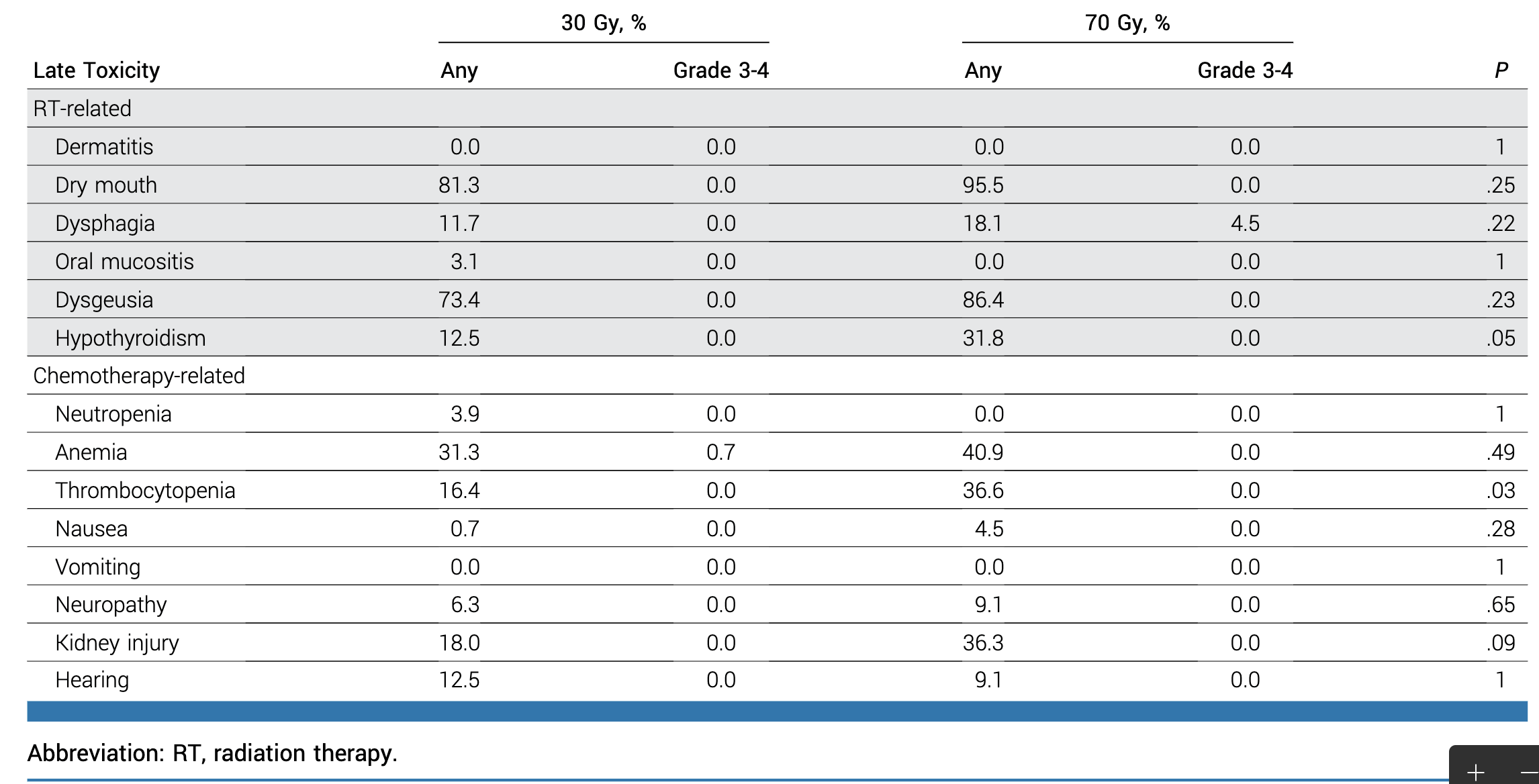
- Grade 3-4 late toxicity only in 70 Gy cohort (4.5% dysphagia)
- Grade 2 late toxicity (entire cohort):
- Xerostomia: 2.6%
- Dysgeusia: 5.2%
- Dysphagia: 1.4%
- Modified barium swallow at 1 year: No moderate dysphagia in 30 Gy cohort
- MDADI scores improved from baseline to 1 year
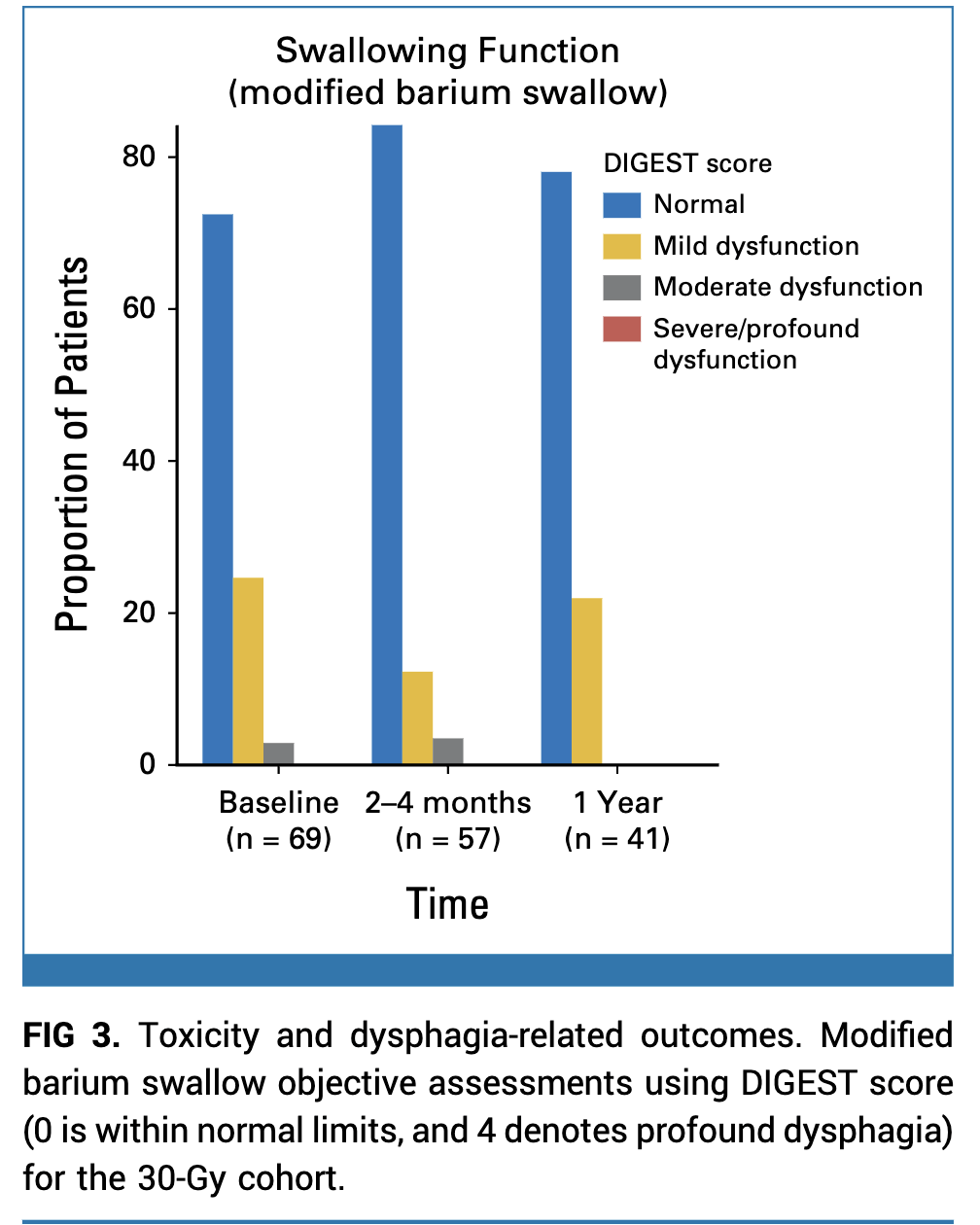
Late Toxicity and PROs
Exploratory Biomarkers
- MRI parameters (ADC, volume) did not predict hypoxia status
- ctDNA detectable in 64% at 2 weeks → cannot replace FMISO PET
- Financial toxicity: 63% reduction in healthcare costs (30 Gy vs 70 Gy)
Surgery in HPV+ OPC: Why 30 ROC Went Against the Grain
The Radiation Oncology View of Surgery:
- ORATOR2 terminated early: 4% surgical mortality (2/35 patients died from post-op hemorrhage)
- ECOG 3311: Only 0.2% mortality (1/495) - but was this cherry-picked excellence?
- Definitive chemoRT: Zero surgical mortality risk, 85-95% cure rates
- The verdict: Why risk surgery when radiation works perfectly?
