Managing pathogen spillover at the wildlife-livestock interface
Kezia Manlove, Paul Cross, Ryan Miller, Steve Sweeney, Tom Besser, & Frances Cassirer
Goal
Develop a general quantitative model to describe pathogen spillover risk at the wildlife-livestock interface





Reservoir
Recipient
Index case
Onward transmission
Spillover process


Reservoir
Recipient
Index case
Onward transmission




(...or livestock into wildlife)

Plowright et al. 2017 Nat Rev Micro
Potentially relevant factors
Between-host transmission in reservoir
Interspecific contact
Pathogen establishment
Between-host processes
Pathogen movement and amplification in reservoir
Epidemic growth rate in recipient
Movement restrictions in reservoir
Attractant management
Density reductions
Zoning in recipient
Depopulation/Stamping out
Phytosanitary controls
Biosecurity
Preventive vaccination
Test/Cull reservoir
Treatment
Retroactive vaccination
Management Strategies
Less certainty in space
Less time to act
Distance host could move while infected
Epidemic growth rate
Reduce reservoir prevalence or density
Stop onward transmission:
stamping out or targeted vaccination
Reduce interspecific contact: biosecurity
Two questions
2. Where do actual systems fit on these axes?
1. Do management predictions hold up in silico?
Distance host moves while infected
Epidemic growth rate
Biosecurity
Manage in reservoir
Stamp-out in recipient
Does the framework hold up?
Reservoir movement and disease
Interspecific contact & spillover
Recipient movement and disease
Parameters:
Epidemic growth rate
Contact structure
Natural disease process
Management experiments

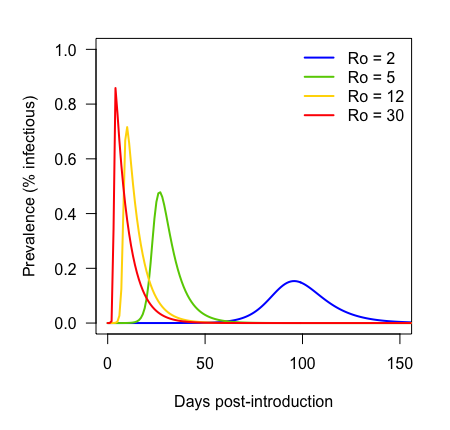
Disease parameter space
Epidemic growth rate
Dispersion of movement kernel

Increasing dispersion
Increasing epidemic growth rate
Colors ramp according to first day cell became infected
Outputs
1) Number of reservoir patches infected
2) Number of recipient patches infected
3) Total recipients infected over whole simulation
Management
Vaccination:
Depopulation:
Test-cull:
Biosecurity:
Adjust recipient SIR state-space so that R goes up, and S goes down
Reset recipient patch to S = 1, I = 0, R = 0
If prevalence exceeds some threshold...
Adjust reservoir SIR state-space so that R goes up, and S goes down
Decrease interspecific contact rates at designated patches
Apply management up to threshold investment (in $$), then stop managing


Increasingly aggregated
Increasingly aggregated
Transmission coefficient
(prop. to epidemic growth rate)
Transmission coefficient
(prop. to epidemic growth rate)
Does this hold empirically?
Where do actual systems fit?
Wildlife-Livestock systems
African swine fever
Canine distemper virus
Rinderpest*
Tularemia
Rabies
Brucellosis
M.ovi
Avian influenza
Classical swine fever
Salmonella
Foot and Mouth disease
Echinococcus
Paratuberculosis
Scrapie
Anthrax
bTB
Q fever
West Nile virus
Pseudorabies virus
Bluetongue virus
Human systems
Measles
HIV
SARS
Polio
Rubella
Smallpox
Pertussis
Diphtheria
Chickenpox
Multihost systems
Phocine distemper virus
Lyme disease
Plague

Pathogen type
dsRNA viruses
Bluetongue
dsDNA viruses
African swine fever
Chickenpox
Smallpox
ssRNA viruses
Rinderpest
Rabies
Phocine, Canine distempers
Avian influenza
Classical swine fever
Food and Mouth disease
West Nile virus
Measles
SARS
Polio
Rubella
Tapeworm
Echinococcus
Bacteria
Tularemia
Q fever
Brucellosis
Movi
Salmonella
Movi
Anthrax
Paratuberculosis
Pertussis
Diphtheria
bTB

Pathogen type
Mode of transmission
Exudate discharge
Classical swine fever
Pseudorabies virus
Canine distemper virus
Saliva
Diphtheria
Bites
Rabies
Aerosolized
Foot and Mouth disease
Q fever
Respiratory droplets
Chickenpox
Rinderpest
Phocine distemper
M.ovi
Measles
SARS
Rubella
Smallpox
Pertussis
Fecal-oral
Echinococcus
Avian influenza
Salmonella
Polio
Vectored
African swine fever
Tularemia
West Nile virus
Bluetongue
Environmental
Anthrax
Paratuberculosis
Scrapie
bTB
| Pathogen | Reservoir host | Time to recovery/death (days) | Potential movement during I |
|---|---|---|---|
| Avian influenza | mallards | 7 | 360 km |
| Rabies | raccoons | ~ 35 | 1-2 km |
| Brucellosis | elk | 730 | (100 km?) |
| BHS pneumonia | (bighorn?) sheep | 20 | 25 km |
| bTB | white-tailed deer | 730 | 15 km |
| Bluetongue | Culicoides spp. | 20.6 | 150 km |
| ASF | warthogs | 20-40 |
Movement potential while infected

Depopulation
Test-cull

Distance host could move while infected
Reducing reservoir prevalence or density
Halting onward transmission
Reducing interspecific contact through biosecurity on recipient
Epidemic growth rate
Is this useful?
To-do:
- Finish parameterization
- Expand empirical dataset
- Sensitivities to investment, premise size, reservoir density
Questions?
t = 1
X

Deterministic disease updates
t = 1
t = 2
Hosts move according to kernel
Mover's infection status depends on prevalence
Stochastic movement updates


t = 2
Deterministic disease updates
Spillover
Random subset of patches chosen to harbor "recipient hosts"
Recipients get infected following random contacts with infected LOCAL reservoir hosts
Recipient map
Reservoir map
Recipient map
General Framework - V2
By Kezia Manlove
General Framework - V2
Proposed general framework for considering spillover risk at the Wildlife-Livestock interface. June 8, 2017; K. Manlove, preparer.
- 674