Evidence Against Surgery:
- ORATOR2: PFS 100% (RT) vs 84% (surgery)
- Positive margins: 8% requiring adjuvant RT anyway
- Variable surgical quality across centers
- 78% still needed radiation after surgery
- Two treatments = two sources of morbidity
Why Some Still Pursue Surgery:
- ENTs: "ORATOR2 doesn't represent good surgery"
- High-volume centers claim better outcomes
- Pathologic staging information
- Potential to avoid chemotherapy (30-40%)
- Patient preference for "cutting it out"
So why did 30 ROC require surgery? Not because Nancy Lee believes in surgery for HPV+ disease, but because 30 Gy was too radical to test without removing the primary first.
The Context of Dose De-escalation Failures:
- RTOG 1016 & De-ESCALaTE: Cetuximab failed → stick with cisplatin
- NRG HN-002: 60 Gy looked promising in Phase II
- NRG HN-005: 60 Gy arm closed for inferiority → need 70 Gy
- Lesson learned: Even modest de-escalation (60 Gy) can fail
The 30 ROC Gamble:
- Standard: 70 Gy (proven)
- Failed attempt: 60 Gy (14% reduction)
- 30 ROC: 30 Gy (57% reduction!)
- 4x more aggressive than failed trials
- Based on anal cancer data
Why Surgery Made It Possible:
- Primary site failure → catastrophic
- Re-irradiation to 70+ Gy → severe toxicity
- Surgical salvage of primary → high morbidity
- But nodal salvage → feasible
- IRB approval required this safety net
The Investigators' True Intent:
This was NOT a surgery trial. This was a hypoxia-directed dose trial.
- Surgery = enabling tool, not the intervention being tested
- FMISO PET = the real innovation
- 84% could get 30 Gy based on biology
- Future goal: Apply to intact primaries once proven safe
"Given that 60 Gy failed, testing 30 Gy on intact primaries would have been unethical. Surgery wasn't philosophically preferred - it was pragmatically necessary."
What 30 ROC Teaches Us (Despite the Surgery):
Biological Insights:
- FMISO PET reliably identifies hypoxia
- 84% of HPV+ tumors lack persistent hypoxia
- Non-hypoxic disease needs much less dose
- 30 Gy controls non-hypoxic nodes perfectly
- Biology > clinical factors for selection
Technical Advances:
- 30 Gy to elective volumes is safe
- Dramatic toxicity reduction achieved
- FMISO interpretation standardized
- Multi-site feasibility demonstrated
- Financial toxicity reduced 63%
The Path to Non-Surgical Implementation:
Next Steps for Radiation Oncology:
- Dose finding for primaries: Test 50-60 Gy for FMISO-negative intact tumors?
- Selective boost strategy: 50 Gy whole field + 20 Gy to hypoxic volumes only?
- Combine biomarkers: FMISO + ctDNA + MRI for better selection?
- Start conservatively: Small T1N0-1 primaries first?
- EORTC "Best of" results: Will definitive answers on surgery vs RT debate
What NOT to Take from 30 ROC:
• Surgery is necessary for HPV+ OPC
• TORS should be routine
• 30 Gy is ready for intact primaries
• This validates surgical approaches
What TO Take from 30 ROC:
• Biology-based selection works
• Massive de-escalation is possible
• FMISO PET is clinically ready
• Future is personalized dose
Bottom Line: 30 ROC used surgery as a necessary safety measure, not an endorsement. The real advance is proving hypoxia-directed therapy works. Now we must adapt this to our non-surgical patients.
Strengths
- First personalized radiotherapy trial using functional imaging
- Dramatic dose reduction (57%) with maintained efficacy
- Excellent inter-observer agreement for FMISO interpretation
- Comprehensive toxicity assessment (PRO + objective)
- Median follow-up >3 years
- Multi-site feasibility demonstrated
Limitations
- Single-arm design (no randomized comparison)
- Selected population (surgical candidates, T0-2)
- Primary tumor removed (limits generalizability)
- FMISO PET not widely available
- Limited evidence for FMISO as prognostic biomarker in HPV+ disease
- Surgical morbidity not accounted for
Discussion Points
- Is the 7% non-inferiority margin appropriate for this dramatic dose reduction?
- How does primary tumor resection impact the generalizability?
- What are the barriers to implementing FMISO PET in clinical practice?
- Should we wait for Phase III results or consider early adoption?
- How does this compare to other de-escalation strategies (immunotherapy, protons)?
- What is the role of salvage therapy planning in de-escalation trials?
Hypoxia-Directed Treatment of HPV-Related Oropharyngeal Carcinoma
By RadMedSkiier
Hypoxia-Directed Treatment of HPV-Related Oropharyngeal Carcinoma
Journal club presentation on the 30 ROC trial by Lee et al., JCO 2024
- 49



